3.1. Analysis of Tannins Remaining in Solution (Measured by Phloroglucinolysis) after the Interactions with Polysaccharides in the Experiments with Model Solutions
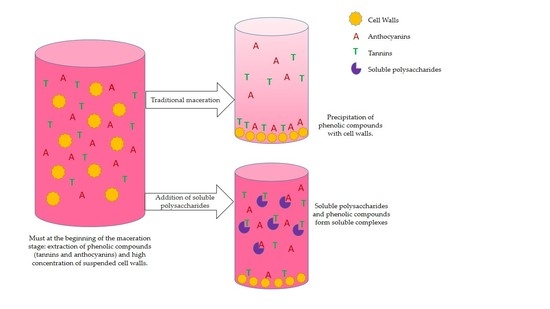
As a first part of the experiment, commercial skin and seed tannins were first led to interact in model solution with three different polysaccharide samples and two commercial polysaccharides: PEC, MAN, and CW-PS. The material solubilized from cell walls after stirring them in a model solution for 90 min to determine whether they formed soluble complexes or whether tannins were lost through precipitation. A second part of the experiment consisted of bringing tannins into contact with cell walls in the absence or presence of the two commercial polysaccharides to determine whether they might limit tannin adsorption to cell walls.
Table 1 shows the concentration and characteristics of the tannins remaining in solution after their interaction with the different polysaccharides and also the results of the concentration of the skin tannins remaining in solution when skin cell walls and polysaccharides were both present in the buffer solution.
Previous studies have shown that tannins and some polysaccharides form complexes that remain stable in colloidal suspension [
9,
15]. Moreover, some nephelometric studies have given evidence for the disrupting effect of some polysaccharides on tannins aggregation and, more importantly, on their aggregation by proteins [
15].
In the present study, we worked with two different polysaccharides: a highly esterified pectin and mannan, a neutral polysaccharide. Pectins are some of the most important components in grapes cell walls and some studies have shown that they present a high affinity for tannins [
9]. Mannose polymers exhibit biological activity and beneficial health characteristics, such as providing dietary fiber and satiety [
29]. Linear mannans are homopolysaccharides composed of linear main chains of α-1, 6-linked D-mannose residues. The structure of mannan from
Saccharomyces cerevisiae has been reported as presenting a backbone, composed of α-1,6-linked mannose residues, 83% branched at O-2 by single mannose residues, as well as oligosaccharide side chains mostly in the form of tri-, di-, mono-, and tetramers. Longer side chains, penta- to heptamers are present in lesser amounts. This was confirmed by Nuclear Magnetic Resonance (NMR) spectra that showed that they were formed mostly by mannose residues [
30,
31]. In wines, mannoproteins (proteoglycans formed of different proportions of protein and D-mannose) have been extensively used to improve several taste characteristics of red wines in which they: appear to have a protective effect on the monomeric anthocyanin content [
32], soften the taste [
33], improve protein stability and reduce the quantity of bentonite [
34], and also promote tartrate stability [
35]. However, as mentioned above, this compound is a glycoprotein and the protein part could be responsible for some unexpected results [
18,
19,
36], which is why we chose to test only the polysaccharide fraction of
Saccharomyces cerevisiae glycoprotein MAN was added at a lower concentration than PEC. When looking at the bibliography on Monastrell wine polysaccharides and oligosaccharides, we observed that mannose residues were always present at a lower concentration than galacturonic acid residues in the wine oligosaccharides and the content of RGII normally exceed that of mannoproteins [
17,
37]. Based on these previous results, we decided to work with a smaller concentration of mannan than pectin.
As regards to the two commercial polysaccharides, when they were added with tannins to the buffer solution, the tannin concentration did not change, indicating that no precipitation occurred in the reaction and that any aggregates formed between tannins and these polysaccharides are basically soluble.
