DNA Methylation of PXDN Is Associated with Early-Life Adversity in Adult Mental Disorders
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. PXDN DNA Methylation Analysis in Whole Blood
3. Data Analyses
3.1. Statistical Analysis
3.2. Demographic and Clinical Information
4. Results
4.1. Demographic and Clinical Information
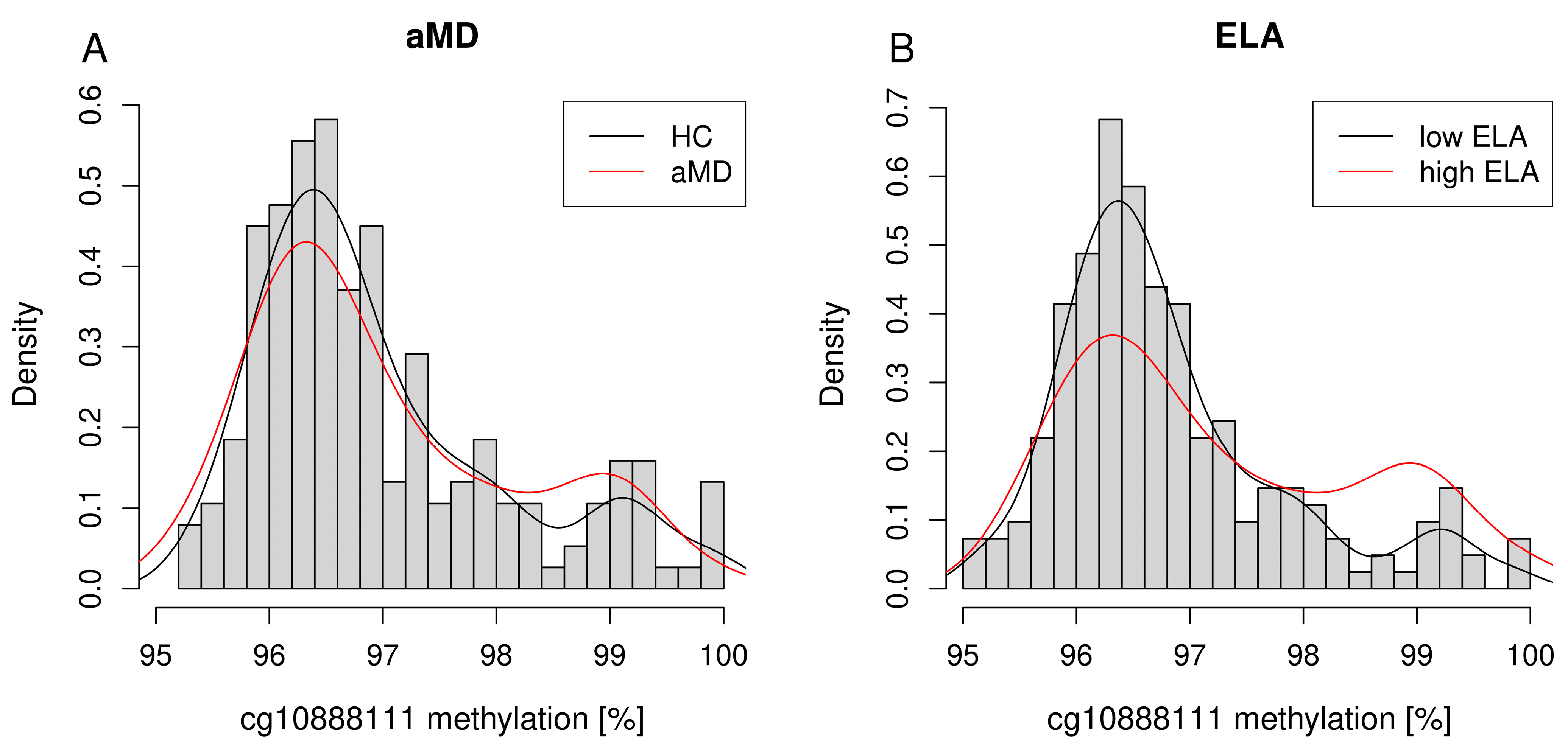
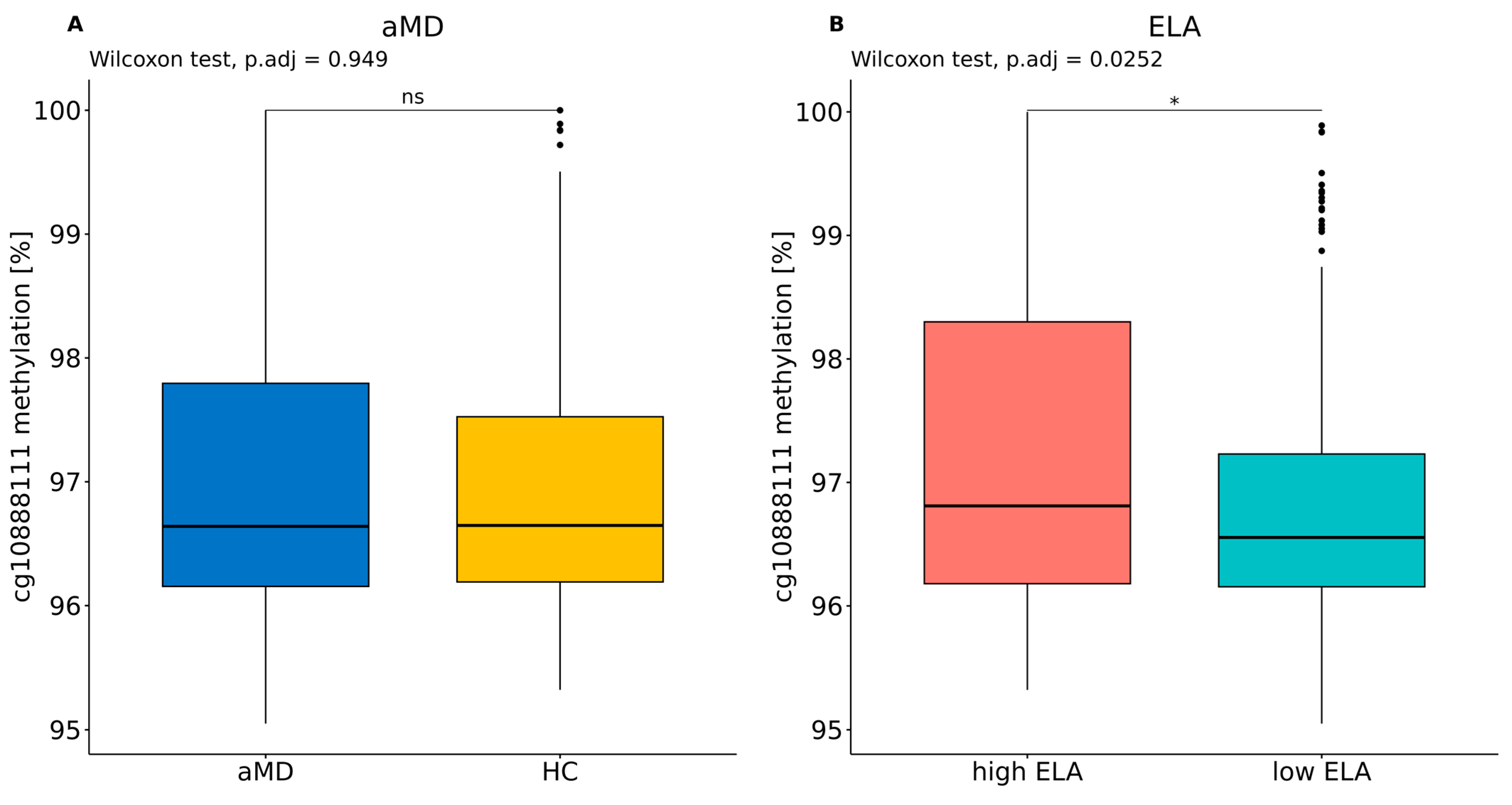
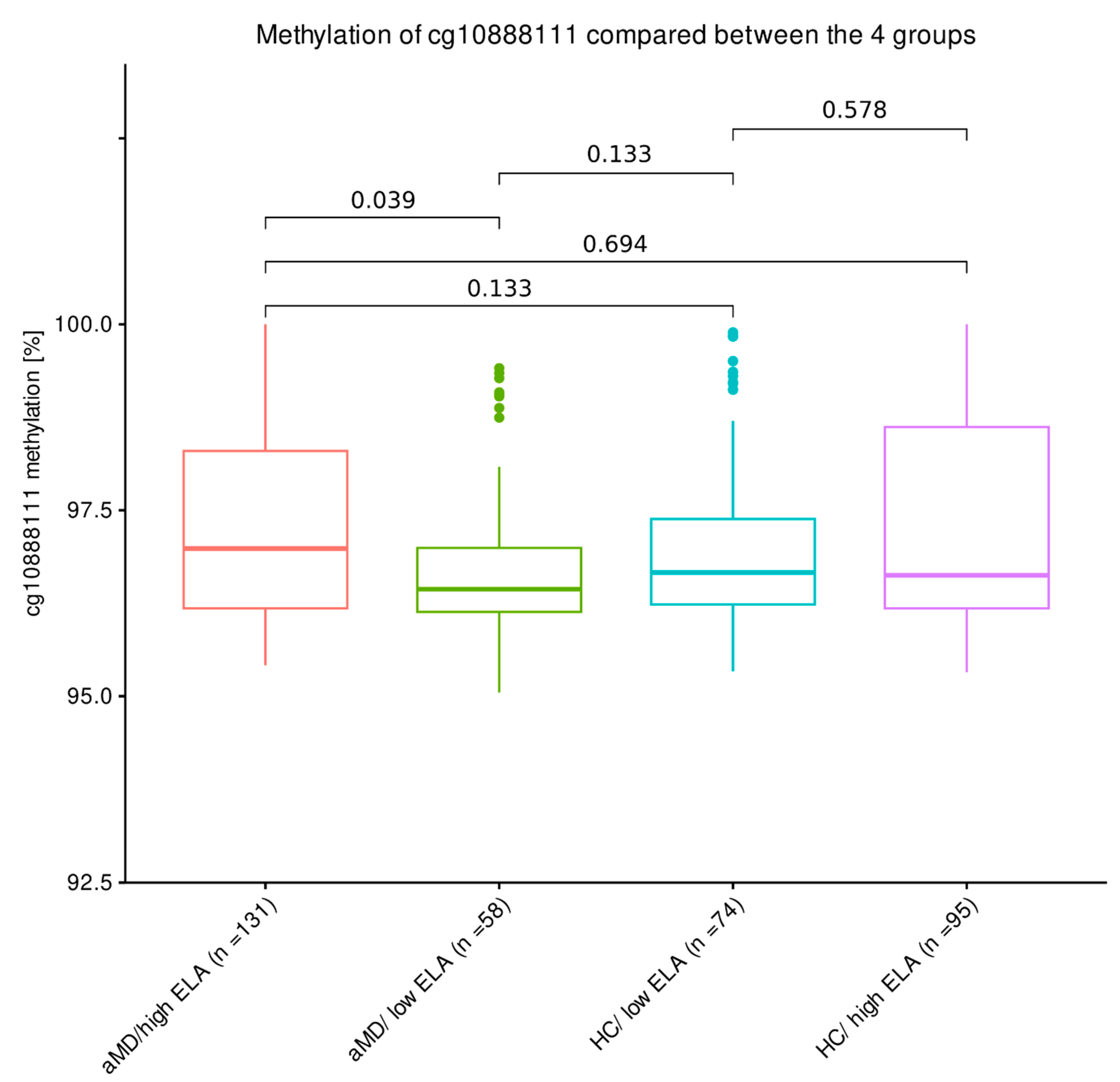
4.2. Distribution of PXDN DNA Methylation Levels between Patients with a Mental Disorder with High or Low Levels of ELA and Healthy Control Individuals with High or Low Levels of ELA
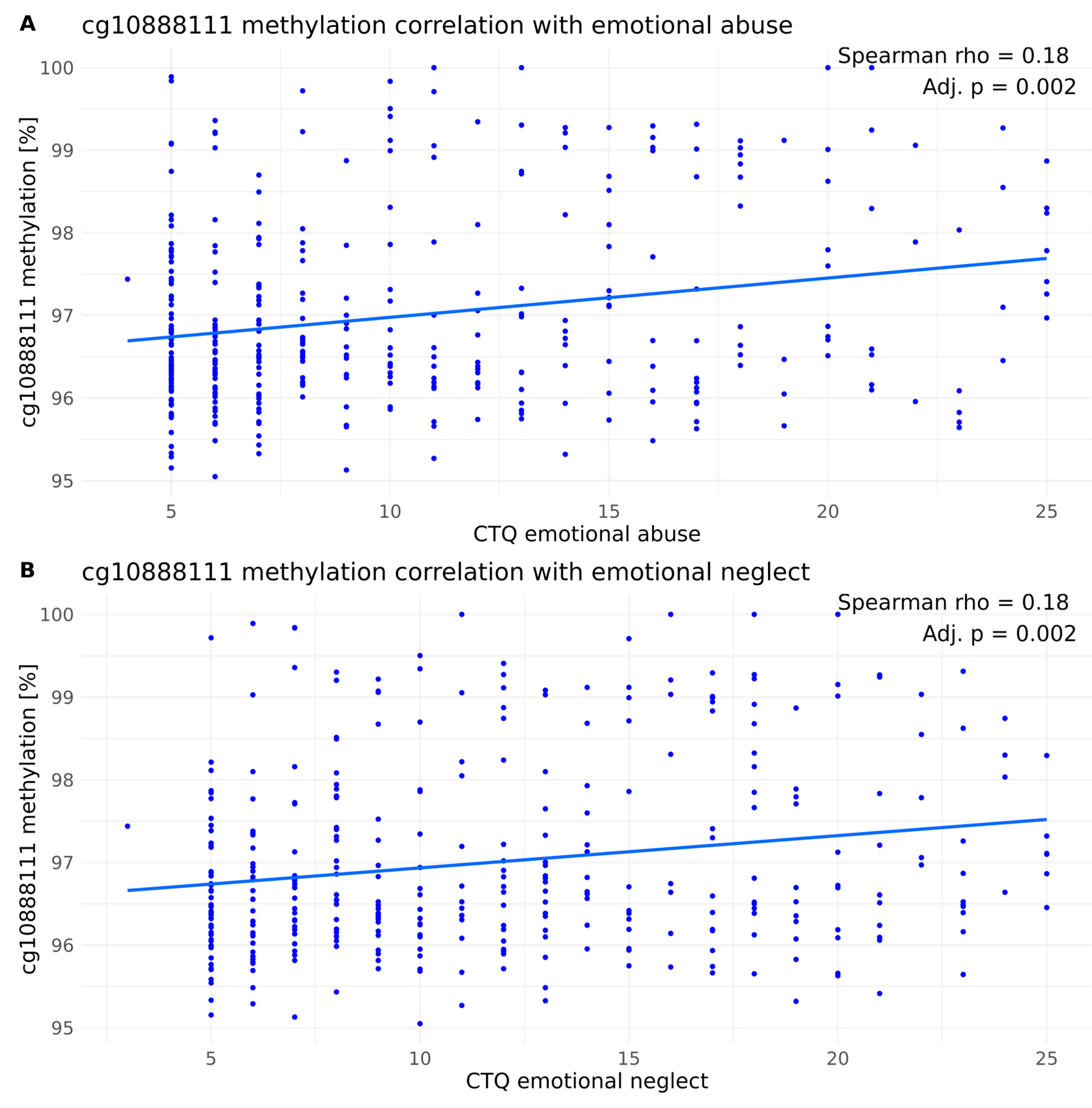
4.3. PXDN DNA Methylation Correlation with Type of ELA
4.4. PXDN DNA Methylation among the Individual Cohorts
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdolmaleky, H. Horizons of Psychiatric Genetics and Epigenetics: Where Are We and Where Are We Heading? Iran. J. Psychiatry Behav. Sci. 2014, 8, 1. [Google Scholar]
- Nestler, E.J.; Pena, C.J.; Kundakovic, M.; Mitchell, A.; Akbarian, S. Epigenetic Basis of Mental Illness. Neuroscientist 2016, 22, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell Dev. Biol. 2014, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Lin, D.; Lea, A.J.; Johnston, R.A.; Voyles, T.; Akinyi, M.Y.; Archie, E.A.; Alberts, S.C.; Tung, J. DNA methylation signatures of early-life adversity are exposure-dependent in wild baboons. Proc. Natl. Acad. Sci. USA 2024, 121, e2309469121. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.-E.; Chay, M.-A.; Pacis, A.; Chen, G.G.; Aouabed, Z.; Maffioletti, E.; Théroux, J.-F.; Grenier, J.-C.; Yang, J.; Aguirre, M.; et al. Non-CG methylation and multiple histone profiles associate child abuse with immune and small GTPase dysregulation. Nat. Commun. 2021, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, E.T.; Berardino, B.G. Epigenetic mechanisms linking early-life adversities and mental health. Biochem. J. 2024, 481, 615–642. [Google Scholar] [CrossRef] [PubMed]
- Alomari, N.A.; Bedaiwi, S.K.; Ghasib, A.M.; Kabbarah, A.J.; Alnefaie, S.A.; Hariri, N.; Altammar, M.A.; Fadhel, A.M.; Altowairqi, F.M. Social Anxiety Disorder: Associated Conditions and Therapeutic Approaches. Cureus 2022, 14, e32687. [Google Scholar] [CrossRef]
