Evaluation of Various Inoculation Methods on the Effect of Beauveria bassiana on the Plant Growth of Kiwi and on Halyomorpha halys Infestation: A Two-Year Field Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Population of H. halys Measurements
2.3. Number of Infested Fruits
2.4. Plant Growth Parameters
2.5. Total Chlorophyll Content of A. deliciosa “Hayward” Leaves
2.6. Proline Determination
2.7. Statistical Analysis
3. Results
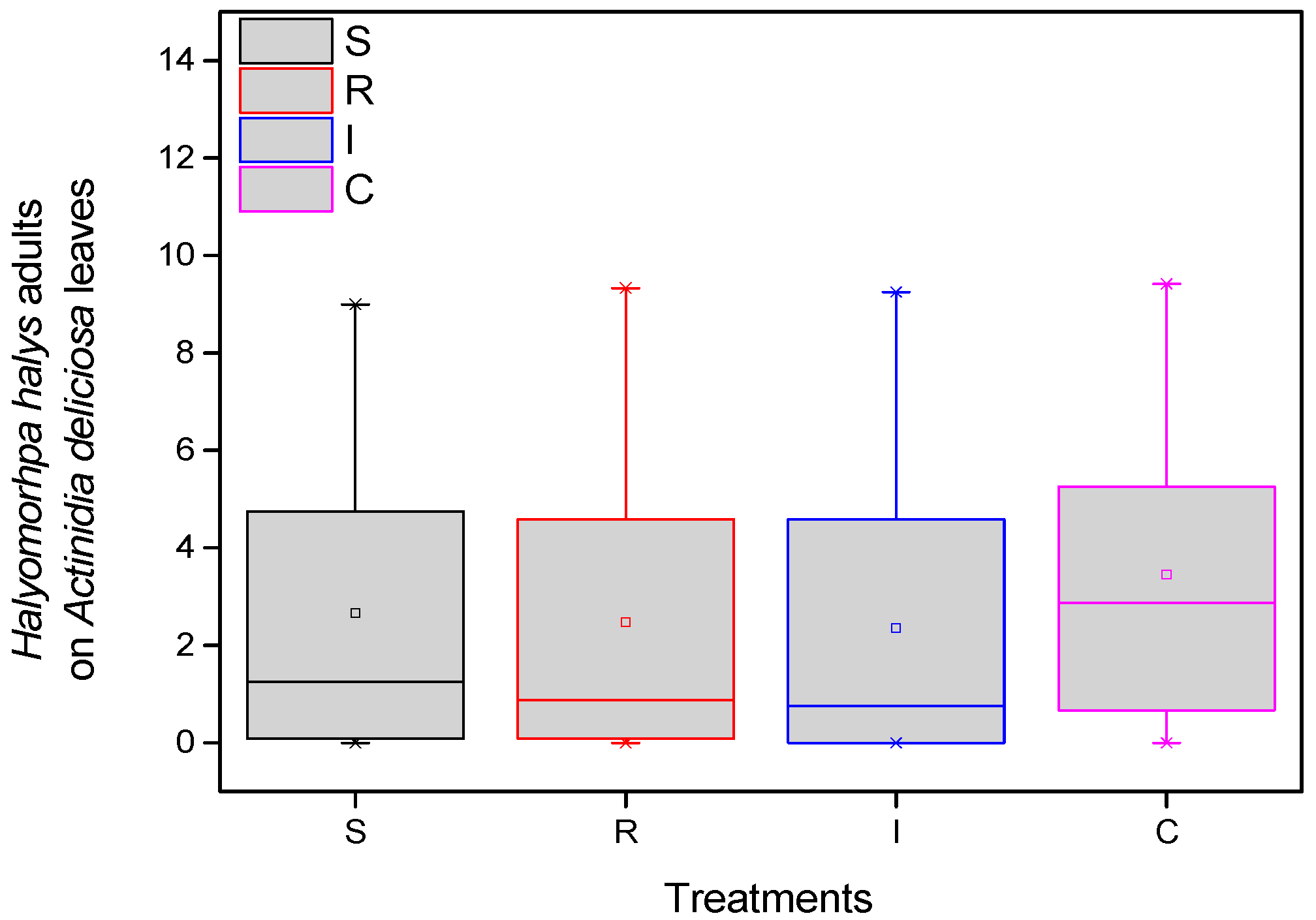
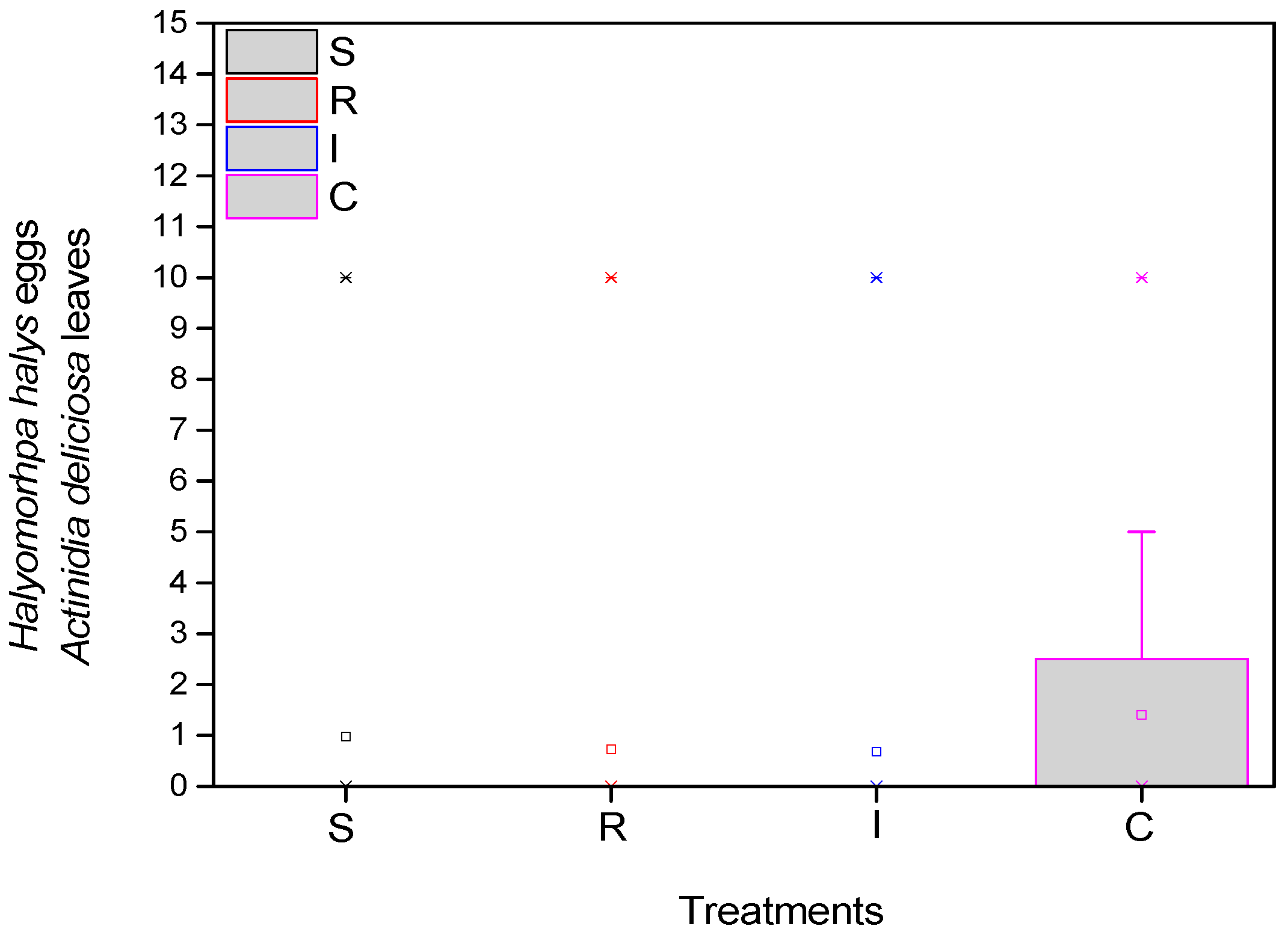
3.1. Population of H. halys (Adults and Eggs) on A. chinensis Leaves
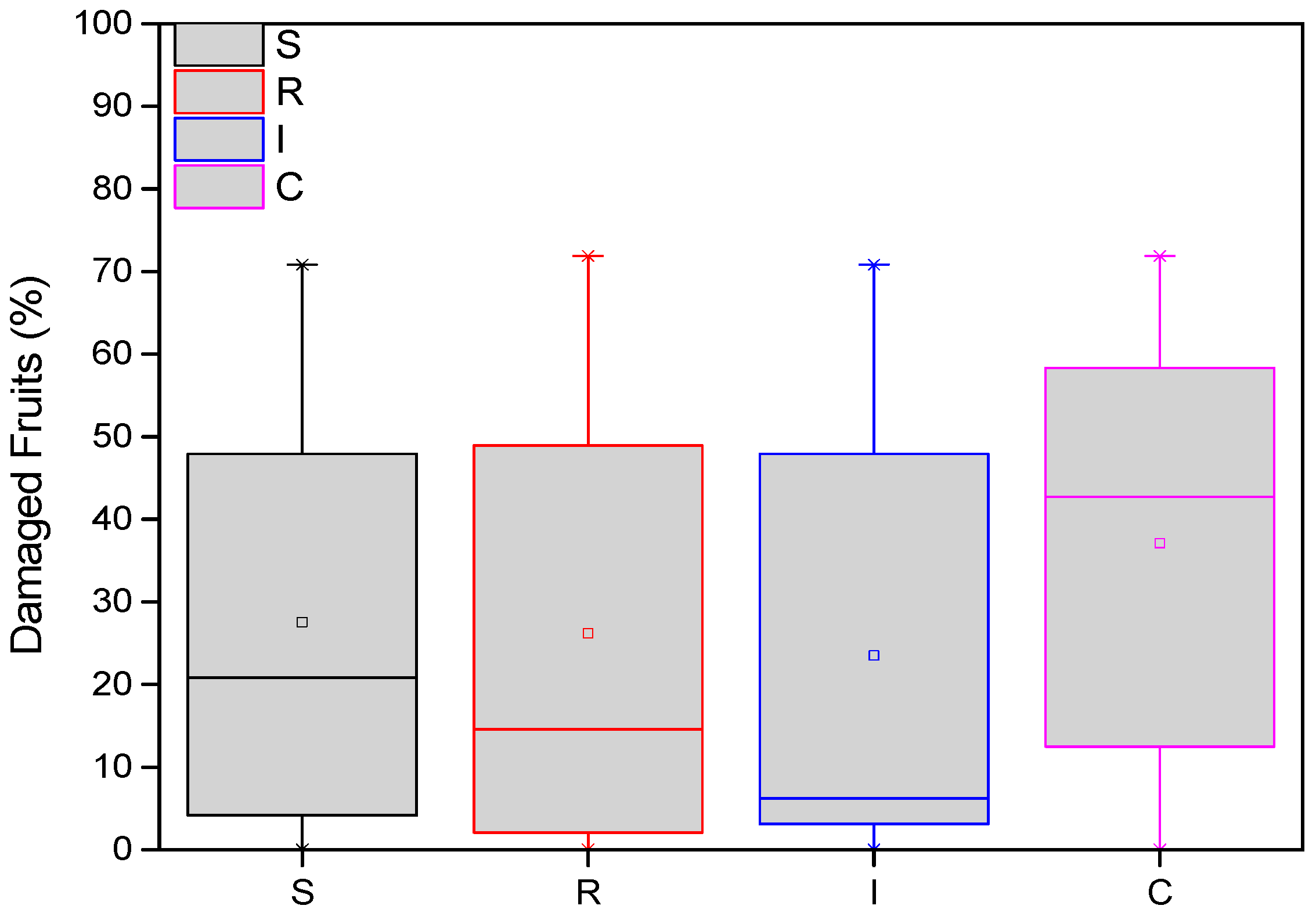
3.2. Damaged Kiwifruits
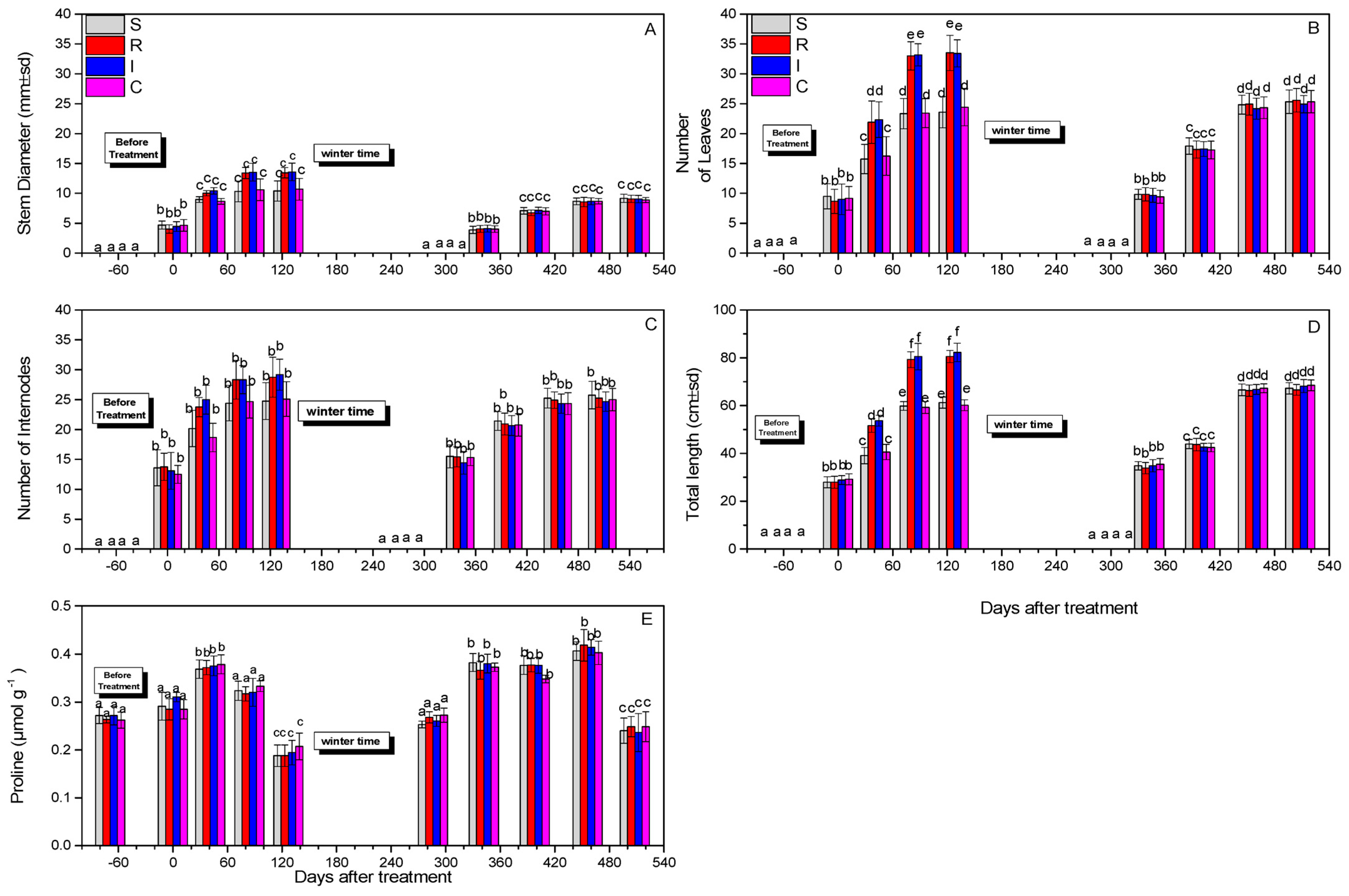
3.3. Effect on Plant Growth and Proline Content
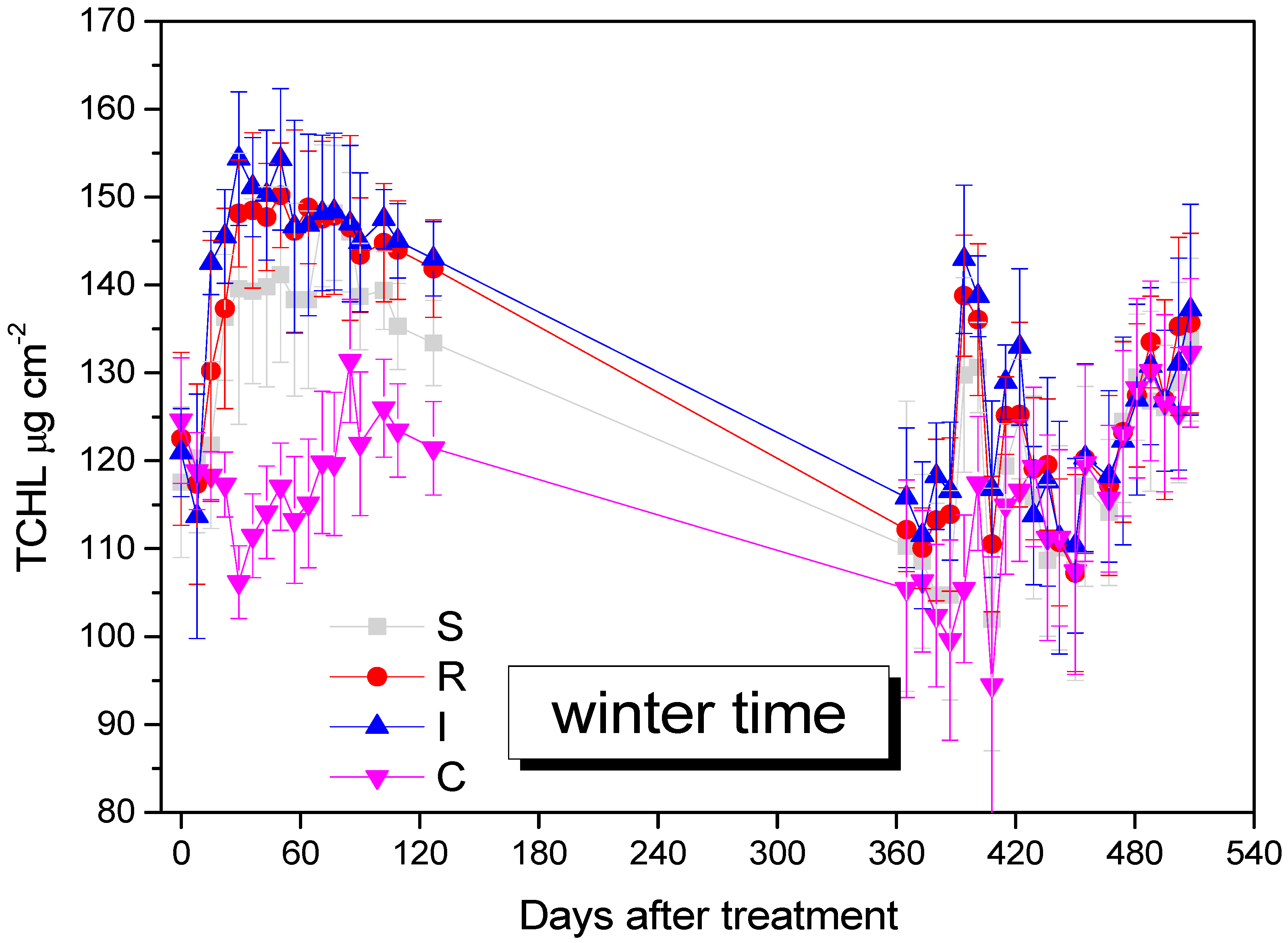
3.4. Effect on A. chinensis Total Chlorophyll Content (TCHL)
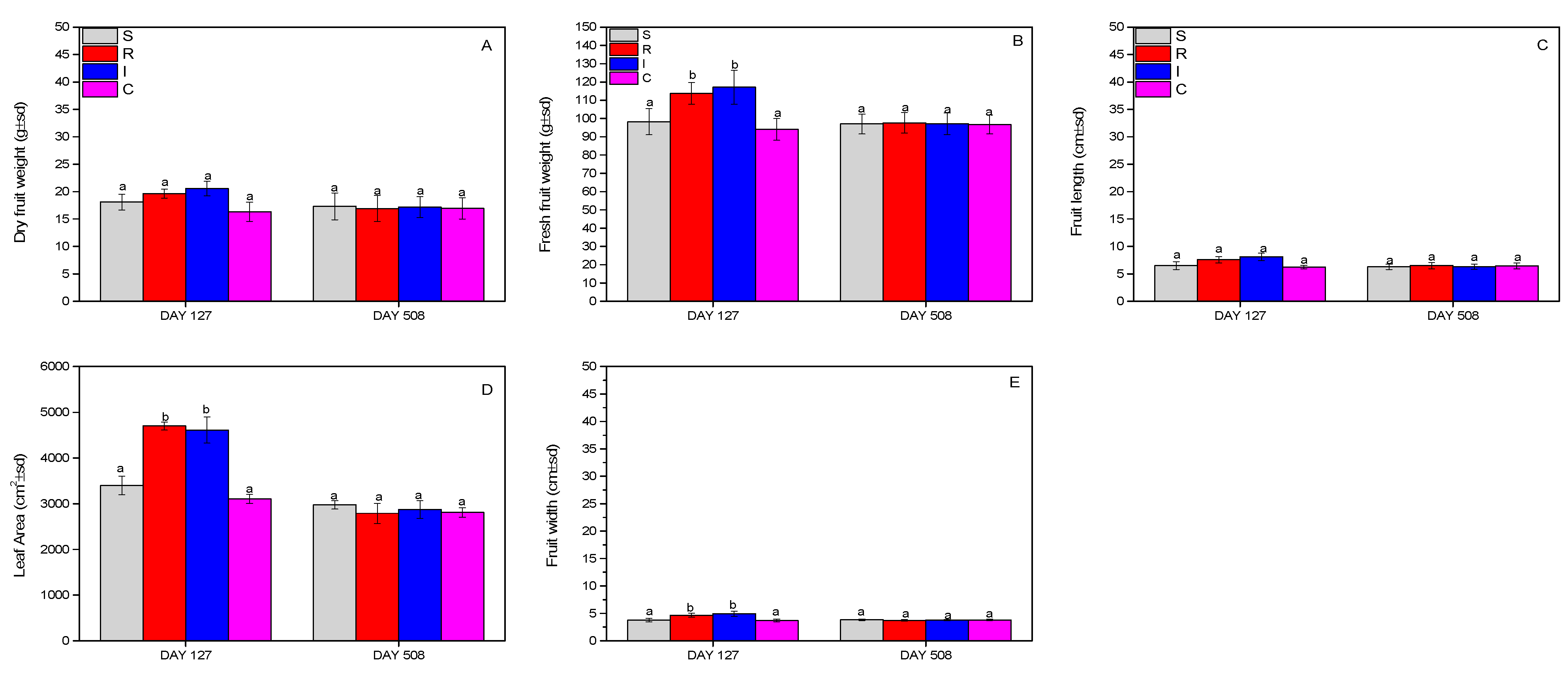
3.5. Effect on A. chinensis Fruits and Leaf Area
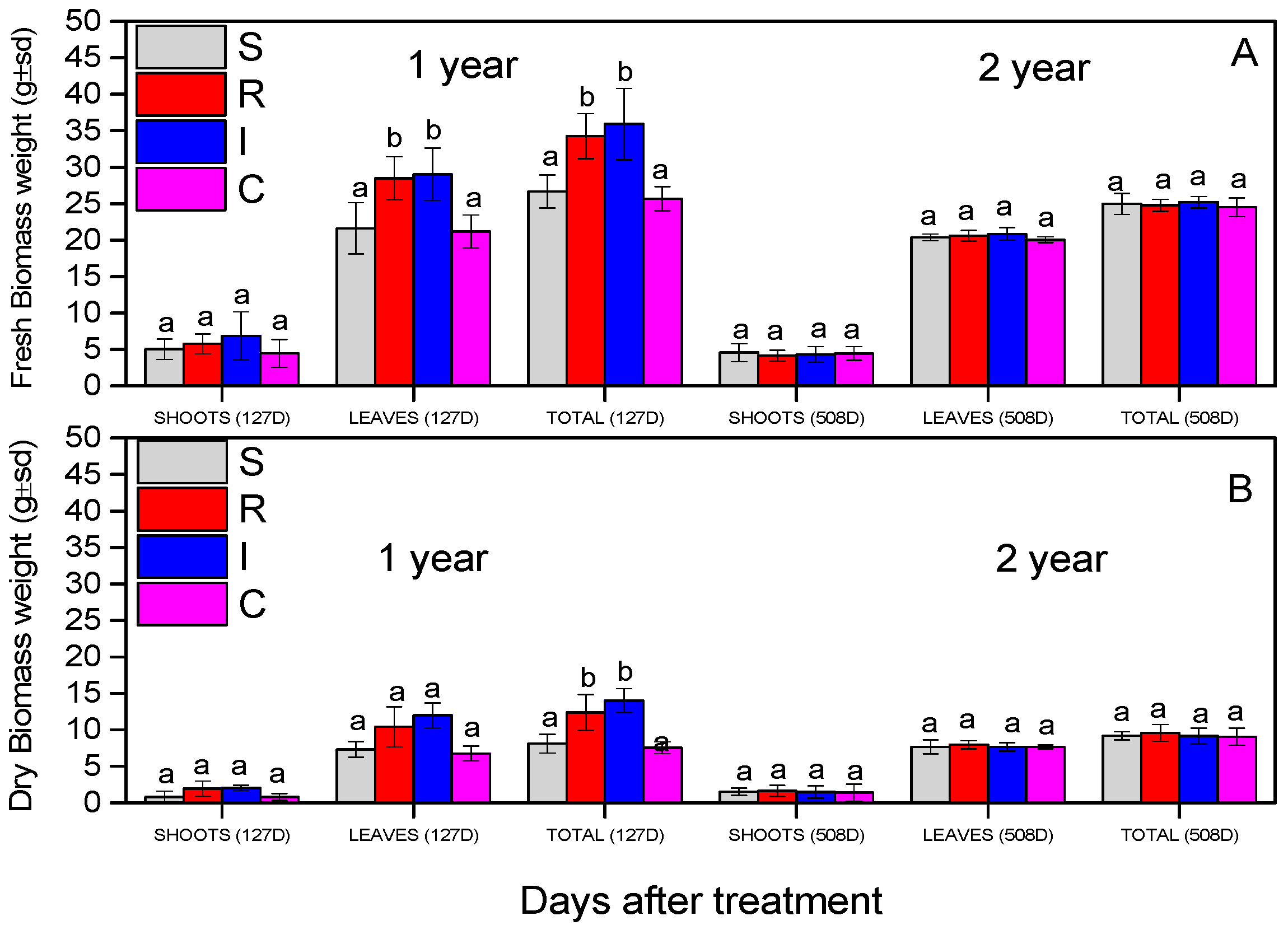
3.6. Effect on A. chinensis Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yonow, T.; Kriticos, D.J.; Ota, N.; Avila, G.A.; Hoelmer, K.A.; Chen, H.; Caron, V. Modelling the Potential Geographic Distribution of Two Trissolcus Species for the Brown Marmorated Stink Bug, Halyomorpha halys. Insects 2021, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Rijal, J.P.; Zalom, F.G. Temperature and Humidity Interact to Influence Brown Marmorated Stink Bug (Hemiptera: Pentatomidae), Survival. Environ. Entomol. 2021, 50, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Bariselli, M.; Bugiani, R.; Maistrello, L. Distribution and Damage Caused by Halyomorpha halys in Italy. EPPO Bulletin 2016, 46, 332–334. [Google Scholar] [CrossRef]
- Özdemir, İ.O.; Yildirim, E.; Uluca, M.; Tuncer, C. Efficacy of Native Beauveria bassiana and B. Pseudobassiana Isolates Against Invasive Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). Black Sea J. Agric. 2022, 5, 227–233. [Google Scholar] [CrossRef]
- Zhu, G.; Bu, W.; Gao, Y.; Liu, G. Potential Geographic Distribution of Brown Marmorated Stink Bug Invasion (Halyomorpha halys). PLoS ONE 2012, 7, e31246. [Google Scholar] [CrossRef] [PubMed]
- Teulon, D.A.J.; Xu, B. Biosecurity Risks from Stink Bugs to New Zealand Kiwifruit Identified in Chinese Language Literature. N. Z. Plant Prot. 2018, 71, 140–150. [Google Scholar] [CrossRef][Green Version]
- Andreadis, S.S.; Navrozidis, E.I.; Farmakis, A.; Pisalidis, A. First Evidence of Halyomorpha halys (Hemiptera: Pentatomidae) Infesting Kiwi Fruit (Actinidia chinensis) in Greece1. J. Entomol. Sci. 2018, 53, 402–405. [Google Scholar] [CrossRef]
- BMSB-Kiwifruit Impacts and on-Orchard Management. 2017. Available online: http://nzkgi.org.nz/wp-content/uploads/2016/12/2017-Kiwifruit-Book-Web-version.pdf (accessed on 28 May 2024).
