Long-Lasting Cognitive Abnormalities after COVID-19
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Descriptive Analysis
3.2. Demographic and Clinical Differences
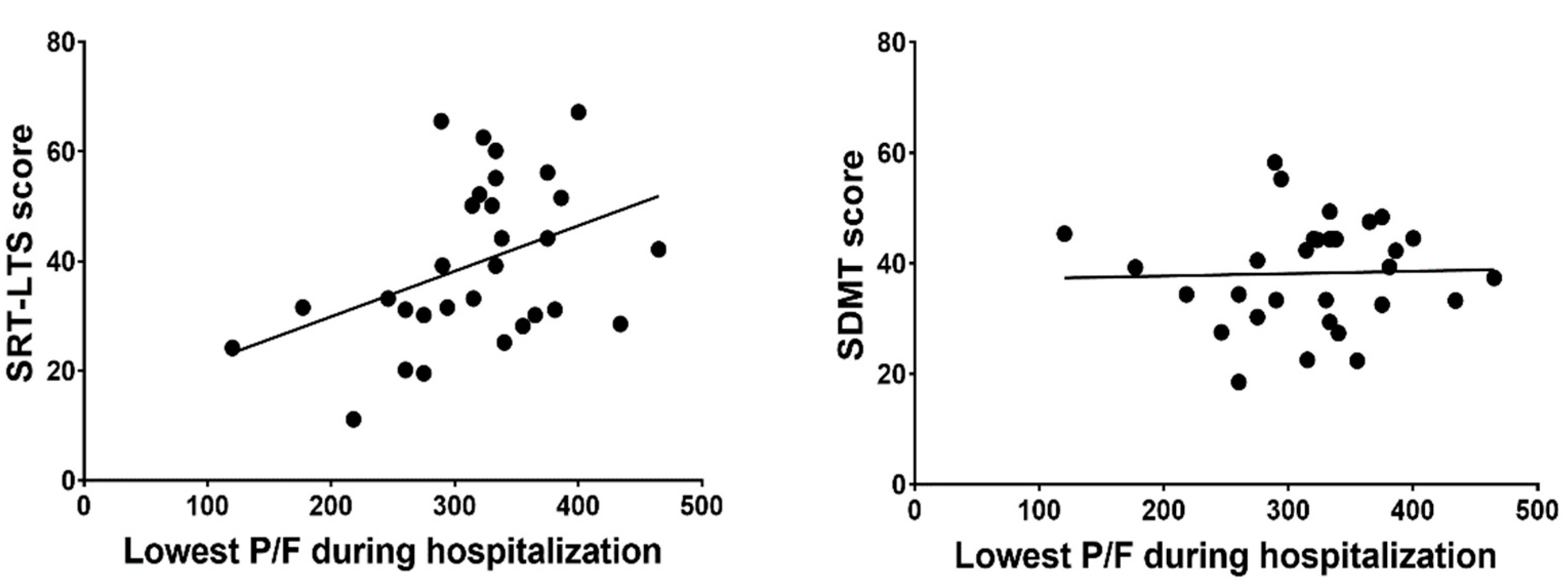
3.3. Correlations
3.4. ARDS vs. No ARDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priori, A.; Baisi, A.; Banderali, G.; Biglioli, F.; Bulfamante, G.; Canevini, M.P.; Cariati, M.; Carugo, S.; Cattaneo, M.; Cerri, A.; et al. The Many Faces of Covid-19 at a Glance: A University Hospital Multidisciplinary Account from Milan, Italy. Front. Public Health 2021, 8, 575029. [Google Scholar] [CrossRef]
- Ferrarese, C.; Silani, V.; Priori, A.; Galimberti, S.; Agostoni, E.; Monaco, S.; Padovani, A.; Tedeschi, G. An Italian multicenter retrospective-prospective observational study on neurological manifestations of COVID-19 (NEUROCOVID). Neurol. Sci. 2020, 41, 1355–1359. [Google Scholar] [CrossRef]
- Leonardi, M.; Padovani, A.; McArthur, J.C. Neurological manifestations associated with COVID-19: A review and a call for action. J. Neurol. 2020, 267, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Moro, E.; Priori, A.; Beghi, E.; Helbok, R.; Campiglio, L.; Bassetti, C.L.; Bianchi, E.; Maia, L.F.; Ozturk, S.; Cavallieri, F.; et al. The international European Academy of Neurology survey on neurological symptoms in patients with COVID-19 infection. Eur. J. Neurol. 2020, 27, 1727–1737. [Google Scholar] [CrossRef]
- Bulfamante, G.; Chiumello, D.; Canevini, M.P.; Priori, A.; Mazzanti, M.; Centanni, S.; Felisati, G. First ultrastructural autoptic findings of sars-cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020, 86, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lana, S.; Marquié, M.; Ruiz, A.; Boada, M. Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals With Dementia. Front. Aging Neurosci. 2020, 12, 588872. [Google Scholar] [CrossRef]
- Al-Obaidi, J.M.M.; Bahadoran, A.; Wang, S.M.; Manikam, R.; Raju, C.S.; Sekaran, S.D. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018, 62, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Michalicová, A.; Bhide, K.; Bhide, M.; Kováč, A. How viruses infiltrate the central nervous system. Acta Virol. 2017, 61, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef]
- Baker, H.A.; Safavynia, S.A.; Evered, L.A. The ‘third wave’: Impending cognitive and functional decline in COVID-19 survivors. Br. J. Anaesth. 2021, 126, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Liotta, E.M.; Batra, A.; Clark, J.R.; Shlobin, N.A.; Hoffman, S.C.; Orban, Z.S.; Koralnik, I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann. Clin. Transl. Neurol. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Gutiérrez-Ocampo, E.; Villamizar-Peña, R.; Holguin-Rivera, Y.; Escalera-Antezana, J.P.; Alvarado-Arnez, L.E.; Bonilla-Aldana, D.K.; Franco-Paredes, C.; Henao-Martinez, A.F.