The Executive Functioning Paradox in Substance Use Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
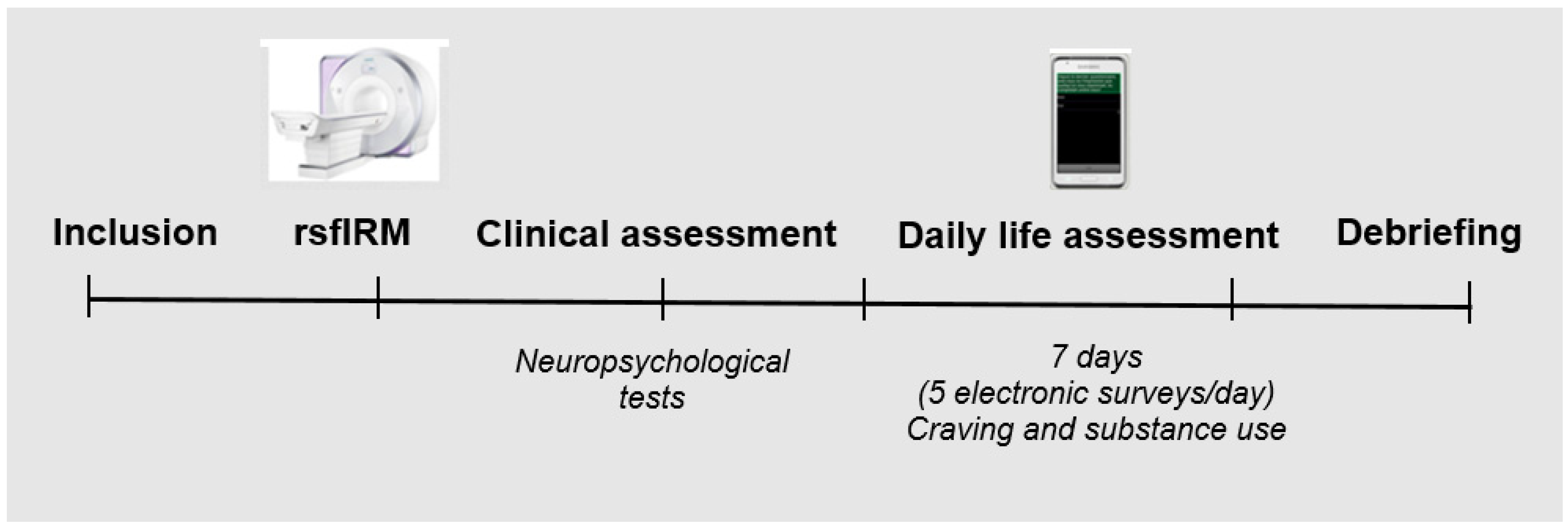
2.2. Procedure
2.3. Clinical Measures
2.3.1. Addiction and Psychiatric Data
2.3.2. Neuropsychological Assessments
2.4. Ecological Momentary Assessment
2.5. Acquisition of Brain Imaging Data
2.5.1. Data Preprocessing
2.5.2. Definition of Functional Connectivity at Two Levels of Topological Organization
2.6. Statistical Analysis
3. Results
3.1. Sample Description
3.2. Associations among Craving, Substance Use and Executive Functioning
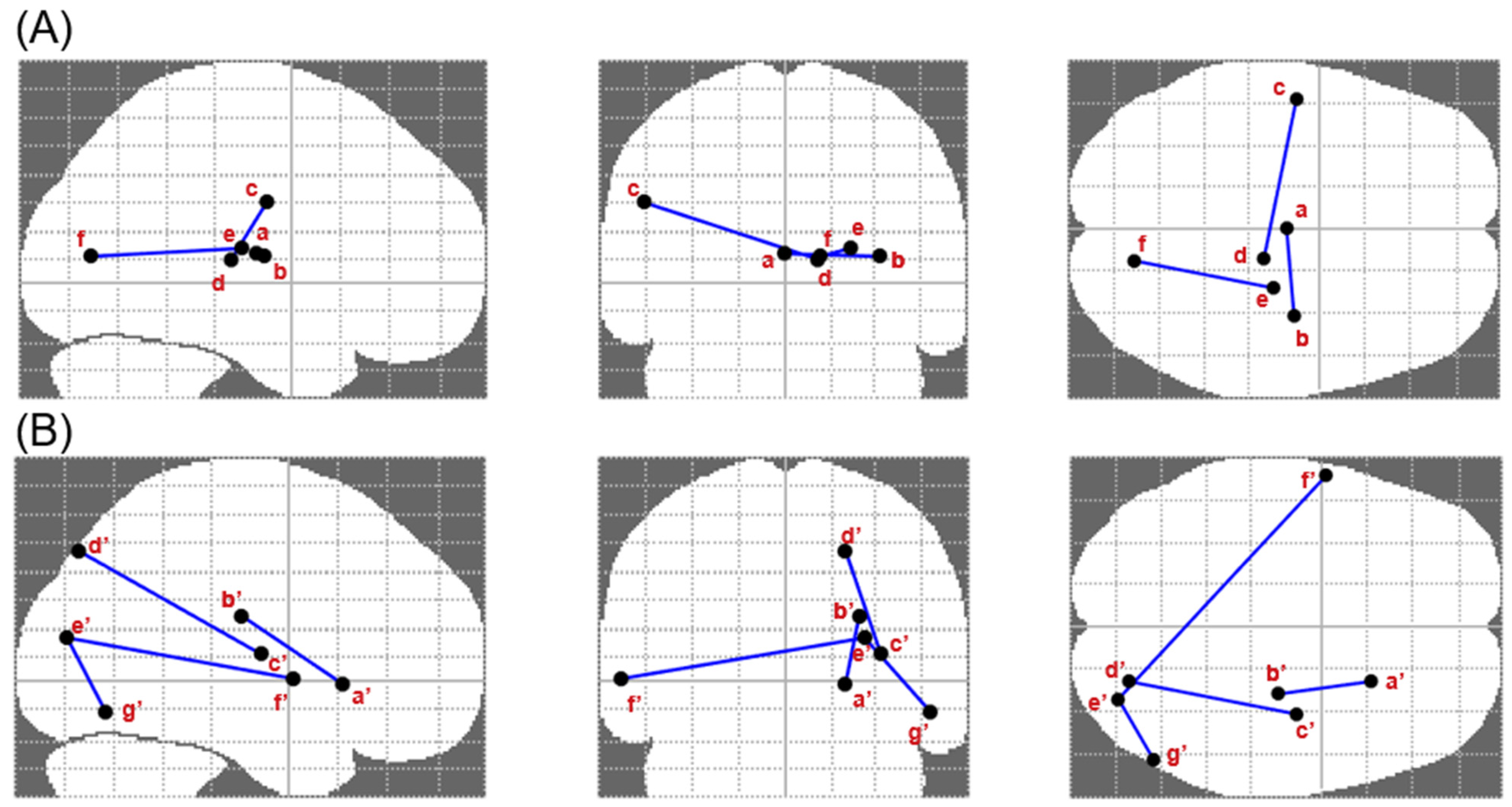
3.3. Altered Brain Connectivity Associated with Executive Performance, Craving and Substance Use
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinha, R. Modeling relapse situations in the human laboratory. Curr. Top. Behav. Neurosci. 2013, 13, 379–402. [Google Scholar]
- Fatseas, M.; Serre, F.; Alexandre, J.M.; Debradant, R.; Auriacombe, M.; Swendsen, J. Craving and substance use among patients with alcohol, tobacco, cannabis or heroin addiction: A comparison of substance- and person-specific cues. Addiction 2015, 110, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Serre, F.; Fatseas, M.; Swendsen, J.; Auriacombe, M. Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug Alcohol Depend. 2015, 148, 1–20. [Google Scholar] [CrossRef]
- Tiffany, S.T.; Wray, J.M. The clinical significance of drug craving. Ann. N. Y. Acad. Sci. 2012, 1248, 1–17. [Google Scholar] [CrossRef]
- Fatseas, M.; Serre, F.; Swendsen, J.; Auriacombe, M. Effects of anxiety and mood disorders on craving and substance use among patients with substance use disorder: An ecological momentary assessment study. Drug Alcohol Depend. 2018, 187, 242–248. [Google Scholar] [CrossRef]
- Dominguez-Salas, S.; Diaz-Batanero, C.; Lozano-Rojas, O.M.; Verdejo-Garcia, A. Impact of general cognition and executive funciton deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neurosci. Biobehav. Rev. 2016, 71, 772–801. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Ann. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [Green Version]
- Sofuoglu, M.; DeVito, E.E.; Waters, A.J.; Carroll, K.M. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 2013, 64, 452–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdejo-Garcia, A.; Garcia-Fernandez, G.; Geert, D. Cognition and addiction. Dialogues Clin. Neurosci. 