Blood Biomarkers in Takotsubo Syndrome Point to an Emerging Role for Inflammaging in Endothelial Pathophysiology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Human Patient Serum
2.2. Ethics Approval and Consent to Participate
2.3. Study Design—Human Patient Serum
2.4. Biochemical Analyses
2.5. Endothelial Cell Cultures
2.6. Human iPSC Maintenance and Cardiac Differentiation
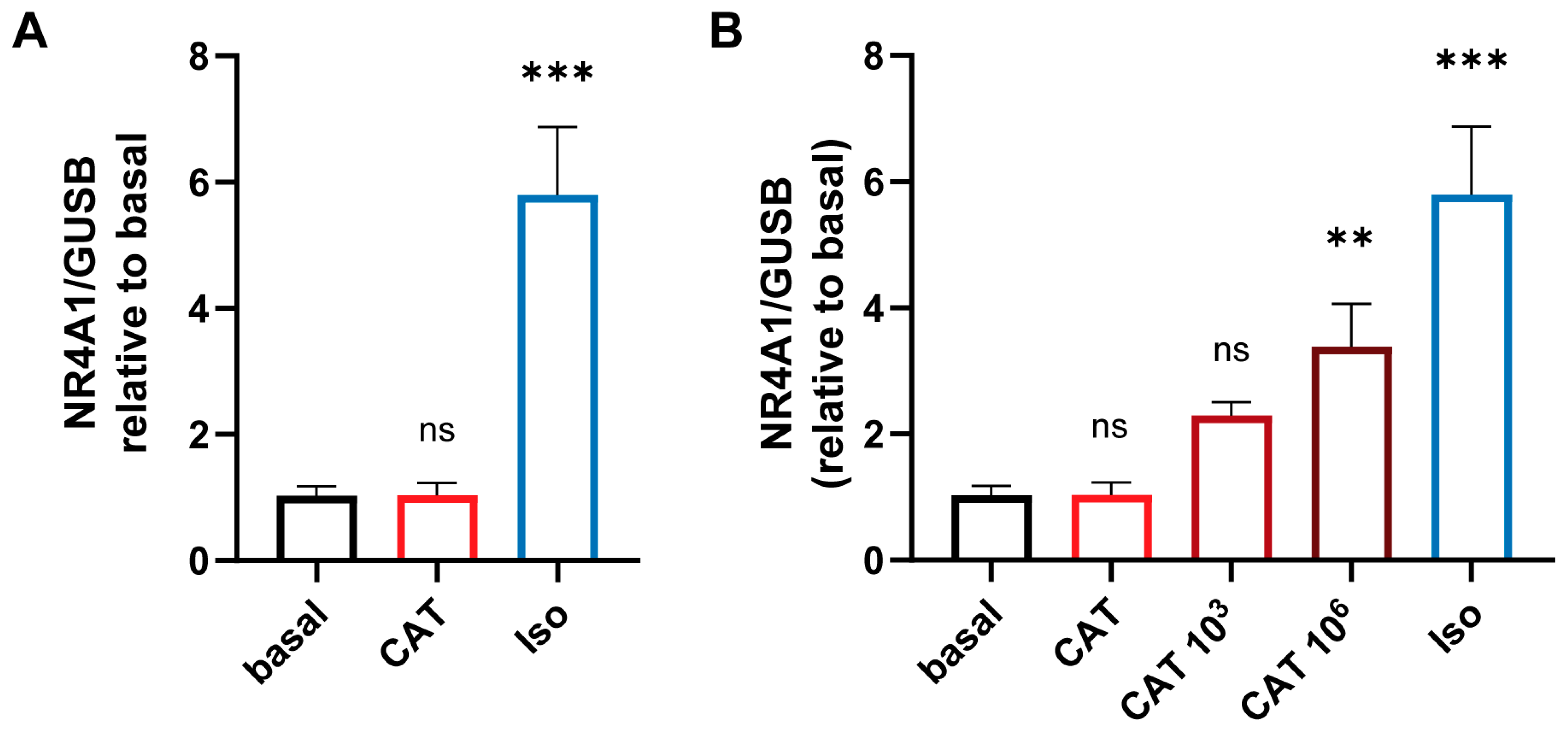
2.7. Catecholamine Treatment In Vitro
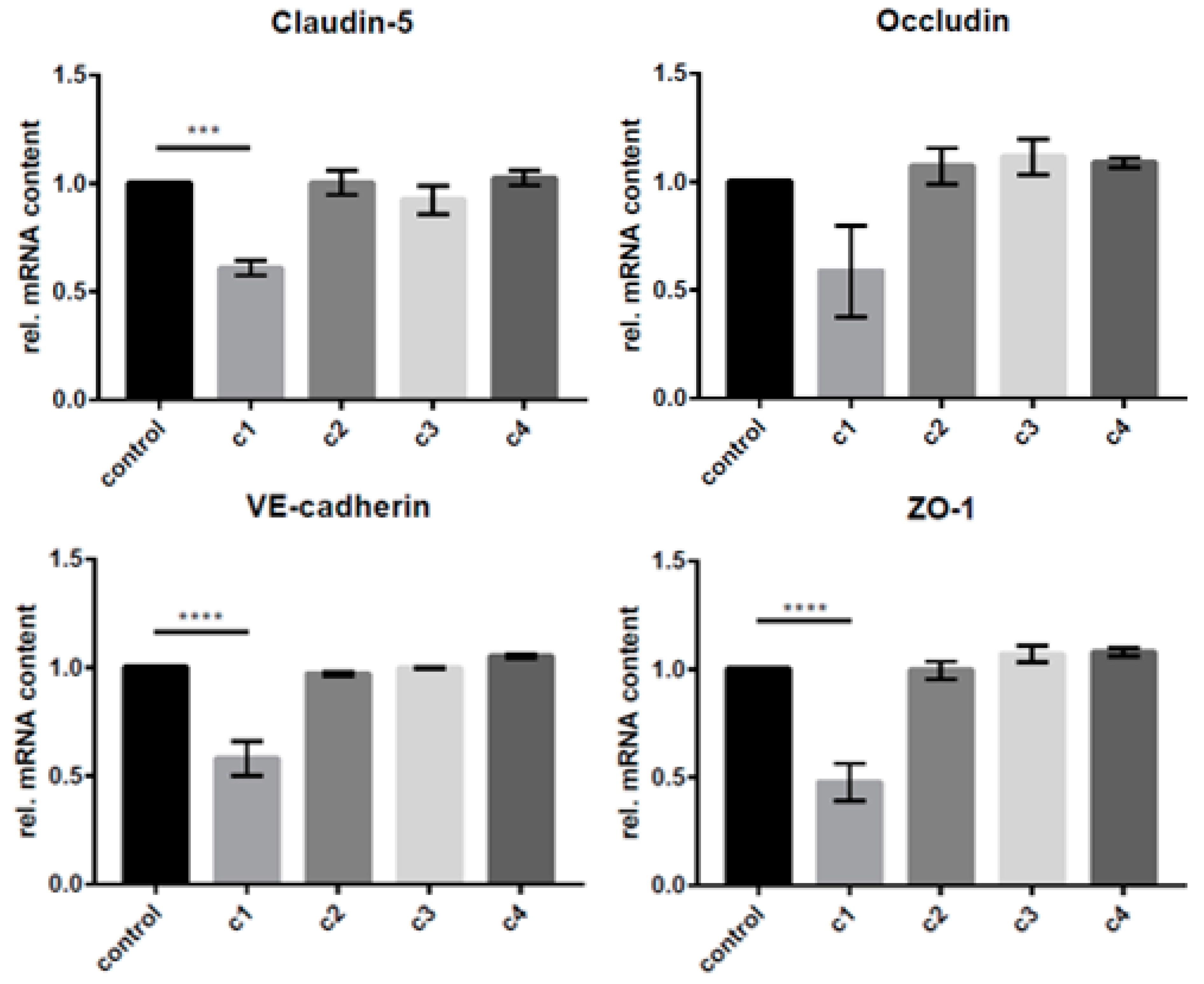
2.8. Gene Expression Analysis in cEND and MyEND Cells
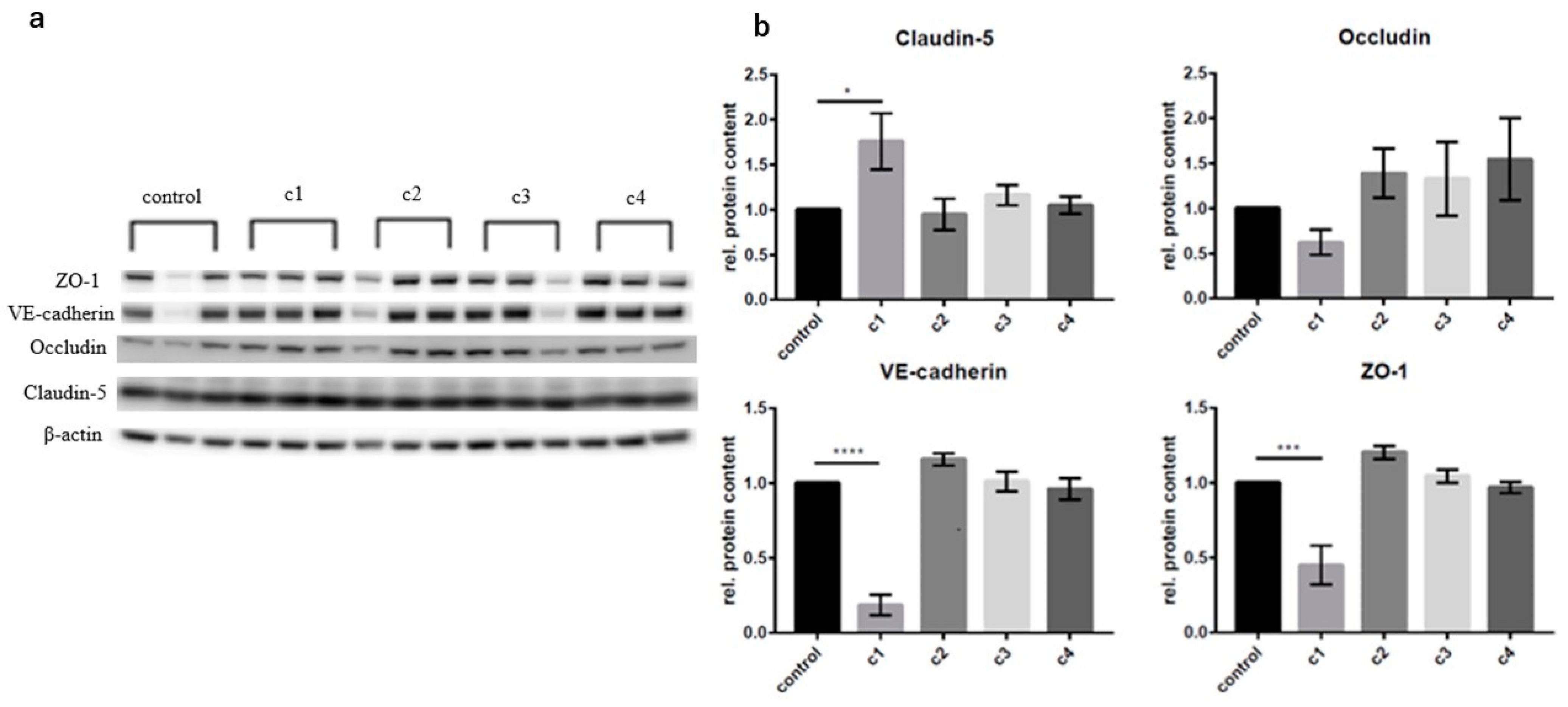
2.9. Western Blot Analysis in cEND and MyEND Cells
2.10. Gene expression Analysis in Cardiomycytes
2.11. Computational Analysis
2.12. Analysis and Statistics
3. Results
3.1. Quantification of Catecholamines in Serum of Patients with Different Heart Diseases
3.2. The Concentration-Dependent Effects of CAT on cEND and MyEND Brain and Myocardial Endothelial Cells In Vitro
3.3. The Concentration-Dependent Effects of CATs on iPSC-Derived Cardiomycytess
3.4. Quantification of Cytokines and Chemokines in Patient Serum
3.5. Quantification of Testosterone and Estradiol in Serum of Patients with Various Heart Diseases and Correlation between Testosterone-to-Estradiol Ratio
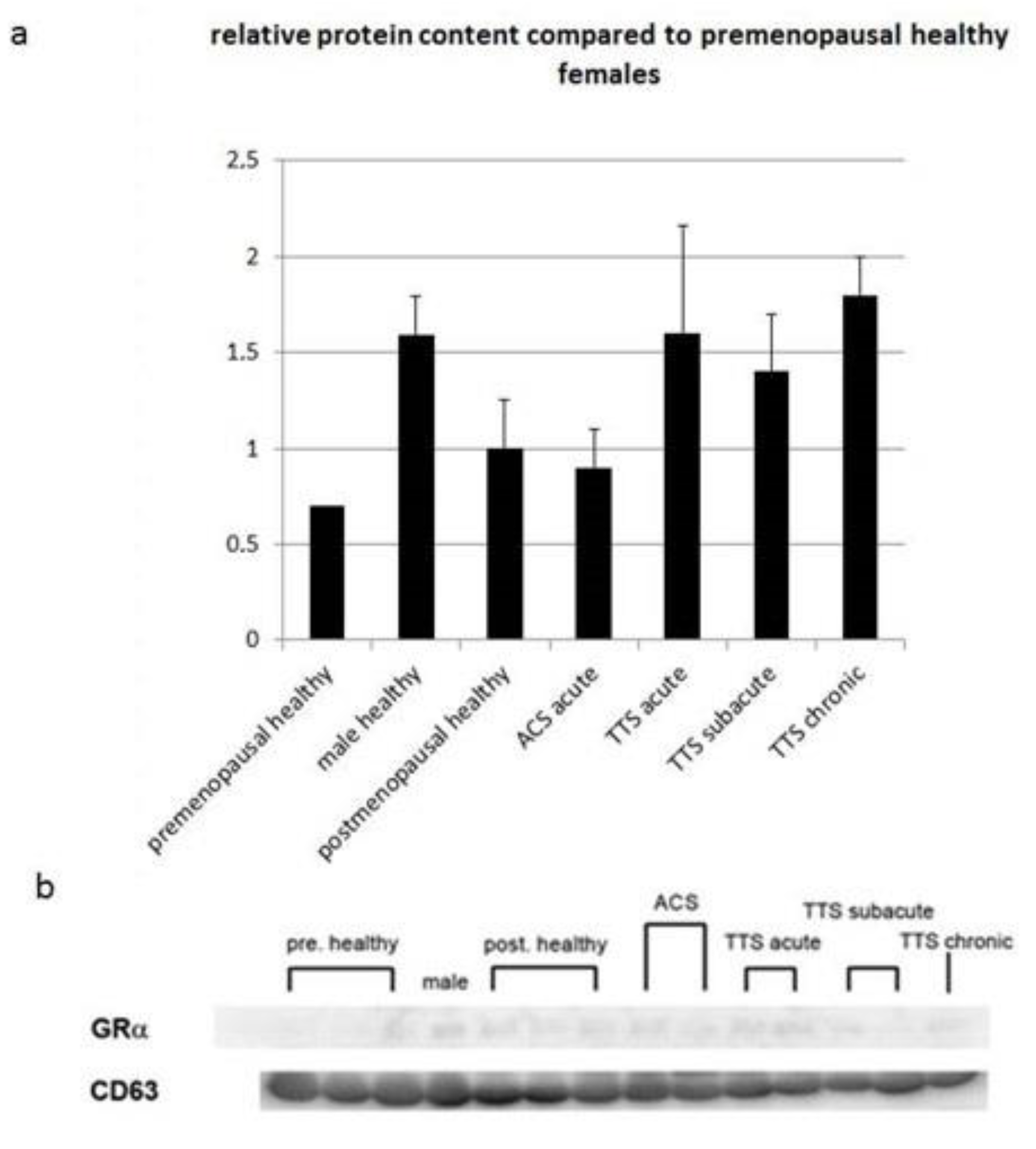
3.6. Protein Expression of Glucocorticoid Receptor α in Exosomes of Serum from Patients with Various Heart Diseases
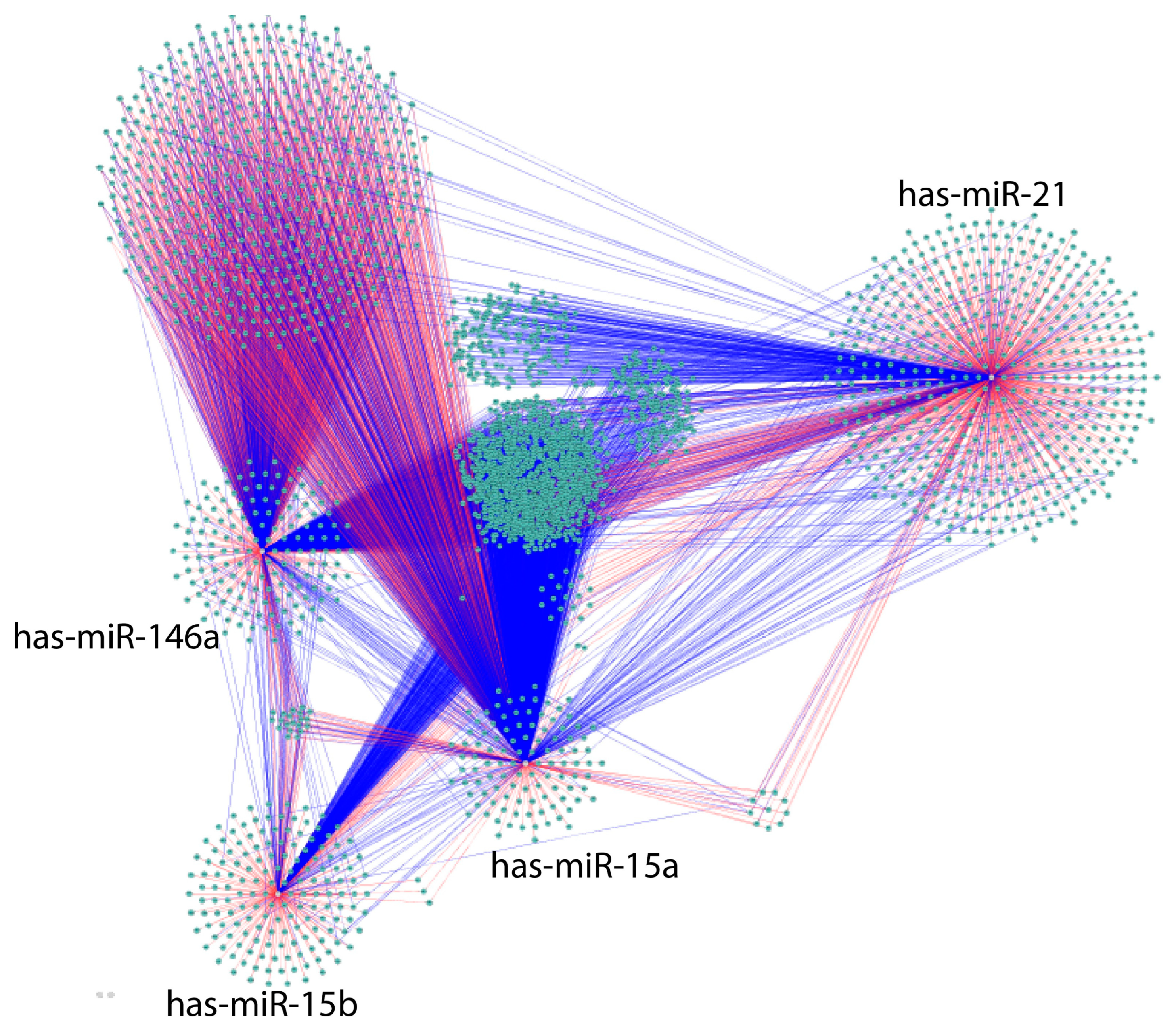
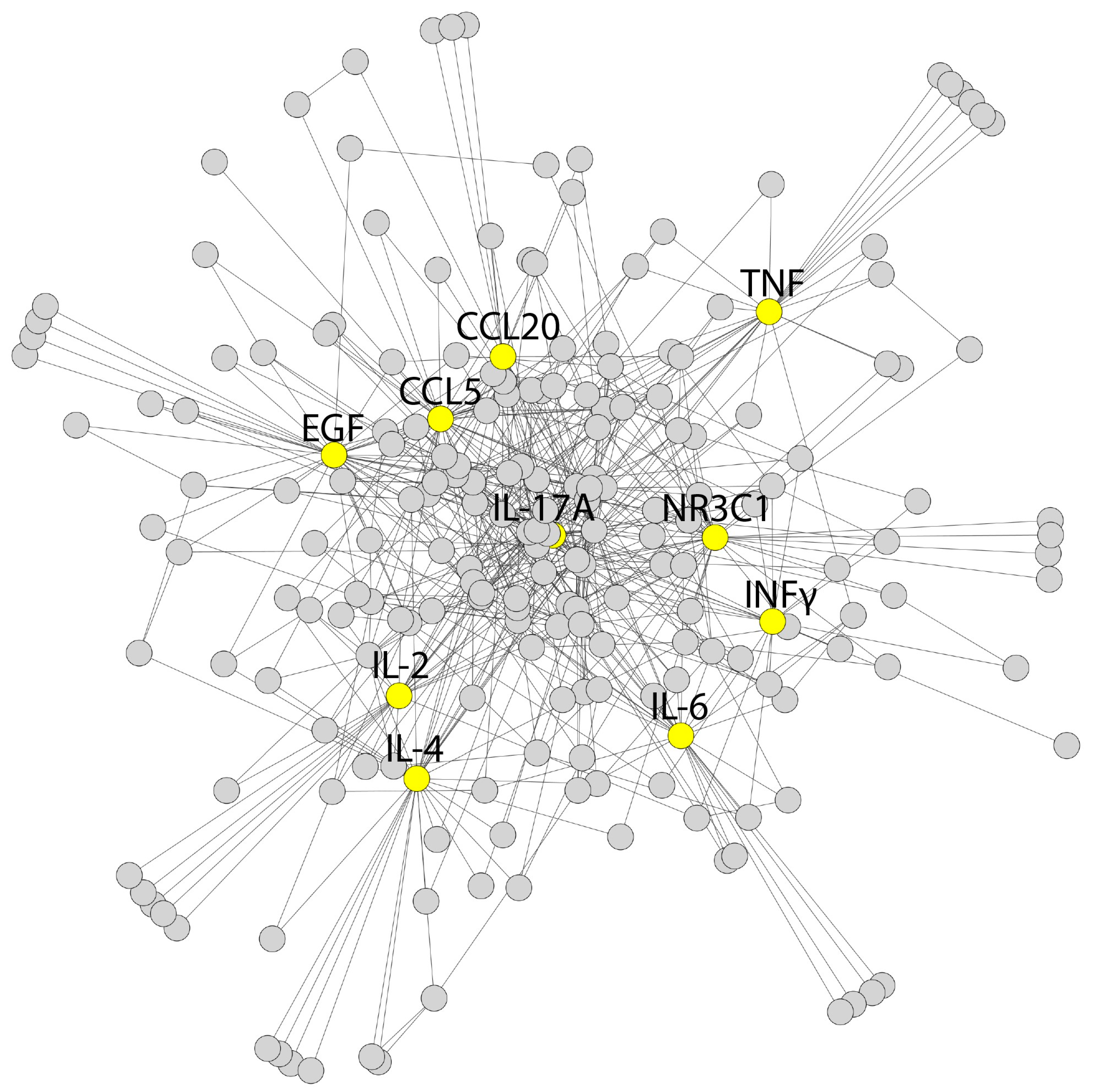
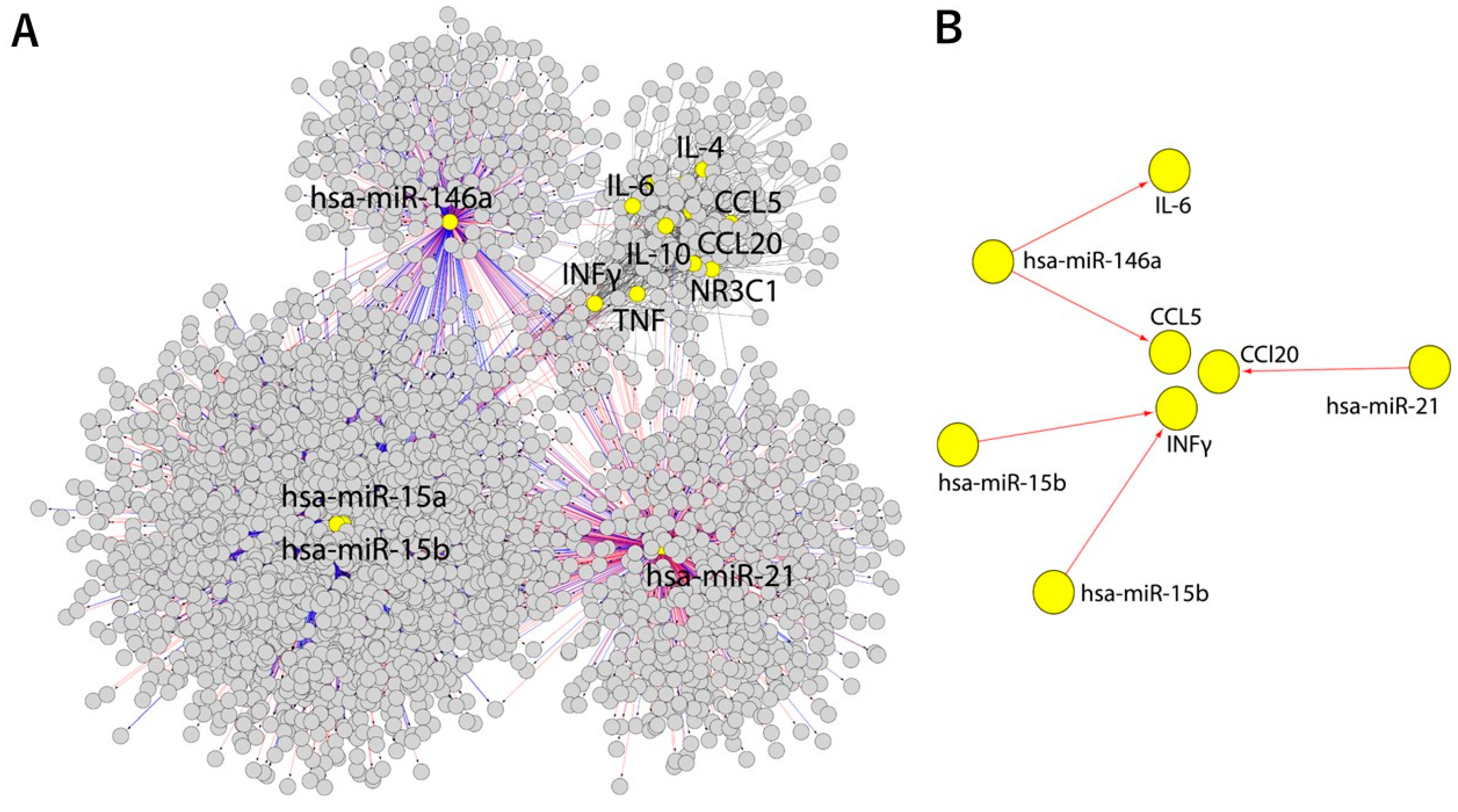
3.7. Bioinformatic Analysis
4. Discussion
4.1. TTS Patient Serum Catecholamine Levels
4.2. TTS Patient GR Serum Levels
4.3. Inflammation: Cytokines and Chemokines in Patient Serum
4.4. Sex Differences
4.5. Bioinformatic Extrapolation: TTS—An Inflammaging Disorder?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Elgendy, A.Y.; Mahmoud, A.N.; Elgendy, I.Y. Takotsubo syndrome: Still a benign entity? Int. J. Cardiol. 2017, 247, 41. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International expert consensus document on Takotsubo syndrome (Part II): Diagnostic workup, outcome, and management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Elgendy, A.Y.; Elgendy, I.Y.; Mansoor, H.; Mahmoud, A.N. Clinical presentations and outcomes of Takotsubo syndrome in the setting of subarachnoid hemorrhage: A systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 236–245. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position statement from the taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef] [Green Version]
- Angelini, P.; Tobis, J.M. Is high-dose catecholamine administration in small animals an appropriate model for takotsubo syndrome? Circ. J. 2015, 79, 897. [Google Scholar] [CrossRef] [Green Version]