- Steffen, A.; Thom, J.; Jacobi, F.; Holstiege, J.; Batzing, J. Trends in prevalence of depression in Germany between 2009 and 2017 based on nationwide ambulatory claims data. J. Affect. Disord. 2020, 271, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, F.; Fonagy, P.; Heim, N.; Kernberg, O.; Leweke, F.; Luyten, P.; Salzer, S.; Spitzer, C.; Steinert, C. Borderline personality disorder: A comprehensive review of diagnosis and clinical presentation, etiology, treatment, and current controversies. World Psychiatry 2024, 23, 4–25. [Google Scholar] [CrossRef]
- Kos, A.; Lopez, J.; Bordes, J.; de Donno, C.; Dine, J.; Brivio, E.; Karamihalev, S.; Luecken, M.; Almeida-Correa, S.; Gasperoni, S.; et al. Early life adversity shapes social subordination and cell type–specific transcriptomic patterning in the ventral hippocampus. Sci. Adv. 2023, 9, eadj3793. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, S.; Wiegand, A.; Hentrich, T.; Pasche, S.; Schulze-Hentrich, J.M.; Munk, M.H.J.; Fallgatter, A.J.; Kreifelts, B.; Nieratschker, V. Blood transcriptome analysis suggests an indirect molecular association of early life adversities and adult social anxiety disorder by immune-related signal transduction. Front. Psychiatry 2023, 14, 1125553. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhu, Y.; Strachan, E.; Fowler, E.; Bacus, T.; Roy-Byrne, P.; Goldberg, J.; Vaccarino, V.; Zhao, J. Childhood Trauma, DNA Methylation of Stress-Related Genes, and Depression: Findings from Two Monozygotic Twin Studies. Psychosom. Med. 2018, 80, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S.; Dwivedi, Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol. Psychiatry 2023, 28, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, epigenetics and depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 102, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Nieratschker, V.; Massart, R.; Gilles, M.; Luoni, A.; Suderman, M.J.; Krumm, B.; Meier, S.; Witt, S.H.; Nothen, M.M.; Suomi, S.J.; et al. MORC1 exhibits cross-species differential methylation in association with early life stress as well as genome-wide association with MDD. Transl. Psychiatry 2014, 4, e429. [Google Scholar] [CrossRef] [PubMed]
- Teschler, S.; Bartkuhn, M.; Kunzel, N.; Schmidt, C.; Kiehl, S.; Dammann, G.; Dammann, R. Aberrant methylation of gene associated CpG sites occurs in borderline personality disorder. PLoS ONE 2013, 8, e84180. [Google Scholar] [CrossRef] [PubMed]