We also studied the effect of the interaction between the skin tannins and the soluble material that resulted from stirring cell walls in a model solution (CW-PS) without the presence of the insoluble CW. Vidal et al. [
38] stated that some characteristics of wine soluble cell wall components are similar to those of pectin isolated from citrus, whose hydrocolloid properties improve the mouthfeel and texture of foods. According to Gao et al. [
39,
40], CW-PS in wine are mostly formed from polysaccharides arising from the degradation of cell wall components due to mechanical and enzymatic actions, mainly rhamnogalacturonan I (RG I), rhamnogalacturonan II (RG II), xyloglucan (XG), arabinogalactan (AG), and arabinogalactan-protein (AGP). Our results showed that this material did not behave in the same way as the commercial polysaccharides, since it caused a substantial decrease in the concentration of skin tannins in solution (46.5%) and the formation of a precipitate. Similar findings were observed by Bindon et al. [
2], who noted that, in the interaction of the mesocarp or skin cell wall solubilized in the supernatant with tannins, the tannin concentration was reduced and a visible precipitate was formed. The cause of this reduction in tannin concentration and precipitate formation was mainly attributed by these authors to the formation of insoluble protein–tannin complexes and their consequent precipitation, especially in the case of mesocarp cell walls. On the other hand, they found that, in the precipitates from the skin cell walls extract, tannins were found to be mainly associated with insoluble polysaccharides although small quantities of proteins were also observed in the precipitate. The precipitated tannins were of high molecular mass, which justifies our observations concerning the reduced concentration of tannins and of the decrease in the mDP of the tannins remaining in solution.
When we simultaneously added grape skin tannin and skin cell walls to the solution, there was an 83.6% reduction in the concentration of the tannins remaining in solution. This effect was not unexpected and was already observed by Bautista-Ortín et al. [
41] working with different varieties (Monastrell and Syrah) and different tannins, and by Bindon et al. [
4]. The addition of soluble polysaccharides to the medium reduced the interactions between skin tannins and the cell walls, increasing the concentration of tannins in solution, pointing to competition between the cell wall bound and soluble polysaccharides for the skin tannins. As a result, losses caused by cell wall adsorption were reduced, although the adsorption to cell walls seems to be the predominant interaction.
The mean degree of polymerization (mDP) of the skin tannin remaining in solution was reduced after the reaction with the cell walls, particularly in the experiments in which commercial soluble polysaccharides were also present. Tannins with the highest degree of polymerization are those with the highest affinity for cell walls [
13,
14,
42,
43], so those remaining in the solution presented a lower mDP. The large decrease observed when the commercial polysaccharides were present may indicate that a slight degree of precipitation of high molecular weight tannin-commercial polysaccharide complexes could have occurred. Since direct interactions with the commercial polysaccharides led to no precipitation, any precipitation, if it existed, can only be attributed to the CW-PS released during this experiment. Mateus et al. [
15] stated that the masses and structures of colloidal tannin−polysaccharide complexes depend on the degree of tannin polymerization; short tannins and polysaccharides are aggregated into loose oligomeric structures whose sizes are comparable to a single polysaccharide molecule. Tannins longer than 10 nm (high mDP tannins) and polysaccharides aggregate into larger microgel-like particles, whose sizes exceed 200 nm and may sometimes precipitate.
The percentage of galloylation did not change when the grape skin tannin interacted with the commercial soluble polysaccharides or with the cell walls, but it did decrease significantly with CW-PS + SkinTan and when both cell walls and soluble polysaccharides were present in the interaction. It is possible that galloylated tannins present a high affinity for the proteins present in the soluble material since these tannins have always been considered as the cause of a high degree of astringency in wines and could explain why it decreases in the presence of solubilized proteins.
The percentage of epigallocatechin in the tannins remaining in solution decreased significantly in all the experiments. Trihydroxylated subunits may present a higher affinity for interactions since epigallocatechin has an additional hydroxyl group on ring B that provides an extra site for hydrogen bonding to occur [
44].
When seed tannins were used, differences could be observed (
Table 2). The addition of soluble polysaccharides to the medium did not decrease the grape seed tannin content in solution with any of the polysaccharides, indicating the formation of soluble complexes.
When seed tannins were added to the soluble material obtained from the cell walls stirred in model solution, the seed tannins in the solution decreased, similarly to that described for skin tannins, although to a lower degree in proportion. Several studies have shown that the reactivity of tannins with different compounds increases with the molecular weight [
45,
46,
47,
48]. In the same way, Mateus et al. [
15] also stated that the affinity of tannins and proteins increased with tannin mDP, which is why a greater reduction in tannins was seen when skin tannins were used.