- Messas, N.; Willemain, R.; Bilger, A.; Rondeau-Lutz, M.; Kuhnert, C.; Mertes, P.; Morel, O.; Collange, O. Takotsubo syndrome triggered by acute intermittent porphyria attack: An unusual stressor for catecholamine-induced cardiomyopathy. Int. J. Cardiol. 2016, 207, 28–30. [Google Scholar] [CrossRef]
- Y-Hassan, S. Catecholamine levels and cardiac sympathetic hyperactivation-disruption in Takotsubo syndrome. JACC Cardiovasc. Imaging 2017, 10, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S.; Sörensson, P.; Ekenbäck, C.; Lundin, M.; Agewall, S.; Brolin, E.B.; Caidahl, K.; Cederlund, K.; Collste, O.; Daniel, M.; et al. Plasma catecholamine levels in the acute and subacute stages of takotsubo syndrome: Results from the Stockholm myocardial infarction with normal coronaries 2 study. Clin. Cardiol. 2021, 44, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Y-Hassan, S. Divergence in the results of plasma catecholamine levels in different studies on patients with takotsubo syndrome: Why? J. Cardiol. 2016, 68, 89. [Google Scholar] [CrossRef] [Green Version]
- Andò, G.; Boretti, I.; Tripodi, R. Stress cardiomyopathies beyond Takotsubo: Does a common catecholaminergic pathophysiology fit all? Expert Rev. Cardiovasc. Ther. 2014, 12, 643–645. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Borggrefe, M.; Akin, I. Catecholamine in takotsubo syndrome. Int. J. Cardiol. 2017, 233, 97. [Google Scholar] [CrossRef] [PubMed]
- Borchert, T.; Hübscher, D.; Guessoum, C.I.; Lam, T.D.; Ghadri, J.R.; Schellinger, I.N.; Tiburcy, M.; Liaw, N.Y.; Li, Y.; Haas, J.; et al. Catecholamine-dependent β-adrenergic signaling in a pluripotent stem cell model of Takotsubo cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 975–991. [Google Scholar] [CrossRef]
- Madias, J.E. Pathophysiology of Takotsubo syndrome: An adrenergic cardiac “chemical neuritis/myocarditis”? Cardiovasc. Revasc. Med. 2014, 15, 50. [Google Scholar] [CrossRef]
- Martins, J.B.; Kerber, R.E.; Marcus, M.L.; Laughlin, D.L.; Levy, D.M. Inhibition of adrenergic neurotransmission in ischaemic regions of the canine left ventricle. Cardiovasc. Res. 1980, 14, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Feola, M.; Chauvie, S.; Rosso, G.L.; Biggi, A.; Ribichini, F.; Bobbio, M. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: A myocardial PET study. J. Nucl. Cardiol. 2008, 15, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Palla, A.R.; Dande, A.S.; Petrini, J.; Wasserman, H.S.; Warshofsky, M.K. Pretreatment with low-dose β-adrenergic antagonist therapy does not affect severity of Takotsubo cardiomyopathy. Clin. Cardiol. 2012, 35, 478–481. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: A new model of Takotsubo cardiomyopathy. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Salemi, V.M.; Atik, E.; Kairalla, R.A.; Queiroz, E.L.; Rosa, L.V.; Kalil Filho, R. Takotsubo cardiomyopathy triggered by β(2) adrenergic agonist. J. Bras. Pneumol. 2011, 37, 560–562. [Google Scholar] [CrossRef] [Green Version]