2019, 21, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.T.; Martin, L.E.; Bruce, J.; Moreno, J.L.; Staggs, V.S.; Lee, H.S.; Goggin, K.; Harris, K.J.; Richter, K.; Patten, C.; et al. Executive function fails to predict smoking outcomes in a clinical trial to motivate smokers to quit. Drug Alcohol Depend. 2017, 175, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Tomasi, D. Addiction circuitry in the human brain. Ann. Rev. Pharmacol. Toxicol. 2012, 52, 321–336. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Cox, W.M. Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug Alcohol Depend. 2008, 97, 1–20. [Google Scholar] [CrossRef]
- Field, M.; Mogg, K.; Mann, B.; Bennett, G.A.; Bradley, B.P. Attentional biases in abstinent alcoholics and their association with craving. Psychol. Addict. Behav. 2013, 27, 71–80. [Google Scholar] [CrossRef]
- Flaudias, V.; Picot, M.C.; Lopez-Castroman, J.; Llorca, P.-M.; Schmitt, A.; Perriot, J.; Georgescu, V.; Courtet, P.; Quantin, X.; Guillaume, S. Executive Functions in Tobacco Dependence: Importance of Inhibitory Capacities. PLoS ONE 2016, 11, e0150940. [Google Scholar] [CrossRef]
- Zhai, T.; Salmeron, B.J.; Gu, H.; Adinoff, B.; Stein, E.A.; Yang, Y. Functional connectivity of dorsolateral prefrontal cortex predicts cocaine relapse: Implications for neuromodulation treatment. Brain Commun. 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Wilcox, C.E.; Abbott, C.C.; Calhoun, V.D. Alterations in resting-state funcitonal connectivity in substance use disorders and treatment implications. Prog. Neuro-Psychopharmacol. Biol. 2019, 91, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, S.; Yu, R. Brain network dysfunctions in addiction: A meta-analysis of resting- state functional connectivity. Transl. Psychiatry 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pariyadath, V.; Gowin, J.; Elliot, A.; Stein, E. Chapter 8—Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 224, pp. 155–173. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- Denis, C.; Fatseas, M.; Beltran, V.; Serre, F.; Alexandre, J.-M.; Debrabant, R.; Daulouède, J.-P.; Auriacombe, M. Usefulness and validity of the modified Addiction Severity Index: A focus on alcohol, drugs, tobacco, and gambling. Subst. Abus. 2016, 37, 168–175. [Google Scholar] [CrossRef]
- MacLeod, C.M. Half a century of research on the Stroop effect: An integrative review. Psychol. Bull. 1991, 109, 163–203. [Google Scholar] [CrossRef]
- Chafetz, M.D.; Matthews, L.H. A new interference score for the Stroop test. Arch. Clin. Neuropsychol. 2004, 19, 555–567. [Google Scholar] [CrossRef]