- Teschler, S.; Gotthardt, J.; Dammann, G.; Dammann, R.H. Aberrant DNA Methylation of rDNA and PRIMA1 in Borderline Personality Disorder. Int. J. Mol. Sci. 2016, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Arranz, M.J.; Gallego-Fabrega, C.; Martin-Blanco, A.; Soler, J.; Elices, M.; Dominguez-Clave, E.; Salazar, J.; Vega, D.; Briones-Buixassa, L.; Pascual, J.C. A genome-wide methylation study reveals X chromosome and childhood trauma methylation alterations associated with borderline personality disorder. Transl. Psychiatry 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Bonaglia, M.; Giorda, R.; Zanini, S. A new patient with a terminal de novo 2p25.3 deletion of 1.9 Mb associated with early-onset of obesity, intellectual disabilities and hyperkinetic disorder. Mol. Cytogenet. 2014, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, N.; Gundel, F.; Bruckmann, C.; Becker-Sadzio, J.; Frischholz, C.; Nieratschker, V. DNA methylation of APBA3 and MCF2 in borderline personality disorder: Potential biomarkers for response to psychotherapy. Eur. Neuropsychopharmacol. 2018, 28, 252–263. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; text rev.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Thomas, M.; Banet, N.; Wallisch, A.; Glowacz, K.; Becker-Sadzio, J.; Gundel, F.; Nieratschker, V. Differential COMT DNA methylation in patients with Borderline Personality Disorder: Genotype matters. Eur. Neuropsychopharmacol. 2019, 29, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Knoblich, N.; Wallisch, A.; Glowacz, K.; Becker-Sadzio, J.; Gundel, F.; Bruckmann, C.; Nieratschker, V. Increased BDNF methylation in saliva, but not blood, of patients with borderline personality disorder. Clin. Epigenet. 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Coope, A.; Falkenberg, C.; Dunlop, B.W.; Czamara, D.; Provencal, N.; Craighead, W.E.; Mayberg, H.S.; Nemeroff, C.B.; Binder, E.B.; et al. Investigation of MORC1 DNA methylation as biomarker of early life stress and depressive symptoms. J. Psychiatr. Res. 2020, 120, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, A.; Kreifelts, B.; Munk, M.H.J.; Geiselhart, N.; Ramadori, K.E.; MacIsaac, J.L.; Fallgatter, A.J.; Kobor, M.S.; Nieratschker, V. DNA methylation differences associated with social anxiety disorder and early life adversity. Transl. Psychiatry 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Wingenfeld, K.; Spitzer, C.; Mensebach, C.; Grabe, H.J.; Hill, A.; Gast, U.; Schlosser, N.; Höpp, H.; Beblo, T.; Driessen, M. The German version of the Childhood Trauma Questionnaire (CTQ): Preliminary psychometric properties. Psychother. Psychosom. Med. Psychol. 