Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
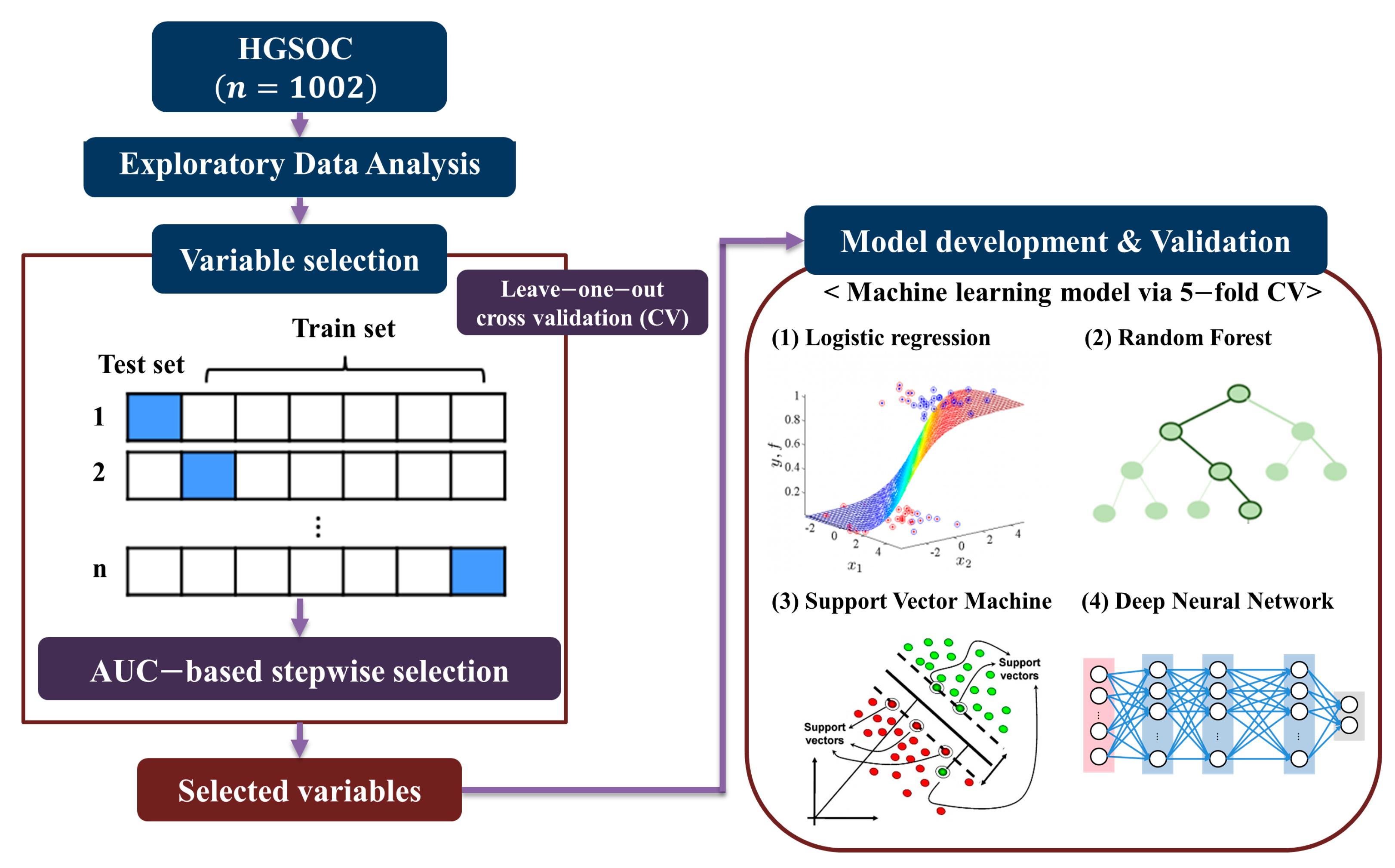
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Exploratory Data Analysis
2.3. Variable Selection
2.4. Model Development and Validation
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Model Development and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Won, Y.-J.; Ko, M.J.; Kim, M.; Shim, S.-H.; Suh, D.H.; Kim, J.-W. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J. Gynecol. Oncol. 2019, 30, e38. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.J.; Bast, R.C., Jr.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.L.; Bookman, M.A.; et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer, Version 2.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 31 August 2020).