- Damos, P.; Soulopoulou, P.; Thomidis, T. First Record and Current Status of the Brown Marmorated Sting Bug Halyomorpha halys Damaging Peaches and Olives in Northern Greece. J. Plant Prot. Res. 2020, 60, 323–326. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef]
- Peiffer, M.; Felton, G.W. Insights into the Saliva of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Pentatomidae). PLoS ONE 2014, 9, e88483. [Google Scholar] [CrossRef]
- Tozlu, E.; Saruhan, I.; Tozlu, G.; Kotan, R.; Dadaşoğlu, F.; Tekiner, N. Potentials of Some Entomopathogens against the Brown Marmorated Stink Bug, Halyomorpha halys (Stål, 1855) (Hemiptera: Pentatomidae). Egypt. J. Biol. Pest Control 2019, 29, 76. [Google Scholar] [CrossRef]
- Shan, T.; Wei, J.; Wang, Y.; Zhao, X.; Zhao, Y.; Ge, Q.; Yuan, Y.; Yue, T. Effects of Different Pesticides Treatments on the Nutritional Quality of Kiwifruit. J. Food Sci. 2021, 86, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.L.; Skinner, M.; Gouli, S.; Gouli, V.; Kim, J.S. Virulence of BotaniGard(®) to Second Instar Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Insects 2015, 6, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Short, B.D.; Lee, D.H. Efficacy of Insecticide Residues on Adult Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Mortality and Injury in Apple and Peach Orchards. Pest. Manag. Sci. 2014, 70, 1097–1104. [Google Scholar] [CrossRef]
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana Reduce the Survival and Fecundity of Aphis Gossypii Following Contact with Conidia and Secondary Metabolites. Crop Prot. 2011, 30, 349–353. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Zou, W.X. Endophytes: A Rich Source of Functional Metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Araj, S.E. Interactions among Endophytic Fungal Entomopathogens (Ascomycota: Hypocreales), the Green Peach Aphid Myzus Persicae Sulzer (Homoptera: Aphididae), and the Aphid Endoparasitoid Aphidius Colemani Viereck (Hymenoptera: Braconidae). Biol. Control. 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Burjanadze, M.; Kharabadze, N.; Chkhidze, N. Testing Local Isolates of Entomopathogenic Microorganisms against Brown Marmorated Stink Bug Halyomorpha halys in Georgia. BIO Web Conf. 2020, 18, 00006. [Google Scholar] [CrossRef][Green Version]
- Gouli, V.; Gouli, S.; Skinner, M.; Hamilton, G.; Kim, J.S.; Parker, B.L. Virulence of Select Entomopathogenic Fungi to the Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Pest. Manag. Sci. 2012, 68, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Bruck, D.J. Fungal Entomopathogens in the Rhizosphere. Ecol. Fungal Entomopathog. 2009, 55, 103–112. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Luo, Z.; Keyhani, N.O. Improving Mycoinsecticides for Insect Biological Control. Appl. Microbiol. Biotechnol. 2015, 99, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A. The Split Personality of Beauveria bassiana: Understanding the Molecular Basis of Fungal Parasitism and Mutualism. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic Fungi: New Insights into Host-Pathogen Interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar] [CrossRef]