- Tutgun Onrat, S.; Dural, İ.E.; Yalım, Z.; Onrat, E. Investigating changes in β-adrenergic gene expression (ADRB1 and ADRB2) in Takotsubo (stress) cardiomyopathy syndrome; a pilot study. Mol. Biol. Rep. 2021, 48, 7893–7900. [Google Scholar] [CrossRef]
- Yoneda, H.; Nakamura, T.; Shirao, S.; Tanaka, N.; Ishihara, H.; Suehiro, E.; Koizumi, H.; Isotani, E.; Suzuki, M.; SAH PICCO Study Group. Multicenter prospective cohort study on volume management after subarachnoid hemorrhage: Hemodynamic changes according to severity of subarachnoid hemorrhage and cerebral vasospasm. Stroke 2013, 44, 2155–2161. [Google Scholar] [CrossRef] [Green Version]
- Bybee, K.A.; Motiei, A.; Syed, I.S.; Kara, T.; Prasad, A.; Lennon, R.J.; Murphy, J.G.; Hammill, S.C.; Rihal, C.S.; Wright, R.S. Electrocardiography cannot reliably differentiate transient left ventricular apical ballooning syndrome from anterior ST-segment elevation myocardial infarction. J. Electrocardiol. 2007, 40, 38.e1–38.e6. [Google Scholar] [CrossRef] [PubMed]
- Couch, L.S.; Fiedler, J.; Chick, G.; Clayton, R.; Dries, E.; Wienecke, L.M.; Fu, L.; Fourre, J.; Pandey, P.; Derda, A.A.; et al. Circulating microRNAs predispose to takotsubo syndrome following high-dose adrenaline exposure. Cardiovasc. Res. 2022, 118, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nef, H.M.; Möllmann, H.; Hilpert, P.; Troidl, C.; Voss, S.; Rolf, A.; Behrens, C.B.; Weber, M.; Hamm, C.W.; Elsässer, A. Activated cell survival cascade protects cardiomyocytes from cell death in Tako-Tsubo cardiomyopathy. Eur. J. Heart Fail. 2009, 11, 758–764. [Google Scholar] [CrossRef]
- Nakano, T.; Onoue, K.; Nakada, Y.; Nakagawa, H.; Kumazawa, T.; Ueda, T.; Nishida, T.; Soeda, T.; Okayama, S.; Watanabe, M.; et al. Alteration of β-adrenoceptor signaling in left ventricle of acute phase Takotsubo syndrome: A human study. Sci. Rep. 2018, 8, 12731. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.M.; Brinjikji, W.; Salka, S. Demographic and co-morbid predictors of stress (takotsubo) cardiomyopathy. Am. J. Cardiol. 2012, 110, 1368–1372. [Google Scholar] [CrossRef]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and systemic inflammation in acute stress-induced (Takotsubo) cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
- Karnati, S.; Guntas, G.; Rajendran, R.; Shityakov, S.; Höring, M.; Liebisch, G.; Kosanovic, D.; Ergun, S.; Nagai, M.; Förster, C.Y. Quantitative lipidomic analysis of Takotsubo syndrome patients’ serum. Front. Cardiovasc. Med. 2022, 9, 797154. [Google Scholar] [CrossRef]