- Reitan, R.M.; Wolfson, D. The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Arch. Clin. Neuropsychol. 2004, 19, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Borkowski, J.G.; Benton, A.L.; Spreen, O. Word fluency and brain damage. Neuropsychologia 1967, 5, 135–140. [Google Scholar] [CrossRef]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. fMRIPrep: A robust preprocessing pipeline for funcitonal MRI. Nat. Methods 2018, 16, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Esteban, O.; Blair, R.W.; Nielson, D.M.; Varada, J.C.; Marrett, S.; Thomas, A.G.; Poldrack, R.A.; Gorgolewski, K.J. Crowdsourced MRI quality metrics and expert quality annotations for training of humans and machines. Sci. Data 2019, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Gorgolewski, K.J.; Burns, C.D.; Madison, C.; Clark, D.; Halchenko, Y.O.; Waskom, M.L.; Ghosh, S.S. Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 2011, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Gorgolewski, K.J.; Nichols, T.; Kennedy, D.N.; Poline, J.-B.; Poldrack, R.A.; Zwaan, R.A.; Etz, A.; Lucas, R.E.; Donnellan, M.B.; Howe, P.D.L.; et al. Making replication prestigious. Behav. Brain. Sci. 2018, 41, e131. [Google Scholar] [CrossRef]
- Dadi, K.; Varoquaux, G.; Machlouzarides-Shalit, A.; Gorgolewski, K.J.; Wassermann, D.; Thirion, B.; Mensch, A. Fine-grain atlases of functional modes for fMRI analysis. NeuroImage 2020, 221, 117–126. [Google Scholar] [CrossRef]
- Murphy, K.; Fox, M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage 2017, 154, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J. The organization of the human cerebral cortex estimated by intrinsic funcitonal connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Raudenbush, S.W.; Congdon, R.T. HLM 8: Hierarchical Linear and Nonlinear Modeling; Scientific Software International, Inc.: Chapel Hill, NC, USA, 2021. [Google Scholar]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. NeuroImage 2010, 53, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-García, A.; Betanzos-Espinosa, P.; Lozano, O.; Vergara-Moragues, E.; González-Saiz, F.; Fernández-Calderón, F.; Bilbao-Acedos, I.; Pérez-García, M. Self-regulation and treatment retention in cocaine dependent individuals: A longitudinal study. Drug Alcohol Depend. 2012, 122, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Nuijten, M.; Blanken, P.; Van den Brink, W.; Goudriaan, A.E.; Hendriks, V.M. Impulsivity and attentional bias as predictors of modafinil treatment outcome for retention and drug use in crack-cocaine dependent patients: Results of a randomised controlled trial. J. Psychopharmacol. 2016, 30, 616–626. [Google Scholar] [CrossRef]