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 34, 101623. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020, 191, 145. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Hu, X.; Michael De Silva, T.; Chen, J.; Faraci, F.M. Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke. Circ. Res. 2017, 120, 449–471. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Barker, T.A.; Berk, B.C. Angiotensin II and the endothelium: Diverse signals and effects. Hypertension 2005, 45, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bernard, I.; Limonta, D.; Mahal, L.; Hobman, T. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID. Viruses 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 2021, 44, 13–34. [Google Scholar] [CrossRef]
- Labò, N.; Ohnuki, H.; Tosato, G. Vasculopathy and Coagulopathy Associated with SARS-CoV-2 Infection. Cells 2020, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Res. Ther. 2020, 12, 69. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID. J. Alzheimer’s Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Ricchiuti, L.; De Caro, M.F.; Patti, F.; Vecchio, R.; Sorbi, S.; Trojano, M. The Rao’s Brief Repeatable Battery and Stroop Test: Normative values with age, education and gender corrections in an Italian population. Mult. Scler. 2006, 12, 787–793. [Google Scholar] [CrossRef]
- Beck, A.T. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevik, E.J.; Eikeland, R.A.; Lundervold, A.J. Subthreshold depressive symptoms have a negative impact on cognitive functioning in middle-aged and older males. Front. Psychol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cattelani SSD—Scala Soggettiva di Danno. Neurologia Clinica; Angelini, C., Battistin, L., Eds.; Società Editrice Esculapio: Bologna, Italy, 2010. [Google Scholar]
- Ghisi, M.; Flebus, G.B.; Montano, A.; Sanavio, E.; Sica, C. Beck Depression Inventory—II, 1st ed.; Giunti Psychometrics: Florence, Italy, 2006; ISBN 978-88-09-40277-5. [Google Scholar]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F.; Sabeti, P. Neuropathological Features of Covid. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Egbert, A.R.; Cankurtaran, S.; Karpiak, S. Brain abnormalities in COVID-19 acute/subacute phase: A rapid systematic review. Brain. Behav. Immun. 2020, 89, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Brugulat-Serrat, A.; Salvadó, G.; Operto, G.; Cacciaglia, R.; Sudre, C.H.; Grau-Rivera, O.; Suárez-Calvet, M.; Falcon, C.; Sánchez-Benavides, G.; Gramunt, N.; et al. White matter hyperintensities mediate gray matter volume and processing speed relationship in cognitively unimpaired participants. Hum. Brain Mapp. 2020, 41, 1309–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herridge, M.S.; Moss, M.; Hough, C.L.; Hopkins, R.O.; Rice, T.W.; Bienvenu, O.J.; Azoulay, E. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016, 42, 725–738. [Google Scholar] [CrossRef]