2010, 60, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, S.S.; Zejnelagic, A.; Farrugia, R.; Wettinger, S.B. Comparison of DNA extraction methods for samples from old blood collections. Biotechniques 2021, 70, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Lapaire, O.; Johnson, K.L.; Bianchi, D.W. Method for extraction of high-quantity and -quality cell-free DNA from amniotic fluid. Methods Mol. Biol. 2008, 444, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Magri, E.; Bianchini, E.; Montinari, E.; Corazza, M.; Virgili, A.; Tognon, M.; Martini, F. Hypermethylation-Induced Inactivation of the IRF6 Gene as a Possible Early Event in Progression of Vulvar Squamous Cell Carcinoma associated with Lichen Sclerosus. JAMA Dermatol. 2016, 152, 928–933. [Google Scholar] [CrossRef] [PubMed]
- El-Maarri, O.; Leontiou, C.A.; Hadjidaniel, M.D.; Mina, P.; Antoniou, P.; Ioannides, M.; Patsalis, P.C. Bisulfite Conversion of DNA: Performance Comparison of Different Kits and Methylation Quantitation of Epigenetic Biomarkers that Have the Potential to Be Used in Non-Invasive Prenatal Testing. PLoS ONE 2015, 10, e0135058. [Google Scholar] [CrossRef]
- Kottaridi, C.; Koureas, N.; Margari, N.; Terzakis, E.; Bilirakis, E.; Pappas, A.; Chrelias, C.; Spathis, A.; Aga, E.; Pouliakis, A.; et al. A Pyrosequencing Assay for the Quantitative Methylation Analysis of GALR1 in Endometrial Samples: Preliminary Results. Biomed. Res. Int. 2015, 2015, 756359. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Amoroso, M.G.; Gesualdi Montesano, N.; Viscardi, M. Development of a pyrosequencing assay for the typing of alphaherpesviruses. MethodsX 2015, 2, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M.L.; Fleckhaus, J.; Borsting, C.; Jurtikova, H.; Piters, A.; Papin, J.; Gauthier, Q.; Ghemrawi, M.; Doutremepuich, C.; McCord, B.; et al. Collaborative exercise: Analysis of age estimation using a QIAGEN protocol and the PyroMark Q48 platform. Forensic Sci. Res. 2024, 9, owad055. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots, R package version 0.6.0. 2023. Available online: https://github.com/kassambara/ggpubr (accessed on 29 June 2024).