- Ledermann, J.; Raja, F.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C. Corrections to “Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Ann. Oncol. 2018, 29, iv259. [Google Scholar] [CrossRef] [Green Version]
- Colombo, N.; Sessa, C.; Du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.; Trimble, E.; Tinker, A.; Alberts, D.; Avall-Lundqvist, E.; Brady, M.; Harter, P.; Pignata, S.; Lauraine, E.P.-; Sehouli, J.; et al. Clinical Trials in Recurrent Ovarian Cancer. Int. J. Gynecol. Cancer 2011, 21, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [Green Version]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Christensen, R.D.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Paik, E.S.; Sohn, I.; Baek, S.-Y.; Shim, M.; Choi, H.J.; Kim, T.-J.; Choi, C.H.; Lee, J.-W.; Kim, B.-G.; Lee, Y.-Y.; et al. Nomograms Predicting Platinum Sensitivity, Progression-Free Survival, and Overall Survival Using Pretreatment Complete Blood Cell Counts in Epithelial Ovarian Cancer. Cancer Res. Treat. 2017, 49, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Chi, D.S.; Palayekar, M.J.; Sonoda, Y.; Abu-Rustum, N.R.; Awtrey, C.S.; Huh, J.; Eisenhauer, E.L.; Barakat, R.R.; Kattan, M.W. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol. Oncol. 2008, 108, 191–194. [Google Scholar] [CrossRef]
- Teramukai, S.; Ochiai, K.; Tada, H.; Fukushima, M. PIEPOC: A new prognostic index for advanced epithelial ovarian cancer—Japan Multinational Trial Organization OC01-01. J. Clin. Oncol. 2007, 25, 3302–3306. [Google Scholar] [CrossRef]
- Barlin, J.N.; Yu, C.; Hill, E.K.; Zivanovic, O.; Kolev, V.; Levine, U.A.; Sonoda, Y.; Abu-Rustum, N.R.; Huh, J.; Barakat, R.R.; et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol. Oncol. 2012, 125, 25–30. [Google Scholar] [CrossRef]
- Gerestein, C.G.; Eijkemans, M.J.C.; De Jong, D.; Burg, M.E.L.V.D.; Dykgraaf, R.H.M.; Kooi, G.S.; Baalbergen, A.; Burger, C.W.; Ansink, A.C. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG: Int. J. Obstet. Gynaecol. 2009, 116, 372–380. [Google Scholar] [CrossRef]
- Kim, S.I.; Song, M.; Hwangbo, S.; Lee, S.; Cho, U.; Kim, J.-H.; Lee, M.; Kim, H.S.; Chung, H.H.; Suh, D.-S.; et al. Development of Web-Based Nomograms to Predict Treatment Response and Prognosis of Epithelial Ovarian Cancer. Cancer Res. Treat. 2019, 51, 1144–1155. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Rustin, G.J.S.; Vergote, I.; Eisenhauer, E.; Pujade-Lauraine, E.; Quinn, M.; Thigpen, T.; Du Bois, A.; Kristensen, G.; Jakobsen, A.; Sagae, S.; et al. Definitions for Response and Progression in Ovarian Cancer Clinical Trials Incorporating RECIST 1.1 and CA 125 Agreed by the Gynecological Cancer Intergroup (GCIG). Int. J. Gynecol. Cancer 2011, 21, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kwon, M.S.; Choi, Y.; Yi, S.G.; Namkung, J.; Han, S.; Kwon, W.; Kim, S.W.; Jang, J.Y.; Kim, H.; et al. Comparative studies for developing protein based cancer prediction model to maximise the ROC-AUC with various variable selection methods. IJDMB 2016, 16, 64–76. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News. 2002, 2, 18–22. [Google Scholar]