- Thal, S.C.; Smetak, M.; Hayashi, K.; Förster, C.Y. Hemorrhagic cerebral insults and secondary Takotsubo syndrome: Findings in a novel in vitro model using human blood samples. Int. J. Mol. Sci. 2022, 23, 11557. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International expert consensus document on Takotsubo syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [Green Version]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565 Pt 2, 475–486. [Google Scholar] [CrossRef]
- Burek, M.; Salvador, E.; Förster, C.Y. Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J. Vis. Exp. 2012, 66, e4022. [Google Scholar]
- Golenhofen, N.; Ness, W.; Wawrousek, E.F.; Drenckhahn, D. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem. Cell Biol. 2002, 117, 203–209. [Google Scholar] [CrossRef]
- Ittner, C.; Burek, M.; Störk, S.; Nagai, M.; Förster, C.Y. Increased catecholamine levels and inflammatory mediators alter barrier properties of brain microvascular endothelial cells in vitro. Front. Cardiovasc. Med. 2020, 7, 73. [Google Scholar] [CrossRef]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef] [Green Version]
- Weksler, B.; Iromero, L.A.; Couraud, P.O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Haase, A.; Göhring, G.; Martin, U. Generation of non-transgenic iPS cells from human cord blood CD34+ cells under animal component-free conditions. Stem Cell Res. 2017, 21, 71–73. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyöngyösi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Hofer, T.; Costa, A.; Lu, D.; Batkai, S.; Gupta, S.K.; Bolesani, E.; Zweigerdt, R.; Megias, D.; Streckfuss-Bomeke, K. Telomerase therapy attenuates cardiotoxic effects of doxorubicin. Mol. Ther. 2021, 29, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, H.M.; Cheyne, L.; Brown, P.A.; Kerr, K.; Hannah, A.; Srinivasan, J.; Duniak, N.; Horgan, G.; Dawson, D.K. Characterization of the myocardial inflammatory response in acute stress-induced (Takotsubo) cardiomyopathy. JACC Basic Transl. Sci. 2018, 3, 766–778. [Google Scholar] [CrossRef]
- Kutmon, M.; Kelder, T.; Mandaviya, P.; Evelo, C.T.; Coort, S.L. CyTargetLinker: A cytoscape app to integrate regulatory interactions in network analysis. PLoS ONE 2013, 8, e82160. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.M.; Ullah, S.; Aziz, S.; Johnsen, T.A.; Shityakov, S. In-silico analysis of non-synonymous single nucleotide polymorphisms in human β-defensin type 1 gene reveals their impact on protein-ligand binding sites. Comput. Biol. Chem. 2022, 98, 107669. [Google Scholar] [CrossRef]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grouzmann, E.; Cavadas, C.; Grand, D.; Moratel, M.; Brunner, H.R.; Mazzolai, L. Blood sampling methodology is crucial for precise measurement of plasma catecholamines concentrations in mice. Pflugers Arch. 2003, 447, 254–258. [Google Scholar] [CrossRef]
- Förster, C.; Kahles, T.; Kietz, S.; Drenckhahn, D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J. Physiol. 2007, 580 Pt 3, 937–949. [Google Scholar] [CrossRef]