- Sharp, R.G. A Review of the Applications of Chitin and Its Derivatives in Agriculture to Modify Plant-Microbial Interactions and Improve Crop Yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Gohel, V.; Singh, A.; Vimal, M.; Ashwini, P.; Chhatpar, H. Bioprospecting and Antifungal Potential of Chitinolytic Microorganisms. Afr. J. Biotechnol. 2006, 5, 54–72. [Google Scholar]
- Proietti, S.; Falconieri, G.S.; Bertini, L.; Pascale, A.; Bizzarri, E.; Morales-Sanfrutos, J.; Sabidó, E.; Ruocco, M.; Monti, M.M.; Russo, A.; et al. Beauveria bassiana Rewires Molecular Mechanisms Related to Growth and Defense in Tomato. J. Exp. Bot. 2023, 74, 4225–4243. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.C.; Sword, G.A. The Endophytic Fungal Entomopathogens Beauveria bassiana and Purpureocillium Lilacinum Enhance the Growth of Cultivated Cotton (Gossypium hirsutum) and Negatively Affect Survival of the Cotton Bollworm (Helicoverpa Zea). Biol. Control. 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Canassa, F.; Tall, S.; Moral, R.A.; de Lara, I.A.R.; Delalibera, I.; Meyling, N.V. Effects of Bean Seed Treatment by the Entomopathogenic Fungi Metarhizium Robertsii and Beauveria bassiana on Plant Growth, Spider Mite Populations and Behavior of Predatory Mites. Biol. Control. 2019, 132, 199–208. [Google Scholar] [CrossRef]
- Sinno, M.; Ranesi, M.; Di Lelio, I.; Iacomino, G.; Becchimanzi, A.; Barra, E.; Molisso, D.; Pennacchio, F.; Digilio, M.C.; Vitale, S.; et al. Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests. Pathogens 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The Entomopathogenic Fungi Metarhizium brunneum and Beauveria bassiana Promote Systemic Immunity and Confer Resistance to a Broad Range of Pests and Pathogens in Tomato. Phytopathology 2022, 112, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Quesada Moraga, E. Entomopathogenic Fungi as Endophytes: Their Broader Contribution to IPM and Crop Production. Biocontrol Sci. Technol. 2020, 30, 864–877. [Google Scholar] [CrossRef]
- Resquín-Romero, G.; Garrido-Jurado, I.; Delso, C.; Ríos-Moreno, A.; Quesada-Moraga, E. Transient Endophytic Colonizations of Plants Improve the Outcome of Foliar Applications of Mycoinsecticides against Chewing Insects. J. Invertebr. Pathol. 2016, 136, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bakr, E.M. A New Software for Measuring Leaf Area, and Area Damaged by Tetranychus Urticae Koch. J. Appl. Entomol. 2005, 129, 173–175. [Google Scholar] [CrossRef]