On the other hand, when the interaction between the grape seed tannins and the cell walls was studied, there was a 64.8% reduction of the tannins remaining in solution, a lower decrease than that observed in the case of skin tannins. This finding was similar to those of Bindon [
4], who also recorded that tannins from grape skin had a greater affinity for Syrah grape cell walls than tannins from seeds.
The addition of soluble polysaccharides to the model solution containing seed tannin and cell walls significantly reduced the adsorption of tannin by these cell walls, with no significant difference being observed between the soluble polysaccharides added, although the reduction was higher than that observed with skin tannins.
The average degree of polymerization of the seed tannin was reduced in the presence of soluble polysaccharides and cell walls, and even more so when both were in contact with the tannin, which coincided with the results obtained with grape skin tannin.
The percentage of galloylation of the seed tannin did not change in the presence of the commercial polysaccharides but decreased in the presence of CW-PS. Those tannins remaining in the solution presented a reduced %Gal in the presence of cell walls, especially if commercial polysaccharides were also present.
3.2. Analysis of the Tannins Measured by SEC in Model Solutions
SEC completes the information obtained with the phloroglucinolysis method, which cannot detect those tannins that cannot be depolymerized by phloroglucinol. This technique gives information about the mass distribution of the tannins in solution and improves our knowledge as to how phenolic compounds are affected by the treatments.
Figure 1 and
Figure 2 and
Table 3 show the mass distribution of the skin tannins that remained in the solution in the different experiments with model solutions. It can be seen that the addition of the two commercial polysaccharides to the tannin solution led to slight changes in the profile of the mass distribution of these tannins. These results confirm that polysaccharide–skin tannin complexes do not form structures that precipitate under our conditions, while the slightly lager areas presented by the tannin–polysaccharide complexes could be due to an increase in the overall particle size of the complexes, as observed by Li et al. [
29]. This phenomenon was not observed in the mDP values, possibly due to that tannin–polysaccharide complexes could be formed mainly with oxidized tannins, which do not show a response by the phloroglucinolysis method.
The results of the interaction between the skin tannin and the soluble material released from cell walls (CW-PS + SkinTan) confirmed that the soluble material dramatically decreases the content of tannins in solution, which agrees with our phloroglucinolysis analysis results.
Figure 2 shows the profile of the tannins remaining in solution after the interactions of skin tannins and cell walls, with or without the commercial soluble polysaccharides. As already observed by the phloroglucinolysis technique, the presence of the soluble polysaccharides increased the concentration of skin tannins in solution, probably as a result of competition with cell walls. In accordance with the results obtained in phloroglucinolysis, MAN was the soluble polysaccharide that maintained the highest amount of skin tannin in solution after the interaction, closely followed by the amounts found when PEC was used. It was surprising that MAN and PEC performed similarly, given the different concentrations used for each of them. Regarding the possible causes behind PEC and MAN resulting in similar capacities of interaction, it can be hypothesized that the low pH of the model solution led to demethylation of the highly methylated pectin [
49], which, in turn, lowers its affinity for tannins [
7].
Figure 3 and
Figure 4 and
Table 4 show the mass distribution of the seed tannins that remained in solution in the different experiments with the model solutions.
Figure 3 shows that the addition of soluble polysaccharides to seed tannins hardly changed the profile of the mass distribution of the seed tannin in solution. As with the grape skin tannin, in the presence of CW-PS, a pronounced decrease in the tannins in solution was observed (especially of the polymeric and oligomeric compounds), confirming the results found with the phloroglucinolysis analysis.
3.3. Analysis of the Soluble Polysaccharides Released in the Tests with Model Solutions by SEC
To further analyze the role played by the polysaccharides solubilized from cell walls in the precipitation of skin and seed tannins, we have studied the changes observed in their concentration after the different interactions using SEC.
Figure 5,
Figure 6,
Figure 7 and
Figure 8 and
Table 5 show the profile of the soluble polysaccharides in the model solution after the different interactions. In this study, the area corresponding to the commercial polysaccharides (PEC and MAN) and the area resulting from the interaction between commercial polysaccharides and tannins (PEC + SkinTan, PEC + SeedTan, MAN + SkinTan, and MAN + SeedTan) clearly showed that, similarly to that observed when tannins were analyzed by SEC, the concentration of polysaccharides did not change, confirming the absence of polysaccharide precipitation and that the complexes formed between these commercial polysaccharides and tannins are soluble.