- Yi, X.; Xu, C.; Huang, P.; Zhang, L.; Qing, T.; Li, J.; Wang, C.; Zeng, T.; Lu, J.; Han, Z. 1-Trifluoromethoxyphenyl-3-(1-Propionylpiperidin-4-yl) urea protects the blood-brain barrier against ischemic injury by upregulating tight junction protein expression, mitigating apoptosis and inflammation In Vivo and In Vitro Model. Front. Pharmacol. 2020, 11, 1197. [Google Scholar] [CrossRef]
- Davies, L.; Paraskevopoulou, E.; Sadeq, M.; Symeou, C.; Pantelidou, C.; Demonacos, C.; Krstic-Demonacos, M. Regulation of glucocorticoid receptor activity by a stress responsive transcriptional cofactor. Mol. Endocrinol. 2011, 25, 58–71. [Google Scholar] [CrossRef]
- Barcena, M.L.; Aslam, M.; Pozdniakova, S.; Norman, K.; Ladilov, Y. Cardiovascular inflammaging: Mechanisms and translational aspects. Cells 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Bleve, A.; Motta, F.; Durante, B.; Pandolfo, C.; Selmi, C.; Sica, A. Immunosenescence, inflammaging, and frailty: Role of myeloid cells in age-related diseases. Clin. Rev. Allergy Immunol. 2023, 64, 123–144. [Google Scholar] [CrossRef]
- Nagai, M.; Kobayashi, Y.; Kobatake, H.; Dote, K.; Kato, M.; Oda, N.; Kunita, E.; Kagawa, E.; Yamane, A.; Osawa, A.; et al. Happy heart syndrome: A case of Takotsubo syndrome with left internal carotid artery occlusion. Clin. Auton. Res. 2020, 30, 347–350. [Google Scholar] [CrossRef]
- Osawa, A.; Nagai, M.; Dote, K.; Kato, M.; Oda, N.; Kunita, E.; Kagawa, E.; Yamane, A.; Kobatake, H.; Shiota, H. A mid-ventricular variant of Takotsubo syndrome: Was it triggered by insular cortex damage? ESC Heart Fail. 2021, 8, 3408–3412. [Google Scholar] [CrossRef]
- Bybee, K.A.; Prasad, A. Stress-related cardiomyopathy syndromes. Circulation 2008, 118, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Nagai, M.; Dote, K.; Kato, M.; Sasaki, S.; Oda, N.; Kagawa, E.; Nakano, Y.; Yamane, A.; Higashihara, T.; Miyauchi, S.; et al. The insular cortex and Takotsubo cardiomyopathy. Curr. Pharm. Des. 2017, 23, 879–888. [Google Scholar] [CrossRef]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo syndrome: Pathophysiology, emerging concepts, and clinical implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Nazir, S.; Lohani, S.; Tachamo, N.; Ghimire, S.; Poudel, D.R.; Donato, A. Takotsubo cardiomyopathy associated with epinephrine use: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 229, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Marcelino, M.; Piechnik, S.K.; Marini, C.; Karamitsos, T.; Ntusi, N.A.; Francis, J.M.; Robson, M.D.; Arnold, J.; Mihai, R.; et al. Pheochromocytoma is characterized by catecholamine-mediated myocarditis, focal and diffuse myocardial fibrosis, and myocardial dysfunction. J. Am. Coll. Cardiol. 2016, 67, 2364–2374. [Google Scholar] [CrossRef] [Green Version]
- Grippo, A.J.; Scotti, M.A. Stress and neuroinflammation. Mod. Trends Pharm. 2013, 28, 20–32. [Google Scholar]