- Hopkins, R.O.; Weaver, L.K.; Pope, D.; Orme, J.F.; Bigler, E.D.; Larson-Lohr, V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Christie, J.D.; Lanken, P.N.; Biester, R.C.; Thompson, B.T.; Bellamy, S.L.; Localio, A.R.; EjigayehuDemissie; Hopkins, R.O.; Angus, D.C. The adult respiratory distress syndrome cognitive outcomes study: Long-term neuropsychological function in survivors of acute lung injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Cervós-Navarro, J.; Sampaolo, S.; Hamdorf, G. Brain changes in experimental chronic hypoxia. Exp. Pathol. 1991, 42, 205–212. [Google Scholar] [CrossRef]
- Squire, L.R.; Zola-Morgan, S. The medial temporal lobe memory system. Science 1991, 253, 1380–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, R.O.; Gale, S.D.; Weaver, L.K. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006, 20, 263–271. [Google Scholar] [CrossRef]
- Fries, M.; Bickenbach, J.; Henzler, D.; Beckers, S.; Dembinski, R.; Sellhaus, B.; Rossaint, R.; Kuhlen, R. S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury. Anesthesiology 2005, 102, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Radkowski, M.; Demkow, U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J. Neuroinflam. 2014, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Sasannejad, C.; Ely, E.W.; Lahiri, S. Long-term cognitive impairment after acute respiratory distress syndrome: A review of clinical impact and pathophysiological mechanisms. Crit. Care 2019, 23, 352. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, R.; Averna, A.; Marino, D.; Reitano, M.R.; Ruggiero, F.; Mameli, F.; Dini, M.; Poletti, B.; Barbieri, S.; Priori, A.; et al. Psychological Impact During the First Outbreak of COVID-19 in Italy. Front. Psychiatry 2020, 11, 1–9. [Google Scholar] [CrossRef]

| Females (n = 11) | Males (n = 27) | Total (n = 38) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Age | 53.82 | 12.62 | 53.30 | 12.88 | 53.45 | 12.64 | 0.910 |
| Education (years) | 11.00 | 3.71 | 12.96 | 2.92 | 12.39 | 3.24 | 0.087 |
| Days of hospitalization | 9.55 | 4.11 | 9.96 | 3.95 | 9.84 | 3.95 | 0.772 |
| SpO2 upon hospital arrival | 96.33 | 1.97 | 96.73 | 2.25 | 96.62 | 2.15 | 0.430 |
| Lowest P/F during hospitalization | 326.71 | 46.44 | 314.43 | 78.44 | 317.30 | 71.70 | 0.699 |
| Days between hospital discharge and cognitive assessment | 154.18 | 40.12 | 123.08 | 31.08 | 132.86 | 36.62 | 0.016 |
| MoCA adjusted score | 25.96 | 2.20 | 25.90 | 2.68 | 25.92 | 2.53 | 0.953 |
| Mean | SD | Normative Cutoff | % Under Normative Cutoff | |
|---|---|---|---|---|
| SRT-LTS score | 40.11 | 13.88 | ≥23.3 | 10.5% |
| SRT-CLTR score | 31.77 | 14.09 | ≥15.5 | 10.5% |
| SPART score | 17.63 | 5.08 | ≥12.7 | 15.8% |
| SDMT score | 39.37 | 10.07 | ≥37.9 | 42.1% |
| PASAT-3 score | 43.39 | 10.64 | ≥28.4 | 10.5% |