- Amari, S.; Wu, S. Improving support vector machine classifiers by modifying kernel functions. Neural Netw. 1999, 12, 783–789. [Google Scholar] [CrossRef]
- Le Cun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Kurman, R. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann. Oncol. 2013, 24, x16–x21. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef] [Green Version]
- Gaitskell, K.; Green, J.; Pirie, K.; Barnes, I.; Hermon, C.; Reeves, G.K.; Beral, V.; Milion Women Study Collaborators. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. Int. J. Cancer 2018, 142, 281–289. [Google Scholar] [CrossRef]
- Shipe, M.E.; Deppen, S.A.; Farjah, F.; Grogan, E.L. Developing prediction models for clinical use using logistic regression: An overview. J. Thorac. Dis. 2019, 11, S574–S584. [Google Scholar] [CrossRef]
- Richter, A.N.; Khoshgoftaar, T.M. A review of statistical and machine learning methods for modeling cancer risk using structured clinical data. Artif. Intell. Med. 2018, 90, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrillo, M.; Ferrandina, G.; Fagotti, A.; Vizzielli, G.; Margariti, P.A.; Pedone, A.L.; Nero, C.; Fanfani, F.; Scambia, G. Timing and pattern of recurrence in ovarian cancer patients with high tumor dissemination treated with primary debulking surgery versus neoadjuvant chemotherapy. Ann. Surg. Oncol. 2013, 20, 3955–3960. [Google Scholar] [CrossRef]
- da Costa, A.A.B.A.; Valadares, C.V.; Baiocchi, G.; Mantoan, H.; Saito, A.; Sanches, S.; Guimarães, A.P.; Achatz, M.I.W. Neo-adjuvant Chemotherapy Followed by Interval Debulking Surgery and the Risk of Platinum Resistance in Epithelial Ovarian Cancer. Ann. Surg. Oncol. 2015, 22, S971–S978. [Google Scholar] [CrossRef]
- Luo, Y.; Lee, M.; Kim, H.S.; Chung, H.H.; Song, Y.S. Effect of neoadjuvant chemotherapy on platinum resistance in stage IIIC and IV epithelial ovarian cancer. Medicine 2016, 95, e4797. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiao, X.; Gao, Q. Neoadjuvant chemotherapy-related platinum resistance in ovarian cancer. Drug Discov. Today 2020, 25, 1232–1238. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, C.; Song, W.; Choo, W.; Lee, S.; Lee, S.; Han, Y.; Bassi, C.; Salvia, R.; Marchegiani, G.; et al. Risk prediction for malignant intraductal papillary mucinous neoplasm of the pancreas: Logistic regression versus machine learning. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Frizzell, J.D.; Liang, L.; Schulte, P.J.; Yancy, C.W.; Heidenreich, P.A.; Hernandez, A.F.; Bhatt, D.L.; Fonarow, G.C.; Laskey, W.K. Prediction of 30-day all-cause readmis-sions in patients hospitalized for heart failure: Comparison of machine learning and other statistical approaches. JAMA Cardiol. 2017, 2, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no per-formance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Missing Rate (%) | All (n = 1002, %) | Platinum-Sensitive (n = 779, %) | Platinum-Resistant (n = 223, %) | p |