- Finnell, J.E.; Moffitt, C.M.; Hesser, L.A.; Harrington, E.; Melson, M.N.; Wood, C.S.; Wood, S.K. The contribution of the locus coeruleus-norepinephrine system in the emergence of defeat-induced inflammatory priming. Brain Behav. Immun. 2019, 79, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar]
- Bardo, M.T.; Chandler, C.M.; Denehy, E.D.; Carper, B.A.; Prendergast, M.A.; Nolen, T.L. Effect of the glucocorticoid receptor antagonist PT150 on acquisition and escalation of fentanyl self-administration following early-life stress. Exp. Clin. Psychopharmacol. 2023, 31, 362–369. [Google Scholar] [CrossRef]
- Duma, D.; Collins, J.B.; Chou, J.W.; Cidlowski, J.A. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010, 3, ra74. [Google Scholar] [CrossRef] [Green Version]
- González Ramírez, C.; Villavicencio Queijeiro, A.; Jiménez Morales, S.; Bárcenas López, D.; Hidalgo Miranda, A.; Ruiz Chow, A.; Tellez Cardenas, L.; Guardado Estrada, M. The NR3C1 gene expression is a potential surrogate biomarker for risk and diagnosis of posttraumatic stress disorder. Psychiatry Res. 2020, 284, 112797. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Förster, C.Y.; Dote, K. Sex hormone-specific neuroanatomy of Takotsubo syndrome: Is the insular cortex a moderator? Biomolecules 2022, 12, 110. [Google Scholar] [CrossRef]
- Walsh, C.P.; Bovbjerg, D.H.; Marsland, A.L. Glucocorticoid resistance and β2-adrenergic receptor signaling pathways promote peripheral pro-inflammatory conditions associated with chronic psychological stress: A systematic review across species. Neurosci. Biobehav. Rev. 2021, 128, 117–135. [Google Scholar] [CrossRef]
- Oberbeck, R. Catecholamines: Physiological immunomodulators during health and illness. Curr. Med. Chem. 2006, 13, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Costantino, M.D.; Guastafierro, F.; Triggiani, G.; Ferraretti, A.; Tarantino, N.; Saguner, A.; Di Biase, M.; Brunetti, N.D. Inflammatory patterns in Takotsubo cardiomyopathy and acute coronary syndrome: A propensity score matched analysis. Atherosclerosis 2018, 274, 157–161. [Google Scholar] [CrossRef]
- Šerstņova, K.; Pilmane, M.; Vitenberga-Verza, Z.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maroti, G.; Prieto-Simon, B. Expression of anti-inflammatory markers IL-2, IL-10, TGF-β1, βDEF-2, βDEF-3 and Cathelicidin LL37 in dairy cattle milk with different health status of the udder. Pol. J. Vet. Sci. 2022, 25, 237–248. [Google Scholar] [PubMed]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [Green Version]