We also studied the concentration of the polysaccharides remaining in solution after the reaction of suspended CWs and the tannins in the presence or absence of the commercial PEC and MAN. The area of these polysaccharides (CW) in the absence of tannins was very large compared with the area representing the added commercial polysaccharides. When the skin or seed tannins were added to the model solution containing the suspended cell walls and were allowed to interact for 90 min, the area slightly decreased in the case of skin tannins (10%) and in the case of seed tannins (7%), but more so when only the CW-PS (the solution after stirring the cell walls followed by the elimination of the insoluble and suspended cell walls) interacted with the tannins, especially when seed tannins were used (15%). We hypothesized that in the presence of suspended cell walls, part of the tannins binds to this material (this was already observed to be the predominant reaction) and, therefore, does not interact with the soluble polysaccharides. Only when the soluble polysaccharides are present do all the added tannins interact with them and the probability of precipitation of this soluble material increases. Bindon et al. [
2] stated that although pectic polysaccharides have the capacity to interact with tannins in solution to form a stable, soluble complex, coprecipitation of soluble material and tannins has also been observed probably resulting from complexes formed with the polysaccharides. Riou et al. [
50] reported that with Rhamnogalacturonan (II) dimers a co-aggregation with tannins was observed and that could lead to precipitation.
However, it is important to note that the decrease in the concentration of polysaccharides after the interaction with tannins is very small compared with the decrease observed in the concentration of tannins (
Figure 1,
Figure 2,
Figure 3 and
Figure 4). This difference can only be attributed to the fact that other compounds beside polysaccharides are responsible for a large part of tannin precipitation (probably proteins, which although present in low concentrations may play an important role).
When the commercial polysaccharides PEC and MAN were added to the solution containing the suspended cell walls and the tannins, the area corresponding to the polysaccharides increased significantly, indicating that these commercial products did not precipitate in the medium, in agreement with previous results.
3.4. The Role of Soluble Polysaccharides in the Chromatic and Sensory Characteristics of Finished Wines
After the studies made with model solutions, it was decided to gain more knowledge of the behavior of these soluble polysaccharides in real vinifications, to ascertain if using them just after crushing (instead of using them in finished wines, as it is common with other polysaccharides such as mannoprotein) could reduce the loss of tannins and improve the chromatic and sensory characteristics of a wine.
We already knew that the transfer rate of phenolic compounds from grape to must is limited due to, among other reasons, the interactions that occur between phenolic compounds and the skin and pulp cell walls present in the must in large concentrations, interactions that prevent these pigments from contributing to the final wine phenolic content [
2,
3]. Another potential mechanism has been identified in which extracted grape tannins may be lost from must/wine during vinification, and this is as a precipitate with solubilized grape proteins [
51,
52]. These authors stated that although protein is, in general, a minor component in terms of total concentration, losses of tannins via precipitation with proteins could be in the order of 50% of available tannins.
If the capacity of polysaccharides to interact with tannins in solution to form a stable soluble complex can be confirmed in wines, this could be used to reduce polyphenol fining during vinification (by limiting must protein–polyphenol precipitation and/or competing with the adsorption onto cell walls). These interactions could also modulate wine astringency since the formation of tannin and polysaccharide complexes may influence their reactivity with salivary proteins and lead to changes in the perception of astringency [
53]. As an example, soluble pectins have been described as reducing the astringency of persimmon [
54] and other carbohydrates such as xanthan or Arabic gum could also have the same effect [
55].
Therefore, we added the two studied polysaccharides at the beginning of a red wine vinification to favor competition between the adsorption of tannins by the insoluble cell wall material in suspension in the must and their reaction with proteins. For this, wine chromatic characteristics were measured at the end of alcoholic fermentation and after six months in the bottle (
Table 6).
With regards to the chromatic parameters, the color intensity at the end of alcoholic fermentation and after six months in the bottle of the two wines to which soluble polysaccharides were added just after crushing significantly increased. The wine with added PEC showed the highest color intensity.