- Carpenter, K.M.; Schreiber, E.; Church, S.; McDowell, D. Drug Stroop performance: Relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict. Behav. 2006, 31, 174–181. [Google Scholar] [CrossRef]
- Brewer, J.A.; Worhunsky, P.D.; Carroll, K.; Rounsaville, B.J.; Potenza, M.N. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol. Psychiatry 2008, 64, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, D.J.; Andrade, J.; May, J. Imaginary relish and exquisite torture: The Elaborated Intrusion Theory of desire. Psychol. Rev. 2005, 112, 446–467. [Google Scholar] [CrossRef] [Green Version]
- Caselli, G.; Spada, M.M. Desire thinking: What is it and what drives it? Addict. Behav. 2015, 44, 71–79. [Google Scholar] [CrossRef]
- Adinoff, B.; Carmody, T.J.; Walker, R.; Donovan, D.M.; Brigham, G.S.; Winhusen, T.M. Decision-making processes as predictors of relapse and subsequent use in stimulant-dependent patients. Am. J. Drug Alcohol Abus. 2016, 42, 88–97. [Google Scholar] [CrossRef]
| Healthy Controls (N = 40) | Any Addiction (N = 86) | Alcohol (N = 36) | Nicotine (N = 34) | Cannabis (N = 16) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD % | M | SD % | M | SD % | M | SD % | M | SD % | |
| Age | 33.62 | 8.27 | 39.60 | 11.65 ** | 43.67 | 10.94 B | 38.82 | 11.84 | 32.13 | 8.99 |
| Sex (% female) | 50 | 43 | 36 A | 62 | 19 | |||||
| Education (years) | 14.45 | 3.00 | 13.05 | 2.54 * | 13.25 | 2.25 | 113.21 | 2.91 | 12.25 | 2.32 |
| Addiction severity | ||||||||||
| ISR | 6.13 | 1.13 | 6.5 | 0.66 A | 5.65 | 1.37 | 6.31 | 1.08 | ||
| Current comorbidity (%) | ||||||||||
| Mood disorder | - | 16 | 25 A | 6 | 19 | |||||
| Anxiety disorder | - | 26 | 19 B | 18 C | 56 | |||||
| Psychotic disorder | - | 20 | 8 A | 32 | 19 | |||||
| Any current | - | 29 | 39 | 44 | 63 | |||||
| Neuropsychological tests | ||||||||||
| Stroop interference | 15.50 | 15.19 | 6.77 | 21.33 *** | 7.78 | 30.29 | 6.70 | 12.57 | 4.68 | 9.02 |
| TMT BA time | 24.01 | 18.16 | 40.23 | 48.00 *** | 38.23 | 34.97 | 46.70 | 66.91 | 31.00 | 12.63 |
| IGT net score | 12.35 | 28.72 | 10.22 | 25.08 | 11.91 | 27.30 | 5.24 | 21.82 | 17.20 | 25.87 |
| Verbal/phonemic fluency | 23.28 | 5.68 | 23.20 | 6.99 | 23.50 | 6.38 | 23.13 | 7.66 | 22.73 | 7.21 |
| EMA | ||||||||||
| Compliance | 32.87 | 1.92 | 29.87 | 3.64 *** | 30.61 | 2.96 | 29.88 | 3.22 | 28.19 | 5.26 |
| Craving intensity | 1.03 | 0.09 | 2.76 | 1.18 | 2.46 | 0.94 B | 2.73 | 1.22 | 3.49 | 1.34 |