- Blasko, I.; Stampfer-Kountchev, M.; Robatscher, P.; Veerhuis, R.; Eikelenboom, P.; Grubeck-Loebenstein, B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: The role of microglia and astrocytes. Aging Cell 2004, 3, 169–176. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Nagai, M.; Ergün, S.; Braunger, B.M.; Förster, C.Y. The Protective effects of neurotrophins and microRNA in diabetic retinopathy, nephropathy and heart failure via regulating endothelial function. Biomolecules 2022, 12, 1113. [Google Scholar] [CrossRef] [PubMed]
- Baylis, D.; Bartlett, D.B.; Patel, H.P.; Roberts, H.C. Understanding how we age: Insights into inflammaging. Longev. Healthspan 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S.; Pawelec, G. The immune system and its dysregulation with aging. Subcell Biochem. 2019, 91, 21–43. [Google Scholar]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Karnati, S.; Garikapati, V.; Liebisch, G.; Van Veldhoven, P.P.; Spengler, B.; Schmitz, G.; Baumgart-Vogt, E. Quantitative lipidomic analysis of mouse lung during postnatal development by electrospray ionization tandem mass spectrometry. PLoS ONE 2018, 13, e0203464. [Google Scholar] [CrossRef] [Green Version]
- Bolayırlı, I.M.; Önal, B.; Adıgüzel, M.; Konukoğlu, D.; Demirdağ, Ç.; Kurtuluş, E.M.; Türegün, F.A.; Uzun, H. The clinical significance of circulating miR-21, miR-142, miR-143, and miR-146a in patients with prostate cancer. J. Med. Biochem. 2022, 41, 191–198. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lee, C.H.; Li, Y.S.; Huang, J.T.; Lan, S.H.; Wang, Y.F.; Lai, W.W.; Wang, Y.C.; Lin, Y.J.; Liu, H.S.; et al. MicroRNA-146a suppresses tumor malignancy via targeting vimentin in esophageal squamous cell carcinoma cells with lower fibronectin membrane assembly. J. Biomed. Sci. 2020, 27, 102. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Calin, G.A.; Croce, C.M. miR-15a and miR-16-1 in cancer: Discovery, function and future perspectives. Cell Death Differ. 2010, 17, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Komiyama, T.; Kobayashi, H.; Ikari, Y. Gender Differences in Takotsubo Syndrome. Biology 2022, 11, 653. [Google Scholar] [CrossRef]
- Pattisapu, V.K.; Hao, H.; Liu, Y.; Nguyen, T.T.; Hoang, A.; Bairey Merz, C.N.; Cheng, S. Sex- and Age-Based Temporal Trends in Takotsubo Syndrome Incidence in the United States. J. Am. Heart Assoc. 2021, 10, e019583. [Google Scholar] [CrossRef]
- Zweiker, D.; Pogran, E.; Gargiulo, L.; El-Razek, A.A.; Lechner, I.; Vosko, I.; Rechberger, S.; Bugger, H.; Christ, G.; Bonderman, D.; et al. Neutrophile-Lymphocyte Ratio and Outcome in Takotsubo Syndrome. Biology 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Rudd, A.; Gamble, D.T.; Mezincescu, A.M.; Cheyne, L.; Horgan, G.; Dhaun, N.; Newby, D.E.; Dawson, D.K. Renin-Angiotensin and Endothelin Systems in Patients Post Takotsubo Cardiomyopathy. J. Am. Heart Assoc. 2022, 11, e025989. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Cammann, V.L.; Kato, K.; Szawan, K.A.; Di Vece, D.; Rossi, A.; Wischnewsky, M.; Hermes-Laufer, J.; Gili, S.; Citro, R.; et al. Impact of Atrial Fibrillation on Outcome in Takotsubo Syndrome: Data From the International Takotsubo Registry. J. Am. Heart Assoc. 2021, 10, e014059. [Google Scholar] [CrossRef] [PubMed]