- Razeto, B.; Valdés, G. Fruit Analysis as an Indicator of the Iron Status of Nectarine and Kiwi Plant. Horttechnology 2006, 16, 579–582. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. Prometheus Wiki 2011, 2011, 1–5. [Google Scholar]
- Chen, J.-H.; Avila, G.A.; Zhang, F.; Guo, L.F.; Manoharie, S.; Mi, Q.-Q.; Shi, S.-S.; Zhang, J.-P.; Org, Z. Field Cage Assessment of Feeding Damage by Halyomorpha halys on Kiwifruit Orchards in China. J. Pest Sci. 2020, 93, 953–963. [Google Scholar] [CrossRef]
- Francati, S.; Masetti, A.; Martinelli, R.; Mirandola, D.; Anteghini, G.; Busi, R.; Dalmonte, F.; Spinelli, F.; Burgio, G.; Dindo, M.L. Halyomorpha halys (Hemiptera: Pentatomidae) on Kiwifruit in Northern Italy: Phenology, Infestation, and Natural Enemies Assessment. J. Econ. Entomol. 2021, 114, 1733–1742. [Google Scholar] [CrossRef]
- Dumbadze, G.; Gokturk, T.; Jgenti, L.; Chelidze, N. Distribution, Biology, Ecology and Control Mechanisms of Halyomorpha halys Stål in Batumi (Georgia). SETSCI-Conf. Proc. 2019, 9, 539–542. [Google Scholar] [CrossRef]
- Tamburini, G.; Laterza, I.; Nardi, D.; Mele, A.; Mori, N.; Pasini, M.; Scaccini, D.; Pozzebon, A.; Marini, L. Effect of Landscape Composition on the Invasive Pest Halyomorpha halys in Fruit Orchards. Agric. Ecosyst. Environ. 2023, 353, 108530. [Google Scholar] [CrossRef]
- Mele, A.; Scaccini, D.; Zanolli, P.; Pozzebon, A.; Mele, A.; Scaccini, D.; Zanolli, P.; Pozzebon, A. Semi-Natural Habitats Promote Biological Control of Halyomorpha halys (Stål) by the Egg Parasitoid Trissolcus Mitsukurii (Ashmead). Biol. Control 2022, 166, 104833. [Google Scholar] [CrossRef]
- Karpun, N.N.; Borisov, B.A.; Zhuravleva, E.N.; Borisova, I.P.; Nadykta, V.D.; Musolin, D.L. Range expansion and increasing damage potential of phytophagous shield bugs (Heteroptera: Pentatomidae) (Review). Sel’skokhozyaistvennaya Biol. 2022, 57, 542–554. [Google Scholar] [CrossRef]
- Ramanujam, B.; Poornesha, B.; Shylesha, A.N. Effect of Entomopathogenic Fungi against Invasive Pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in Maize. Egypt. J. Biol. Pest. Control 2020, 30, 1–5. [Google Scholar] [CrossRef]
- Dara, S.K. Non-Entomopathogenic Roles of Entomopathogenic Fungi in Promoting Plant Health and Growth. Insects 2019, 10, 277. [Google Scholar] [CrossRef]
- Jordan, C.; dos Santos, P.L.; dos Oliveira, L.R.S.; Domingues, M.M.; Gêa, B.C.C.; Ribeiro, M.F.; Mascarin, G.M.; Wilcken, C.F. Entomopathogenic Fungi as the Microbial Frontline against the Alien Eucalyptus Pest Gonipterus platensis in Brazil. Sci. Rep. 2021, 11, 7233. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Koutsogeorgiou, E.I.; Lagogiannis, I.; Gogolashvili, N.; Fifis, G.T.; Navrozidis, E.I.; Thomidis, T.; Andreadis, S.S. Effect of Entomopathogenic Fungi to Eggs and Nymphs Survival of Halyomorpha halys (Hemiptera: Pentatomidae) Under Laboratory Conditions. Curr. Microbiol. 2024, 81, 48. [Google Scholar] [CrossRef]