| Use of treated substance | - | - | 15.83 | 10.24 | 10.36 | 8.34 A | 22.56 | 8.89 C | 13.81 | 8.89 |
| Use of any substance | 1.90 | 2.35 | 23.01 | 8.66 | 23.50 | 8.98 A | 23.35 | 8.67 | 21.19 | 8.16 |
| Variable | Use of Any Substance | Use of Treated Substance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| γ | SE | df | T Ratio | p | γ | SE | df | T Ratio | p | |
| Unadjusted within-person association Craving/substance | 0.158 | 0.031 | 78 | 5.069 | <0.001 | 0.181 | 0.036 | 78 | 5.020 | <0.001 |
| Between-person moderators | ||||||||||
| Age | −0.0003 | 0.003 | 78 | −0.083 | 0.934 | 0.0002 | 0.003 | 78 | 0.061 | 0.951 |
| Sex | −0.005 | 0.067 | 78 | −0.073 | 0.942 | −0.003 | 0.076 | 78 | −0.040 | 0.968 |
| Education | 0.014 | 0.014 | 78 | 0.970 | 0.335 | 0.004 | 0.015 | 78 | 0.266 | 0.791 |
| Nicotine (vs. alcohol) | −0.167 * | 0.070 | 78 | −2.422 | 0.018 | −0.287 * | 0.076 | 78 | −3.790 | <0.001 |
| Cannabis (vs. alcohol) | −0.025 | 0.091 | 78 | −0.271 | 0.787 | −0.131 | 0.104 | 78 | −1.259 | 0.212 |
| Comorbidity | −0.056 | 0.077 | 78 | −0.728 | 0.469 | −0.041 | 0.080 | 78 | 0.535 | 0.594 |
| Stroop test interference | 0.003 | 0.001 | 78 | 2.225 | 0.029 | 0.004 | 0.002 | 78 | 2.462 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubiec, L.; Chirokoff, V.; Abdallah, M.; Sanz-Arigita, E.; Dupuy, M.; Swendsen, J.; Berthoz, S.; Gierski, F.; Guionnet, S.; Misdrahi, D.; et al. The Executive Functioning Paradox in Substance Use Disorders. Biomedicines 2022, 10, 2728. https://doi.org/10.3390/biomedicines10112728
Jakubiec L, Chirokoff V, Abdallah M, Sanz-Arigita E, Dupuy M, Swendsen J, Berthoz S, Gierski F, Guionnet S, Misdrahi D, et al. The Executive Functioning Paradox in Substance Use Disorders. Biomedicines. 2022; 10(11):2728. https://doi.org/10.3390/biomedicines10112728
Chicago/Turabian StyleJakubiec, Louise, Valentine Chirokoff, Majd Abdallah, Ernesto Sanz-Arigita, Maud Dupuy, Joel Swendsen, Sylvie Berthoz, Fabien Gierski, Sarah Guionnet, David Misdrahi, and et al. 2022. "The Executive Functioning Paradox in Substance Use Disorders" Biomedicines 10, no. 11: 2728. https://doi.org/10.3390/biomedicines10112728
APA StyleJakubiec, L., Chirokoff, V., Abdallah, M., Sanz-Arigita, E., Dupuy, M., Swendsen, J., Berthoz, S., Gierski, F., Guionnet, S., Misdrahi, D., Serre, F., Auriacombe, M., & Fatseas, M. (2022). The Executive Functioning Paradox in Substance Use Disorders. Biomedicines, 10(11), 2728. https://doi.org/10.3390/biomedicines10112728