| PASAT-2 score | 32.53 | 9.56 | ≥17.1 | 5.3% |
| SRT-D score | 7.53 | 2.74 | ≥4.9 | 26.3% |
| SPART-D score | 5.76 | 1.91 | ≥3.6 | 18.4% |
| WLG score | 25.65 | 5.23 | ≥17.0 | 7.9% |
| Lowest P/F | SpO2 upon Hospital Arrival | Days of Hospitalization | BDI-II | ||
|---|---|---|---|---|---|
| SRT-LTS | Correlation coefficient | 0.404 | 0.240 | −0.206 | −0.160 |
| p | 0.027 | 0.201 | 0.222 | 0.344 | |
| SRT-CLTR | Correlation coefficient | 0.241 | 0.230 | −0.108 | −0.186 |
| p | 0.199 | 0.221 | 0.524 | 0.270 | |
| SRT-D | Correlation coefficient | 0.318 | 0.373 | −0.020 | −0.372 |
| p | 0.087 | 0.042 | 0.906 | 0.023 | |
| SPART | Correlation coefficient | −0.044 | −0.007 | −0.063 | 0.194 |
| p | 0.817 | 0.971 | 0.713 | 0.250 | |
| SPART-D | Correlation coefficient | 0.080 | −0.013 | −0.088 | 0.064 |
| p | 0.674 | 0.944 | 0.604 | 0.709 | |
| SDMT | Correlation coefficient | 0.032 | −0.024 | 0.028 | 0.139 |
| p | 0.866 | 0.902 | 0.868 | 0.414 | |
| PASAT-3 | Correlation coefficient | 0.163 | 0.158 | −0.242 | 0.193 |
| p | 0.389 | 0.406 | 0.149 | 0.254 | |
| PASAT-2 | Correlation coefficient | 0.216 | 0.134 | −0.141 | 0.009 |
| p | 0.251 | 0.479 | 0.404 | 0.960 | |
| WLG | Correlation coefficient | 0.179 | 0.328 | 0.194 | −0.252 |
| p | 0.345 | 0.077 | 0.250 | 0.133 | |
| No ARDS | ARDS | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| SRT-LTS | 44.50 | 13.16 | 30.63 | 13.33 | 0.007 |
| SRT-CLTR | 34.42 | 14.46 | 25.59 | 14.68 | 0.103 |
| SRT-D | 8.10 | 2.62 | 5.95 | 2.56 | 0.029 |
| SPART | 17.49 | 4.89 | 17.49 | 4.87 | 0.998 |
| SPART-D | 5.73 | 1.86 | 5.30 | 1.89 | 0.526 |
| SDMT | 37.15 | 8.57 | 38.73 | 11.49 | 0.658 |
| PASAT-3 | 43.70 | 1.78 | 41.13 | 9.89 | 0.503 |
| PASAT-2 | 33.52 | 1.23 | 3.20 | 8.80 | 0.355 |
| WLG | 26.99 | 4.47 | 23.62 | 5.84 | 0.073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; D’Arminio Monforte, A.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. https://doi.org/10.3390/brainsci11020235
Ferrucci R, Dini M, Groppo E, Rosci C, Reitano MR, Bai F, Poletti B, Brugnera A, Silani V, D’Arminio Monforte A, et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sciences. 2021; 11(2):235. https://doi.org/10.3390/brainsci11020235
Chicago/Turabian StyleFerrucci, Roberta, Michelangelo Dini, Elisabetta Groppo, Chiara Rosci, Maria Rita Reitano, Francesca Bai, Barbara Poletti, Agostino Brugnera, Vincenzo Silani, Antonella D’Arminio Monforte, and et al. 2021. "Long-Lasting Cognitive Abnormalities after COVID-19" Brain Sciences 11, no. 2: 235. https://doi.org/10.3390/brainsci11020235
APA StyleFerrucci, R., Dini, M., Groppo, E., Rosci, C., Reitano, M. R., Bai, F., Poletti, B., Brugnera, A., Silani, V., D’Arminio Monforte, A., & Priori, A. (2021). Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sciences, 11(2), 235. https://doi.org/10.3390/brainsci11020235