- Husain, M.; Sutanto, K.D.; Rasool, K.G.; Qureshi, J.A.; Aldawood, A.S. Translocation and Survival of Trunk Injected Beauveria bassiana (Hypocreales: Cordycipitaceae) in Healthy Date Palm Trees. J. King Saud. Univ. Sci. 2024, 36, 103077. [Google Scholar] [CrossRef]
- Yerukala, S.; Butler, D.M.; Bernard, E.C.; Gwinn, K.D.; Grewal, P.S.; Ownley, B.H. Colonization Efficacy of the Endophytic Insect-Pathogenic Fungus, Beauveria bassiana, Across the Plant Kingdom: A Meta-Analysis. CRC Crit. Rev. Plant Sci. 2022, 41, 241–270. [Google Scholar] [CrossRef]
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.B.; Salinas, J. Endophytic Colonization of Date Palm (Phoenix dactylifera L.) Leaves by Entomopathogenic Fungi. Micron 2006, 37, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.L.; Lewis, L.C. Colonization of Corn, Zea Mays, by the Entomopathogenic Fungus Beauveria bassiana. Appl. Environ. Microbiol. 2000, 66, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Bing, L.A.; Lewis, L.C. Endophytic Beauveria bassiana (Balsamo) Vuillemin in Corn: The Influence of the Plant Growth Stage and Ostrinia nubilalis (Hübner). Biocontrol Sci. Technol. 1992, 2, 39–47. [Google Scholar] [CrossRef]
- Biswas, C.; Dey, P.; Satpathy, S.; Satya, P. Establishment of the Fungal Entomopathogen Beauveria bassiana as a Season Long Endophyte in Jute (Corchorus olitorius) and Its Rapid Detection Using SCAR Marker. BioControl 2012, 57, 565–571. [Google Scholar] [CrossRef]
- Yerukala, S.; Bernard, E.C.; Gwinn, K.D.; Butler, D.M.; Grewal, P.S.; Ownley, B.H. Endophytic Beauveria bassiana Increases Galling of Rutgers Tomato Roots with Meloidogyne Incognita. J. Nematol. 2021, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.R. Beauveria bassiana, A Cotton Endophyte with Biocontrol Activity Against Seedling Disease. 2007. Available online: https://trace.tennessee.edu/utk_graddiss/180/ (accessed on 28 May 2024).
- Abdrabo, K.A.E.S.; Phang, G.J.; Rahmadani, S.Y.; Huang, Y.T. Insights into the Complexities of Fungus-Insect-Plant Interaction: The Laurel Wilt Disease. J. Phytopathol. 2024, 172, e13263. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Pérez-Martínez, J.M.; Evans, E.A.; Inch, S.A. Toward Fungicidal Management of Laurel Wilt of Avocado. Plant Dis. 2011, 95, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C.; Konkol, J.L.; Pérez-Martínez, J.M.; Fernandez, R. Management of Laurel Wilt of Avocado, Caused by Raffaelea Lauricola. Eur. J. Plant Pathol. 2017, 149, 133–143. [Google Scholar] [CrossRef]