|---|---|---|---|---|---|
| Age, years | 0 | 55.8 ± 10 | 55.2 ± 10 | 58.3 ± 10 | <0.001 |
| BMI, kg/m2 | 3.2 | 23.6 ± 3 | 23.5 ± 3 | 23.9 ± 3 | 0.135 |
| Parity | 0.9 | 0.884 | |||
| 0 | 97 (9.8) | 77 (10.0) | 20 (9.1) | ||
| 1–2 | 592 (59.6) | 458 (59.2) | 134 (60.9) | ||
| ≥3 | 304 (30.6) | 238 (30.8) | 66 (30.0) | ||
| Menopause | 0.4 | 691 (69.2) | 513 (66.1) | 178 (80.2) | <0.001 |
| Comorbidities | |||||
| Hypertension | 22.2 | 153 (19.6) | 107 (18.1) | 46 (24.5) | 0.069 |
| Diabetes | 22.2 | 53 (6.8) | 36 (6.1) | 17 (9.0) | 0.215 |
| Dyslipidemia | 22.3 | 35 (4.5) | 29 (4.9) | 6 (3.2) | 0.431 |
| Personal history of breast cancer | 3.3 | 72 (7.4) | 59 (7.8) | 13 (6.0) | 0.453 |
| Familial history of breast cancer * | 5.5 | 51 (5.4) | 42 (5.7) | 9 (4.2) | 0.486 |
| No. of family members with cancer | |||||
| Median (range) | 5.5 | 0 (0–3) | 0 (0–3) | 0 (0–2) | 0.295 |
| Familial history of gynecologic cancer * | 5.5 | 21 (2.2) | 18 (2.5) | 3 (1.4) | 0.511 |
| No. of family members with cancer | |||||
| Median (range) | 5.5 | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.702 |
| Origin | 0 | 0.532 | |||
| Ovary | 911 (90.9) | 708 (90.9) | 203 (91.0) | ||
| Tube | 51 (5.1) | 42 (5.4) | 9 (4.0) | ||
| Peritoneum | 40 (4.0) | 29 (3.7) | 11 (4.9) | ||
| FIGO stage | 0 | <0.001 | |||
| I | 40 (4.0) | 40 (5.1) | 0 | ||
| II | 56 (5.6) | 54 (6.9) | 2 (0.9) | ||
| III | 628 (62.7) | 496 (63.7) | 132 (59.2) | ||
| IV | 278 (27.7) | 189 (24.3) | 89 (39.9) | ||
| Ln (Serum CA-125 [IU/mL]) | 4.3 | 6.7 ± 2 | 6.6 ± 2 | 7.1 ± 1 | <0.001 |
| Hemoglobin (g/dL) | 11.9 | 12.3 ± 1 | 12.3 ± 1 | 12.2 ± 1 | 0.192 |
| Primary treatment strategy | 0 | <0.001 | |||
| PDS | 764 (76.2) | 629 (80.7) | 135 (60.5) | ||
| NAC | 238 (23.8) | 150 (19.3) | 88 (39.5) | ||
| Residual tumor size after PDS/IDS | 4.4 | <0.001 | |||
| Complete cytoreduction | 549 (57.3) | 455 (61.2) | 94 (43.9) | ||
| Gross residual tumor | 409 (42.7) | 289 (38.8) | 120 (56.1) | ||
| Frontline chemotherapy regimen | 4.0 | 0.870 | |||
| Paclitaxel-Carboplatin | 872 (90.6) | 676 (90.5) | 196 (91.2) | ||
| Docetaxel-Carboplatin | 90 (9.4) | 71 (9.5) | 19 (8.8) | ||
| Total cycle of frontline chemotherapy | 0 | <0.001 | |||
| ≤6 | 694 (69.3) | 564 (72.4) | 130 (58.3) | ||
| >6 | 308 (30.7) | 215 (27.6) | 93 (41.7) | ||
| Recurrence | 0 | 734 (73.3) | 511 (65.6) | 223 (100.0) | <0.001 |
| Treatment-free interval, months | 0 | ||||
| Median (range) | 12.9 (0.1–153.4) | 17.2 (6.1–153.4) | 3.4 (0.1–6.0) | <0.001 |
| No. of Variables | List | Machine Learning | AUC | Sensitivity | Specificity | Balanced Accuracy | Threshold |
|---|---|---|---|---|---|---|---|
| 1 | FIGO stage | LR | 0.556 | 1.000 | 0.111 | 0.556 | 0.025 |
| RF | 0.500 | 0 | 1.000 | 0.500 | 0 | ||
| SVM | 0.556 | 1.000 | 0.167 | 0.583 | 0.241 | ||
| DNN | 0.558 | 1.000 | 0.122 | 0.561 | 0.214 | ||
