Significance of TERT Genetic Alterations and Telomere Length in Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
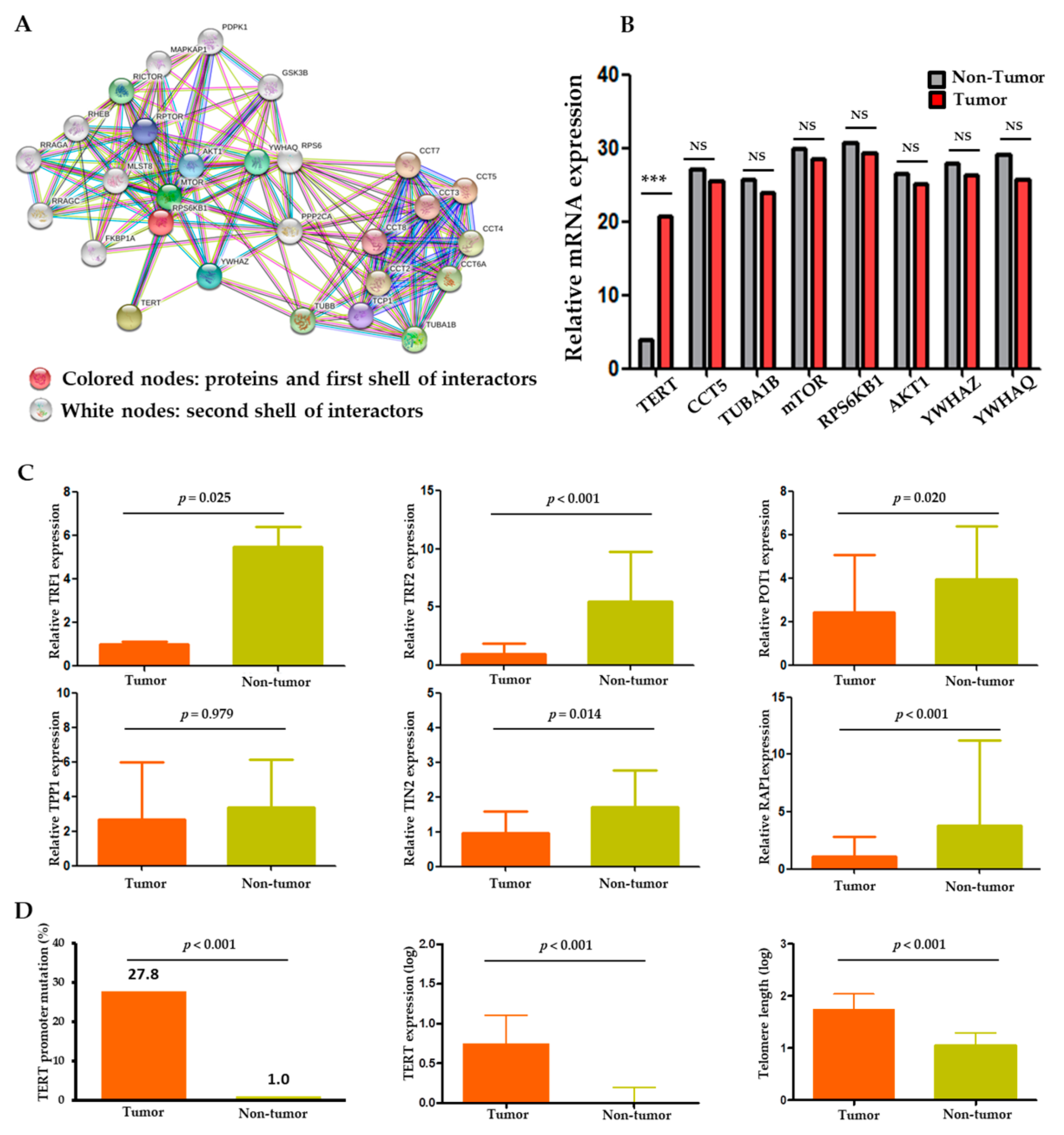