- Pauli Virtanen, R.G.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; van der Walt, S.J. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Koenker, R.; Portnoy, S.; Ng, P.T.; Zeileis, A.; Grosjean, P.; Ripley, B.D. Package ‘Quantreg’. Reference Manual. R-CRAN. Available online: https://cran.r-project.org/web/packages/quantreg/quantreg.pdf (accessed on 1 June 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- de Aquino Ferreira, L.F.; Queiroz Pereira, F.H.; Neri Benevides, A.M.L.; Aguiar Melo, M.C. Borderline personality disorder and sexual abuse: A systematic review. Psychiatry Res. 2018, 262, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Danielsdottir, H.B.; Aspelund, T.; Shen, Q.; Halldorsdottir, T.; Jakobsdottir, J.; Song, H.; Lu, D.; Kuja-Halkola, R.; Larsson, H.; Fall, K.; et al. Adverse Childhood Experiences and Adult Mental Health Outcomes. JAMA Psychiatry 2024, 81, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Akman, K.; Calimport, S.R.; Wuttke, D.; Stolzing, A.; de Magalhaes, J.P. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. 2012, 15, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Zisook, S.; Planeta, B.; Hicks, P.B.; Chen, P.; Davis, L.L.; Villarreal, G.; Sapra, M.; Johnson, G.R.; Mohamed, S. Childhood adversity and adulthood major depressive disorder. Gen. Hosp. Psychiatry 2022, 76, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, L.; Petrelli, C.; Serretti, A. The role of specific early trauma in adult depression: A meta-analysis of published literature. Childhood trauma and adult depression. Eur. Psychiatry 2015, 30, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Hagborg, J.M.; Kalin, T.; Gerdner, A. The Childhood Trauma Questionnaire-Short Form (CTQ-SF) used with adolescents—Methodological report from clinical and community samples. J. Child. Adolesc. Trauma 2022, 15, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Hosang, G.M.; Manoli, A.; Shakoor, S.; Fisher, H.L.; Parker, C. Reliability and convergent validity of retrospective reports of childhood maltreatment by individuals with bipolar disorder. Psychiatry Res. 2023, 321, 115105. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, S.C.; Yang, H.; Oh, D.H. Reliability and validity of the korean version of the childhood trauma questionnaire-short form for psychiatric outpatients. Psychiatry Investig. 2011, 8, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, M.; Cai, J.; Yuan, Y.; He, L.; Liu, J.; Wang, L.; Wang, W. Comparison of ACE-IQ and CTQ-SF for child maltreatment assessment: Reliability, prevalence, and risk prediction. Child Abus. Negl. 2023, 146, 106529. [Google Scholar] [CrossRef] [PubMed]
- Glaesmer, H. Assessing childhood maltreatment on the population level in Germany: Findings and methodological challenges. Child Adolesc. Psychiatry Ment. Health 2016, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Andreassen, O.A.; Lorentzen, S.; Melle, I.; Aas, M. Childhood Trauma and Minimization/Denial in People with and without a Severe Mental Disorder. Front. Psychol. 2017, 8, 1276. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Milot, T.; Rousseau, M.; Lemieux, R.; Garon-Bissonnette, J.; Berthelot, N. Beyond abuse and neglect: Validation of the childhood interpersonal trauma inventory in a community sample of adults. Front. Psychiatry 2024, 15, 1358475. [Google Scholar] [CrossRef] [PubMed]