| 1 | Residual tumor size after PDS/IDS | LR | 0.586 | 0.605 | 0.564 | 0.584 | 0.172 |
| RF | 0.500 | 0 | 1.000 | 0.500 | 0 | ||
| SVM | 0.586 | 0.605 | 0.564 | 0.584 | 0.295 | ||
| DNN | 0.587 | 0.605 | 0.564 | 0.584 | 0.355 | ||
| 2 | FIGO stage + Residual tumor size after PDS/IDS | LR | 0.611 | 0.605 | 0.570 | 0.588 | 0.203 |
| RF | 0.500 | 0 | 1.000 | 0.500 | 0 | ||
| SVM | 0.611 | 0.605 | 0.570 | 0.588 | 0.309 | ||
| DNN | 0.611 | 0.605 | 0.570 | 0.588 | 0.252 | ||
| 6 | Age + Serum CA125 levels * + NAC + Pelvic LN status + Involvement of pelvic tissue other than uterus and tube + Involvement of small bowel and mesentery | LR | 0.741 | 0.778 | 0.622 | 0.700 | 0.175 |
| RF | 0.738 | 0.538 | 0.887 | 0.713 | 0.185 | ||
| SVM | 0.733 | 0.731 | 0.745 | 0.738 | 0.232 | ||
| DNN | 0.721 | 0.857 | 0.556 | 0.706 | 0.357 | ||
| 7 | Age + Serum CA125 levels * + NAC + Pelvic LN status + Involvement of pelvic tissue other than uterus and tube + Involvement of small bowel and mesentery + FIGO stage | LR | 0.748 | 0.920 | 0.476 | 0.698 | 0.141 |
| RF | 0.704 | 0.800 | 0.524 | 0.662 | 0.034 | ||
| SVM | 0.745 | 0.920 | 0.457 | 0.689 | 0.133 | ||
| DNN | 0.646 | 0.655 | 0.625 | 0.640 | 0.218 | ||
| 7 | Age + Serum CA125 levels * + NAC + Pelvic LN status + Involvement of pelvic tissue other than uterus and tube + Involvement of small bowel and mesentery + Residual tumor size after PDS/IDS | LR | 0.741 | 0.793 | 0.563 | 0.678 | 0.144 |
| RF | 0.719 | 0.960 | 0.385 | 0.672 | 0.021 | ||
| SVM | 0.740 | 0.517 | 0.883 | 0.700 | 0.259 | ||
| DNN | 0.735 | 0.680 | 0.654 | 0.667 | 0.461 | ||
| 8 | Age + Serum CA125 levels * + NAC + Pelvic LN status + Involvement of pelvic tissue other than uterus and tube + Involvement of small bowel and mesentery + FIGO stage + Residual tumor size after PDS/IDS | LR | 0.738 | 0.769 | 0.648 | 0.708 | 0.211 |
| RF | 0.738 | 0.897 | 0.519 | 0.708 | 0.065 | ||
| SVM | 0.729 | 0.519 | 0.883 | 0.701 | 0.293 | ||
| DNN | 0.740 | 0.852 | 0.561 | 0.707 | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwangbo, S.; Kim, S.I.; Kim, J.-H.; Eoh, K.J.; Lee, C.; Kim, Y.T.; Suh, D.-S.; Park, T.; Song, Y.S. Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma. Cancers 2021, 13, 1875. https://doi.org/10.3390/cancers13081875
Hwangbo S, Kim SI, Kim J-H, Eoh KJ, Lee C, Kim YT, Suh D-S, Park T, Song YS. Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma. Cancers. 2021; 13(8):1875. https://doi.org/10.3390/cancers13081875
Chicago/Turabian StyleHwangbo, Suhyun, Se Ik Kim, Ju-Hyun Kim, Kyung Jin Eoh, Chanhee Lee, Young Tae Kim, Dae-Shik Suh, Taesung Park, and Yong Sang Song. 2021. "Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma" Cancers 13, no. 8: 1875. https://doi.org/10.3390/cancers13081875
APA StyleHwangbo, S., Kim, S. I., Kim, J.-H., Eoh, K. J., Lee, C., Kim, Y. T., Suh, D.-S., Park, T., & Song, Y. S. (2021). Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma. Cancers, 13(8), 1875. https://doi.org/10.3390/cancers13081875