The total polyphenol index was significantly higher in the wines with added polysaccharides than in the control wine, both at the end of alcoholic fermentation and after six months in the bottle, with no significant differences between the polysaccharides.
The addition of soluble polysaccharides significantly increased total anthocyanins in the wines. At the end of the alcoholic fermentation, there were significant differences among all the wines, the wines with added PEC obtaining the highest results after six months in the bottle, total anthocyanins decreased slightly due to polymerizations, but the differences with the control wine were maintained and no differences between the PEC-containing and the MAN-containing wines were observed.
In terms of the MCPT, it was observed that the addition of soluble polysaccharides led to wines with significantly higher tannin values than control wines, almost doubling the concentration. After six months in the bottle, the vinification with added mannan continued to show a significantly higher tannin concentration than the control wine, although the tannin concentration of this wine decreased much more than in the control vinification or wine with added PEC. It could be hypothesized that this fall in the total tannin values could be due to the precipitation of high molecular tannin–polysaccharide aggregates.
The concentrations of total tannins measured by the phloroglucinolysis method are shown in
Table 7. In the analysis carried out in the wines at the end of AF, the results clearly showed that the addition of both soluble polysaccharides led to a significant increase in the tannin concentration, the highest values being found in the pectin-added wine, with more than double the total tannins measured in the control wine, and mannan-added wines also showed a higher concentration of tannins than control wines. Similarly to the results for MCPT, the mannan-added wines showed a notable loss of tannins after six months in the bottle. This may be due to the complexes that these tannins form with these soluble polysaccharides and which precipitate over time.
The mPD of tannins increased with the addition of soluble polysaccharides in both wines, at the end of AF and after six months in the bottle. This finding was to be expected since the most polymerized tannins are the most susceptible to binding to cell walls and precipitating with proteins. When the soluble polysaccharides were added, they competed with the cell walls and proteins to bind to these tannins so that the amount of polymeric tannins (and hence mDP) in the resulting wine increased.
The distribution of the molecular weights of the proanthocyanidins from the different microvinifications obtained in the SEC analysis is shown in
Figure 9 and
Figure 10.
When the analysis was carried out at the end of AF (
Figure 9), the area of the wines supplemented with polysaccharides was higher for all the molecular masses. The level of polymeric tannins was one of the fractions that was clearly higher in the presence of polysaccharides, coinciding with the higher mDP found in these wines. This profile changed during wine aging (
Figure 10), when polymerization reactions developed, changing the molecular weight distribution profile. After six months of aging in the bottle, the differences between vinifications decreased.
Then, a sensory analysis was conducted with the wines that had been six months in the bottle (
Figure 11). The visual properties of the wines made with added PEC and MAN scored higher than the control wines, which coincides with the results obtained in the colorimetric analysis.
As for the aroma descriptors, the intensity and quality of the aroma of the polysaccharides-supplemented wines scored higher than the control wine, the highest values being observed in the PEC wines, followed by MAN wines. Fruit aroma was also better scored in wines with soluble polysaccharides, whereas the score for vegetal aroma was higher in the control wine. Mitropoulou et al. [
56] studied the effect of commercial tannin extracts, a natural wine polysaccharide extract, pectin, and arabinogalactan on the headspace release of selected aroma compounds from a “model wine” solution observing that, in general, the volatility of esters was generally increased upon tannin addition except at very high addition levels (10 g/L). Both arabinogalactan and pectin addition at low concentrations increased the volatility of the studied aroma compounds, while at higher concentrations pectin exhibited a different behavior by salting out hydrophobic compounds in the vapor phase.
Moreover, Saenz-Navajas et al. [
57] stated that sensory quality was primarily related to wines without defective aroma and secondarily to the presence of some nonvolatile components, among them, proanthocyanidins linked to polysaccharide.
Regarding in-mouth parameters, wines with added polysaccharides scored higher for astringency and bitterness, probably due to the higher tannin content, although persistence, intensity, and mouthfeel quality had higher scores in the wines containing the polysaccharides. It has been described that polysaccharides in wine significantly increase the ‘fullness’ sensation and can potentially add ‘mellowness’ to wines [
16] and this could compensate the higher astringency sensation caused by the higher tannin concentration.