- Seitz, K.I.; Gerhardt, S.; von Schroeder, C.; Panizza, A.; Thekkumthala, D.; Bertsch, K.; Herpertz, S.C.; Schmahl, C.; Schalinski, I. Measuring types and timing of childhood maltreatment: The psychometric properties of the KERF-40. PLoS ONE 2022, 17, e0273931. [Google Scholar] [CrossRef] [PubMed]
- Galione, J.N.; Oltmanns, T.F. The relationship between borderline personality disorder and major depression in later life: Acute versus temperamental symptoms. Am. J. Geriatr. Psychiatry 2013, 21, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Beatson, J.A.; Rao, S. Depression and borderline personality disorder. Med. J. Aust. 2012, 1, 24–27. [Google Scholar] [CrossRef]
- Perroud, N.; Paoloni-Giacobino, A.; Prada, P.; Olie, E.; Salzmann, A.; Nicastro, R.; Guillaume, S.; Mouthon, D.; Stouder, C.; Dieben, K.; et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: A link with the severity and type of trauma. Transl. Psychiatry 2011, 1, e59. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Salzmann, A.; Prada, P.; Nicastro, R.; Hoeppli, M.E.; Furrer, S.; Ardu, S.; Krejci, I.; Karege, F.; Malafosse, A. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl. Psychiatry 2013, 3, e207. [Google Scholar] [CrossRef] [PubMed]
- Zwolinska, W.; Bilska, K.; Tarhonska, K.; Reszka, E.; Skibinska, M.; Pytlinska, N.; Slopien, A.; Dmitrzak-Weglarz, M. Biomarkers of Depression among Adolescent Girls: BDNF and Epigenetics. Int. J. Mol. Sci. 2024, 25, 3281. [Google Scholar] [CrossRef]
- Carlberg, L.; Scheibelreiter, J.; Hassler, M.R.; Schloegelhofer, M.; Schmoeger, M.; Ludwig, B.; Kasper, S.; Aschauer, H.; Egger, G.; Schosser, A. Brain-derived neurotrophic factor (BDNF)-epigenetic regulation in unipolar and bipolar affective disorder. J. Affect. Disord. 2014, 168, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Januar, V.; Ancelin, M.L.; Ritchie, K.; Saffery, R.; Ryan, J. BDNF promoter methylation and genetic variation in late-life depression. Transl. Psychiatry 2015, 5, e619. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, K.; Panagopoulos, V.; Cox, T.R. The role of peroxidasin in solid cancer progression. Biochem. Soc. Trans. 2023, 51, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Dougan, J.; Hawsawi, O.; Burton, L.J.; Edwards, G.; Jones, K.; Zou, J.; Nagappan, P.; Wang, G.; Zhang, Q.; Danaher, A.; et al. Proteomics-Metabolomics Combined Approach Identifies Peroxidasin as a Protector against Metabolic and Oxidative Stress in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 3046. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Liang, L. High expression of PXDN is associated with poor prognosis and promotes proliferation, invasion as well as migration in ovarian cancer. Ann. Diagn. Pathol. 2018, 34, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Paumann-Page, M.; Kienzl, N.F.; Motwani, J.; Bathish, B.; Paton, L.N.; Magon, N.J.; Sevcnikar, B.; Furtmuller, P.G.; Traxlmayr, M.W.; Obinger, C.; et al. Peroxidasin protein expression and enzymatic activity in metastatic melanoma cell lines are associated with invasive potential. Redox Biol. 2021, 46, 102090. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.J.; Axelsen, M.S.; Sheffield, V.C.; Patil, S.R.; Wassink, T.H. Germline mosaic transmission of a novel duplication of PXDN and MYT1L to two male half-siblings with autism. Psychiatr. Genet. 2012, 22, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Dahrendorff, J.; Wani, A.; Keller, T.E.; Armstrong, D.; Qu, A.; Wildmann, D.E.; Valero, M.C.; Kroenen, K.C.; Aiello, A.E.; Uddin, M. Analysis of Post-traumatic Stress Disorder Gene Expression Profiles in a Prospective, Community-based Cohort. medRxiv 2023. [Google Scholar] [CrossRef]