- Zhang, M.D.; Wu, S.Y.; Yan, J.J.; Reitz, S.; Gao, Y.L. Establishment of Beauveria bassiana as a Fungal Endophyte in Potato Plants and Its Virulence against Potato Tuber Moth, Phthorimaea Operculella (Lepidoptera: Gelechiidae). Insect Sci. 2023, 30, 197–207. [Google Scholar] [CrossRef]
- Nishi, O.; Sushida, H.; Higashi, Y.; Iida, Y. Epiphytic and Endophytic Colonisation of Tomato Plants by the Entomopathogenic Fungus Beauveria bassiana Strain GHA. Mycology 2021, 12, 39–47. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Sarmah, S.R.; Roy, S.; Sarma, B.; Nath, B.C.; Bhattacharyya, L.H. Perspectives of Beauveria bassiana, an Entomopathogenic Fungus for the Control of Insect-Pests in Tea [Camellia sinensis (L.) O. Kuntze]: Opportunities and Challenges. Int. J. Trop. Insect Sci. 2022, 43, 1–19. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, P. Fungal Entomopathogens: A Systematic Review. Egypt. J. Biol. Pest. Control 2021, 31, 57. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities With Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 532550. [Google Scholar] [CrossRef]
- Wippel, K. Plant and Microbial Features Governing an Endophytic Lifestyle. Curr. Opin. Plant Biol. 2023, 76, 102483. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhao, X.; Huang, S.; Deng, J.; Li, X.; Luo, Z.; Zhang, Y. Pest Management via Endophytic Colonization of Tobacco Seedlings by the Insect Fungal Pathogen Beauveria bassiana. Pest. Manag. Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Y.; Li, Y.Y.; Xu, C.; Wu, Y.X.; Zhang, Y.R.; Liu, H. Endophytic Colonization by Beauveria bassiana Increases the Resistance of Tomatoes against Bemisia Tabaci. Arthropod Plant Interact. 2020, 14, 289–300. [Google Scholar] [CrossRef]
- Tefera, T.; Vidal, S. Effect of Inoculation Method and Plant Growth Medium on Endophytic Colonization of Sorghum by the Entomopathogenic Fungus Beauveria bassiana. BioControl 2009, 54, 663–669. [Google Scholar] [CrossRef]
- Akter, T.; Mimma, A.A.; Haque, M.A.; Hossain, M.M.; Ghosh, T.K.; Zinan, N.; Chowdhury, M.Z.H.; Islam, S.M.N. Seed Priming with Beauveria bassiana Improves Growth and Salt Stress Response in Rice. Environ. Exp. Bot. 2023, 213, 105427. [Google Scholar] [CrossRef]
- Muola, A.; Birge, T.; Helander, M.; Mathew, S.; Harazinova, V.; Saikkonen, K.; Fuchs, B. Endophytic Beauveria bassiana Induces Biosynthesis of Flavonoids in Oilseed Rape Following Both Seed Inoculation and Natural Colonization. Pest. Manag. Sci. 2023, 80, 2461–2470. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I.; Mpousia, D.; Ntoukas, A.; Karmakolia, K.; Eliopoulos, P.A.; Poulas, K. Beauveria bassiana Endophytic Strain as Plant Growth Promoter: The Case of the Grape Vine Vitis Vinifera. J. Fungi 2021, 7, 142. [Google Scholar] [CrossRef]