| Postmenopausal Healthy n = 3 | TTS Acute n = 4 | TTS Subacute n = 8 | |
|---|---|---|---|
| Noradrenaline | 4.24 ± 1.52 | 3.65 ± 2.37 | 5.23 ± 5.69 * |
| Adrenaline | 0.22 ± 0.11 | 0.13 ± 0.1 | 0.19 ± 0.2 |
| Dopamine | 0.18 ± 0.08 | 0.21 ± 0.12 | 0.80 ± 1.06 * |
| Premenopausal Healthy n = 3 | Postmenopausal Healthy n = 3 | TTS Acute n = 4 | TTS Subacute n = 8 | TTS Chronic n = 3 | ACS n = 4 | |
|---|---|---|---|---|---|---|
| INFγ | 2.41 ± 1.48 | 18.7 ± 7.18 | 65.6 ± 71.99 | 26.79 ± 32.46 | 19.81 ± 30.50 | 18.46 ± 32.03 |
| IL-2 | 0.32 ± 0.33 | 1.49 ± 1.23 | 4.87 ± 8.012 | 2.2 ± 3.263 | 1.53 ± 2.243 | 0.37 ± 0.3641 |
| IL-4 | 2.34 ± 3.38 | 1.59 ± 0.88 | 50.77 ± 8.68 | 2.75 ± 4.29 | 0.80 ± 1.10 | 0.70 ± 0.92 |
| IL-6 | 0.34 ± 0.34 | 6.64 ± 15.41 | 38.01 ± 45.06 | 8.98 ± 15.13 | 1.45 ± 1.45 | 0.97 ± 0.77 |
| IL-10 | 12.13 ± 19.54 | 30.21 ± 27.33 | 24.48 ± 17.52 | 10.55 ± 10.74 | 3.88 ± 6.68 | 3.41 ± 5.79 |
| TNFα | 15 ± 5.67 | 18.53 ± 18.51 | 41.04 ± 47.72 | 25.8 ± 16.62 | 17.02 ± 5.262 | 15.6 ± 12.43 |
| Postmenopausal healthy n = 3 | TTS acute n = 4 | TTS subacute n = 8 | TTS chronic n = 3 | ACS n = 4 | ||
| EGF | 57.05 | 22.19 | 85.14 | 26.14 | 47.85 | |
| IL-17 | 3.40 ± 1.70 | 1.91 ± 0.84 | 25.67 ± 52.31 | 3.13 | 3.52 | |
| MIP3A | 147.3 ± 104.4 | 111.3 ± 68.08 | 249.6 ± 168.5 | 105.3 | 72.69 | |
| Postmenopausal healthy n = 3 | TTS acute n = 4 | TTS subacute n = 8 | ||||
| RANTES | 161,542 ± 190,762 | 69,217 ± 81,541 | 264,518 ± 381,721 |
| Postmenopausal Healthy n = 3 | TTS Acute n = 4 | TTS Subacute n = 8 | TTS Chronic n = 3 | |
|---|---|---|---|---|
| Testosterone | 202.5 ± 120.4 | 160 | 287.5 ± 257.5 | 145 ± 63.6 |
| Estradiol | 35.25 ± 54.04 | 5 | 43.13 ± 66.69 | 9.5 ± 6.364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, M.; Shityakov, S.; Smetak, M.; Hunkler, H.J.; Bär, C.; Schlegel, N.; Thum, T.; Förster, C.Y. Blood Biomarkers in Takotsubo Syndrome Point to an Emerging Role for Inflammaging in Endothelial Pathophysiology. Biomolecules 2023, 13, 995. https://doi.org/10.3390/biom13060995
Nagai M, Shityakov S, Smetak M, Hunkler HJ, Bär C, Schlegel N, Thum T, Förster CY. Blood Biomarkers in Takotsubo Syndrome Point to an Emerging Role for Inflammaging in Endothelial Pathophysiology. Biomolecules. 2023; 13(6):995. https://doi.org/10.3390/biom13060995
Chicago/Turabian StyleNagai, Michiaki, Sergey Shityakov, Manuel Smetak, Hannah Jill Hunkler, Christian Bär, Nicolas Schlegel, Thomas Thum, and Carola Yvette Förster. 2023. "Blood Biomarkers in Takotsubo Syndrome Point to an Emerging Role for Inflammaging in Endothelial Pathophysiology" Biomolecules 13, no. 6: 995. https://doi.org/10.3390/biom13060995