- Cheng, G.; Shi, R. Mammalian peroxidasin (PXDN): From physiology to pathology. Free Radic. Biol. Med. 2022, 182, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Jjingo, D.; Conley, A.; Yi, S.; Lunyak, V.; Jordan, I. On the presence and role of human gene-body DNA methylation. Oncotarget 2012, 3, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.; Walsh, C.; Bestor, T. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997, 13, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Rideout, W.; Coetzee, G.; Olumi, A.; Jones, P. 5-Methylcytosine as an Endogenous Mutagen in the Human LDL Receptor and p53 Genes. Science 1990, 249, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Laurent, L.; Wong, E.; Li, G.; Huynh, T.; Tsirigos, A.; Ong, C.T.; Low, H.M.; Kin Sung, K.W.; Rigoutsos, I.; Loring, J.; et al. Dynamic changes in the human methylome during differentiation. Genome Res. 2010, 20, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Shayevitch, R.; Askayo, D.; Keydar, I.; Ast, G. The importance of DNA methylation of exons on alternative splicing. RNA 2018, 24, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Maunakea, A.K.; Chepelev, I.; Cui, K.; Zhao, K. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013, 23, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Q.; Bakthavachalu, B.; Han, J.; Patil, D.P.; Otsuka, Y.; Guda, C.; Schoenberg, D.R. Identification of the human PMR1 mRNA endonuclease as an alternatively processed product of the gene for peroxidasin-like protein. RNA 2012, 18, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Huang, S.; He, X. Genome-wide analysis reveals that exon methylation facilitates its selective usage in the human transcriptome. Brief. Bioinform. 2018, 19, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009, 37, D412–D416. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Puetz, V.B.; Viding, E.; Gerin, M.I.; Pingault, J.B.; Sethi, A.; Knodt, A.R.; Radtke, S.R.; Brigidi, B.D.; Hariri, A.R.; McCrory, E. Investigating patterns of neural response associated with childhood abuse v. childhood neglect. Psychol. Med. 2020, 50, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, A.; Sheu, Y.S.; Rabi, K.; Suzuki, H.; Navalta, C.P.; Polcari, A.; Teicher, M.H. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 2011, 54 (Suppl. S1), S280–S286. [Google Scholar] [CrossRef] [PubMed]
- Infurna, M.R.; Reichl, C.; Parzer, P.; Schimmenti, A.; Bifulco, A.; Kaess, M. Associations between depression and specific childhood experiences of abuse and neglect: A meta-analysis. J. Affect. Disord. 2016, 190, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lochner, C.; Seedat, S.; Allgulander, C.; Kidd, M.; Stein, D.; Gerdner, A. Childhood trauma in adults with social anxiety disorder and panic disorder: A cross-national study. Afr. J. Psychiatry 2010, 13, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.R.; Goldin, P.R.; Werner, K.; Heimberg, R.G.; Gross, J.J. Childhood trauma and current psychological functioning in adults with social anxiety disorder. J. Anxiety Disord. 2011, 25, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, K.R.; Cole, S.W.; Craske, M.G.; Fuligni, A.J.; Irwin, M.R.; Bower, J.E. Enhanced Immune Activation Following Acute Social Stress Among Adolescents with Early-Life Adversity. Biol. Psychiatry Glob. Open Sci. 2023, 3, 213–221. [Google Scholar] [CrossRef]