- Muhammad, M.; Basit, A.; Ali, K.; Ahmad, H.; Li, W.J.; Khan, A.; Mohamed, H.I. A Review on Endophytic Fungi: A Potent Reservoir of Bioactive Metabolites with Special Emphasis on Blight Disease Management. Arch. Microbiol. 2024, 206, 129. [Google Scholar] [CrossRef] [PubMed]
- Fite, T.; Kebede, E.; Tefera, T.; Bekeko, Z. Endophytic Fungi: Versatile Partners for Pest Biocontrol, Growth Promotion, and Climate Change Resilience in Plants. Front. Sustain. Food Syst. 2023, 7, 1322861. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic Fungi: A Tool for Plant Growth Promotion and Sustainable Agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef] [PubMed]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic Microbes and Their Potential Applications in Crop Management. Pest. Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F. Induction of Abiotic Stress Tolerance in Plants by Endophytic Microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Airin, A.A.; Arafat, M.I.; Begum, R.A.; Islam, M.R.; Seraj, Z.I. Plant Growth-Promoting Endophytic Fungi of the Wild Halophytic Rice Oryza Coarctata. Ann. Microbiol. 2023, 73, 36. [Google Scholar] [CrossRef]
- Behie, S.W.; Bidochka, M.J. Ubiquity of Insect-Derived Nitrogen Transfer to Plants by Endophytic Insect-Pathogenic Fungi: An Additional Branch of the Soil Nitrogen Cycle. Appl. Environ. Microbiol. 2014, 80, 1553–1560. [Google Scholar] [CrossRef]
- Geroh, M.; Gulati, R.; Kanika, T. Beauveria bassiana (Balsamo) Vuillemin (Strain ITCC-4668) as Acaricide against Tetranychus Urticae Koch (Acari: Tetranychidae). Indian. J. Agric. Res. 2014, 48, 384–388. [Google Scholar] [CrossRef]
- Rocha, J.P.L.; Nunes, T.V.; Rodrigues, J.N.; Lima, N.M.P.; Rocha, P.A.L.; de Pinto, I.O.; Sarmento, M.I.; Araújo, W.L.; de Moraes, C.B.; Sarmento, R.A. Morphophysiological Responses in Eucalyptus Demonstrate the Potential of the Entomopathogenic Fungus Beauveria bassiana to Promote Resistance against the Galling Wasp Leptocybe Invasa. Forests 2023, 14, 1349. [Google Scholar] [CrossRef]
- Macuphe, N.; Oguntibeju, O.O.; Nchu, F. Evaluating the Endophytic Activities of Beauveria bassiana on the Physiology, Growth, and Antioxidant Activities of Extracts of Lettuce (Lactuca sativa L.). Plants 2021, 10, 1178. [Google Scholar] [CrossRef]
- Rezkiana, N.; Musa, Y.; Nasaruddin; Ridwan, I.; Kurniawan. Physiological Responses of Clove Seedlings Applied with Different Microbial Consortium in the Rhizosphere and Phyllosphere. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 4. [Google Scholar] [CrossRef]