2.2. Protein–Protein Interaction with TERT Gene Sets and Gene Expression
2.3. Comparison of TERT and Telomere Length in Tumors versus Non-Tumors
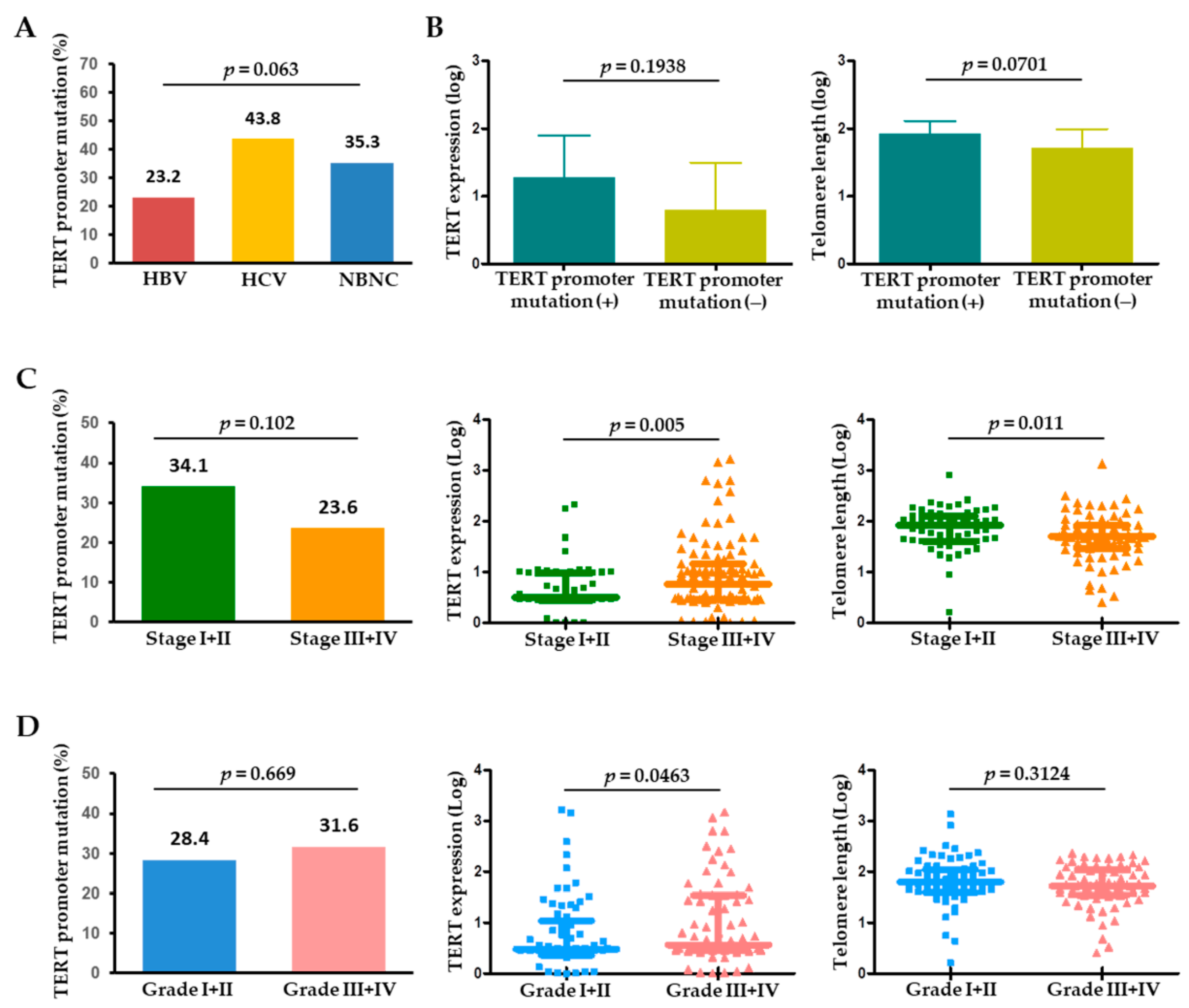