- Kuhlman, K.R.; Cole, S.W.; Irwin, M.R.; Craske, M.G.; Fuligni, A.J.; Bower, J.E. The role of early life adversity and inflammation in stress-induced change in reward and risk processes among adolescents. Brain Behav. Immun. 2023, 109, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, K.R.; Horn, S.R.; Chiang, J.J.; Bower, J.E. Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 86, 30–42. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, A.T.D.; Matsuzaka, C.T.; Neto, J.B.B.; Mello, M.F.; Juruena, M.F.; Mello, A.F. Childhood Sexual Abuse and Indicators of Immune Activity: A Systematic Review. Front. Psychiatry 2018, 9, 354. [Google Scholar] [CrossRef] [PubMed]
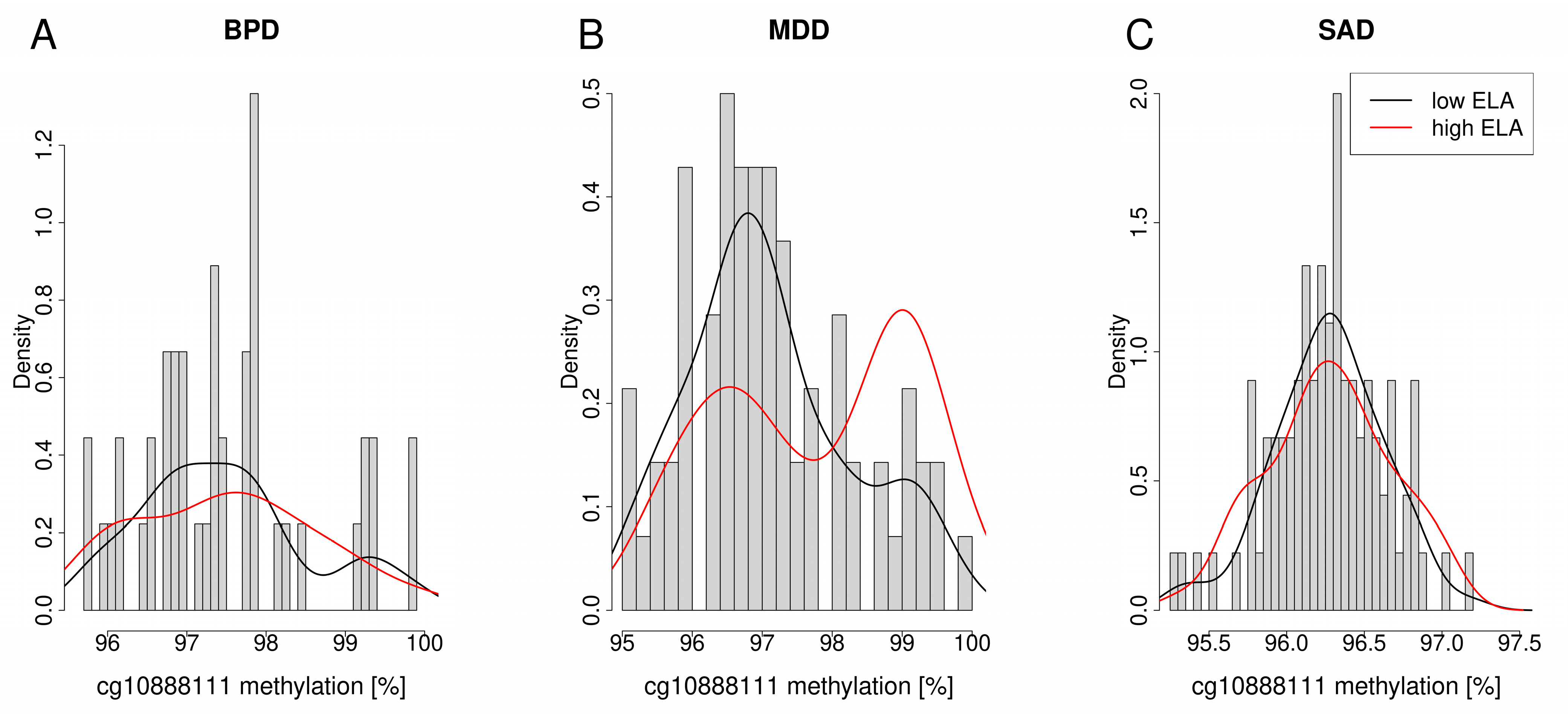

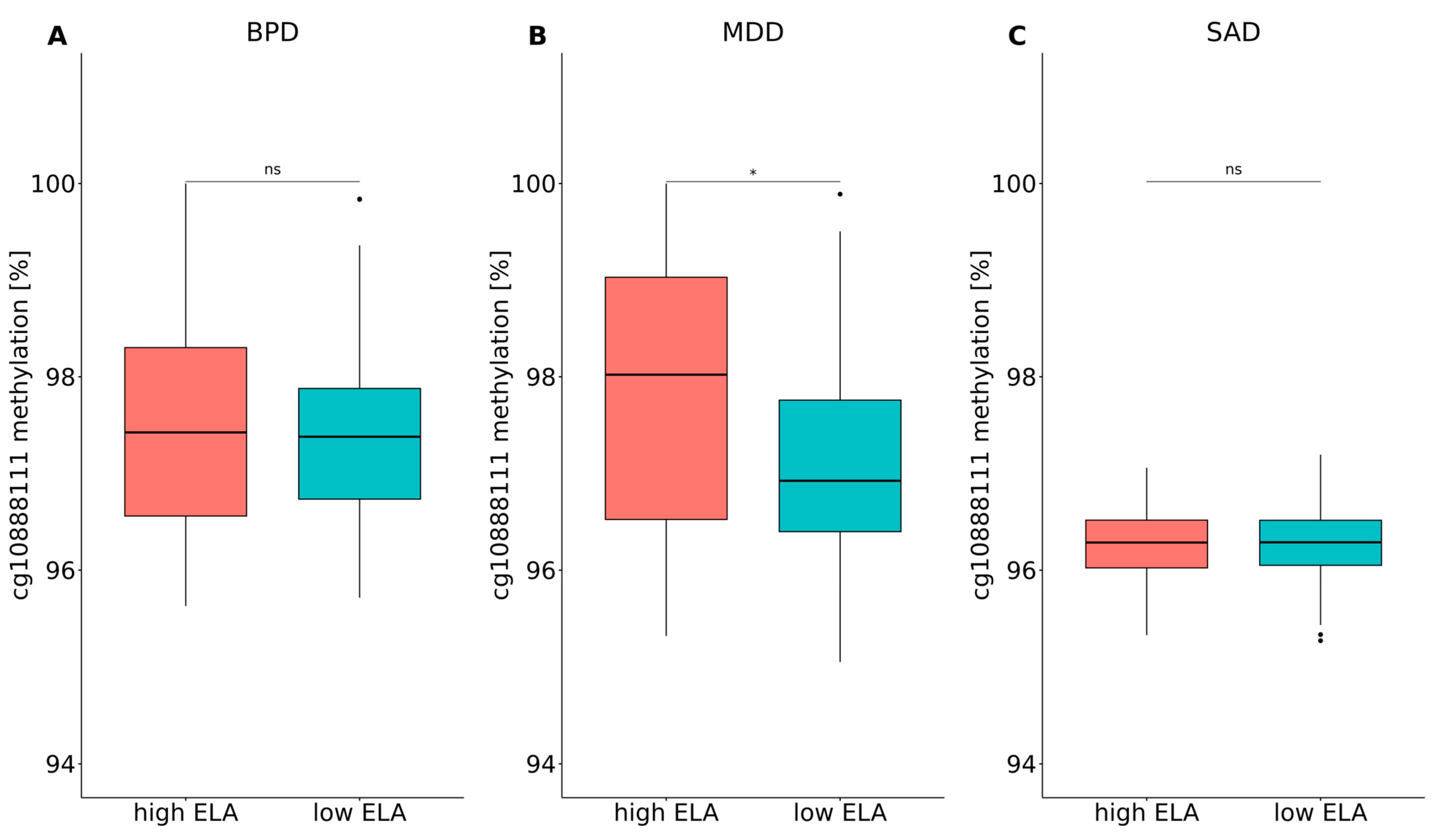
| BPD (n = 93) | MDD (n = 128) | SAD (n = 137) | ||||
|---|---|---|---|---|---|---|
| Patients | HC | Patients | HC | Patients | HC | |
| n | 40 | 53 | 64 | 64 | 65 | 72 |
| Mental Disorder (BPD, MDD and SAD) | Healthy Control Individuals | |||
|---|---|---|---|---|
| High ELA | Low ELA | High ELA | Low ELA | |
| n | 95 | 73 | 58 | 131 |
| CTQ total score | 61.7 ± 18.4 | 32.8 ± 5.1 | 50.9 ± 12.3 | 33.9 ± 4.8 |
| Sex | ♀ 55, ♂ 40 | ♀ 49, ♂ 25 | ♀ 24, ♂ 34 | ♀ 83, ♂ 48 |
| Age [y] | 33 ± 11 | 30 ± 12 | 30 ± 10 | 28 ± 9 |
| BPD Patients | Healthy Control Individuals | |||
|---|---|---|---|---|
| High ELA | Low ELA | High ELA | Low ELA | |
| n | 38 | 2 | 10 | 43 |
| CTQ total score | 66.7 ± 22.4 | NA | 45.7 ± 7.3 | 30.7 ± 4.6 |
| Sex | ♀ 32, ♂ 6 | ♀ 1, ♂ 1 | ♀ 6, ♂ 4 | ♀ 40, ♂ 3 |
| Age [y] | 32 ± 9 | 24 ± 3 | 34 ± 9 | 27 ± 9 |
| MDD Patients | Healthy Control Individuals | |||
|---|---|---|---|---|
| High ELA | Low ELA | High ELA | Low ELA | |
| n | 32 | 32 | 26 | 38 |
| CTQ total score | 59.0 ± 15.5 | 32.9 ± 5.3 | 54.1 ± 8.7 | 31.1 ± 5.6 |
| Sex | ♀ 7, ♂ 25 | ♀ 19, ♂ 13 | ♀ 4, ♂ 22 | ♀ 13, ♂ 25 |
| Age [y] | 39 ± 14 | 38 ± 14 | 32 ± 11 | 30 ± 13 |
| Individuals Suffering from SAD | Healthy Control Individuals | |||
|---|---|---|---|---|
| High ELA | Low ELA | High ELA | Low ELA | |
| n | 25 | 40 | 22 | 50 |
| CTQ total score | 57.5 ± 14.0 | 32.5 ± 5.0 | 49.6 ± 10.5 | 29.7 ±4.7 |
| Sex | ♀ 16, ♂ 9 | ♀ 29, ♂ 11 | ♀ 14, ♂ 8 | ♀ 30, ♂ 20 |
| Age [y] | 29 ± 9 | 24 ± 5 | 28 ± 9 | 25 ± 4 |
| Cg10888111 Methylation | BPD | MDD | SAD |
|---|---|---|---|
| Mean [%] ± SD | 97.5 ± 1.1 | 97.4 ± 1.3 | 96.3 ± 0.4 |
| Median [%] ± IQR | 97.4 ± 1.5 | 97.2 ± 2.3 | 96.3 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelmann, S.; Balaji, J.; Pasche, S.; Wiegand, A.; Nieratschker, V. DNA Methylation of PXDN Is Associated with Early-Life Adversity in Adult Mental Disorders. Biomolecules 2024, 14, 976. https://doi.org/10.3390/biom14080976
Edelmann S, Balaji J, Pasche S, Wiegand A, Nieratschker V. DNA Methylation of PXDN Is Associated with Early-Life Adversity in Adult Mental Disorders. Biomolecules. 2024; 14(8):976. https://doi.org/10.3390/biom14080976
Chicago/Turabian StyleEdelmann, Susanne, Jeysri Balaji, Sarah Pasche, Ariane Wiegand, and Vanessa Nieratschker. 2024. "DNA Methylation of PXDN Is Associated with Early-Life Adversity in Adult Mental Disorders" Biomolecules 14, no. 8: 976. https://doi.org/10.3390/biom14080976
APA StyleEdelmann, S., Balaji, J., Pasche, S., Wiegand, A., & Nieratschker, V. (2024). DNA Methylation of PXDN Is Associated with Early-Life Adversity in Adult Mental Disorders. Biomolecules, 14(8), 976. https://doi.org/10.3390/biom14080976