- Veloz-Badillo, G.M.; Riveros-Ramírez, J.; Angel-Cuapio, A.; Arce-Cervantes, O.; Flores-Chávez, B.; Espitia-López, J.; Loera, O.; Garza-López, P.M. The Endophytic Capacity of the Entomopathogenic Fungus Beauveria bassiana Caused Inherent Physiological Response in Two Barley (Hordeum vulgare) Varieties. 3 Biotech. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Barra-Bucarei, L.; González, M.G.; Iglesias, A.F.; Aguayo, G.S.; Peñalosa, M.G.; Vera, P.V. Beauveria bassiana Multifunction as an Endophyte: Growth Promotion and Biologic Control of Trialeurodes vaporariorum, (Westwood) (Hemiptera: Aleyrodidae) in Tomato. Insects 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, A.; Rey, M.D.; Froussart, E.; Quesada-Moraga, E. Elucidating the Effect of Endophytic Entomopathogenic Fungi on Bread Wheat Growth through Signaling of Immune Response-Related Hormones. Appl. Environ. Microbiol. 2022, 88, e00882-22. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.C.; Ridgway, H.J.; Mendoza Mendoza, A.; Glare, T.R. Growth of Zea Mays in Response to Artificial Inoculation with Endophytic Beauveria bassiana Compared to Trichoderma sp. “Atroviride B”. Biocontrol Sci. Technol. 2023, 33, 155–172. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456. [Google Scholar] [CrossRef]
- Lara, J.R.; Kamiyama, M.; Hernandez, G.; Lewis, M.; Hoddle, M.S. Laboratory Assessment of Feeding Injury and Preference of Brown Marmorated Stink Bug, Halyomorpha halys Stål (Hemiptera: Pentatomidae), for Actinidia chinensis Var. deliciosa ‘Hayward’ (Zespri® Green) and Actinidia chinensis Var. Chinensis ‘Zesy002’ (Zespri® SunGold). N. Z. Entomol. 2018, 41, 12–24. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papantzikos, V.; Mantzoukas, S.; Eliopoulos, P.A.; Servis, D.; Bitivanos, S.; Patakioutas, G. Evaluation of Various Inoculation Methods on the Effect of Beauveria bassiana on the Plant Growth of Kiwi and on Halyomorpha halys Infestation: A Two-Year Field Study. Biology 2024, 13, 470. https://doi.org/10.3390/biology13070470
Papantzikos V, Mantzoukas S, Eliopoulos PA, Servis D, Bitivanos S, Patakioutas G. Evaluation of Various Inoculation Methods on the Effect of Beauveria bassiana on the Plant Growth of Kiwi and on Halyomorpha halys Infestation: A Two-Year Field Study. Biology. 2024; 13(7):470. https://doi.org/10.3390/biology13070470
Chicago/Turabian StylePapantzikos, Vasileios, Spiridon Mantzoukas, Panagiotis A. Eliopoulos, Dimitrios Servis, Stergios Bitivanos, and George Patakioutas. 2024. "Evaluation of Various Inoculation Methods on the Effect of Beauveria bassiana on the Plant Growth of Kiwi and on Halyomorpha halys Infestation: A Two-Year Field Study" Biology 13, no. 7: 470. https://doi.org/10.3390/biology13070470
APA StylePapantzikos, V., Mantzoukas, S., Eliopoulos, P. A., Servis, D., Bitivanos, S., & Patakioutas, G. (2024). Evaluation of Various Inoculation Methods on the Effect of Beauveria bassiana on the Plant Growth of Kiwi and on Halyomorpha halys Infestation: A Two-Year Field Study. Biology, 13(7), 470. https://doi.org/10.3390/biology13070470