2.4. Alterations in TERT and Telomere Length in Relation to Clinical and Tumor Characteristics
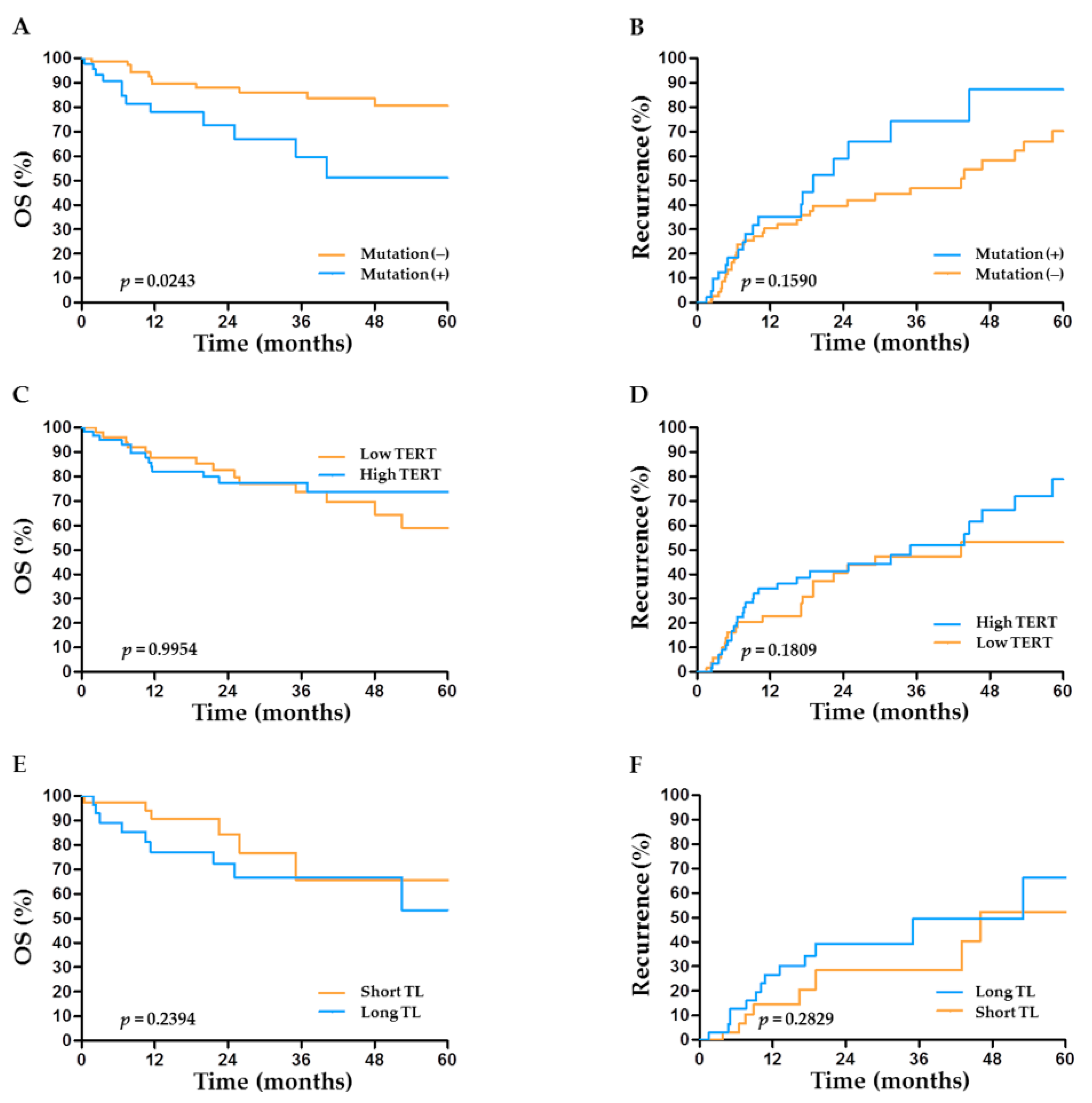
2.5. TERT Abnormalities and Outcome after Hepatectomy
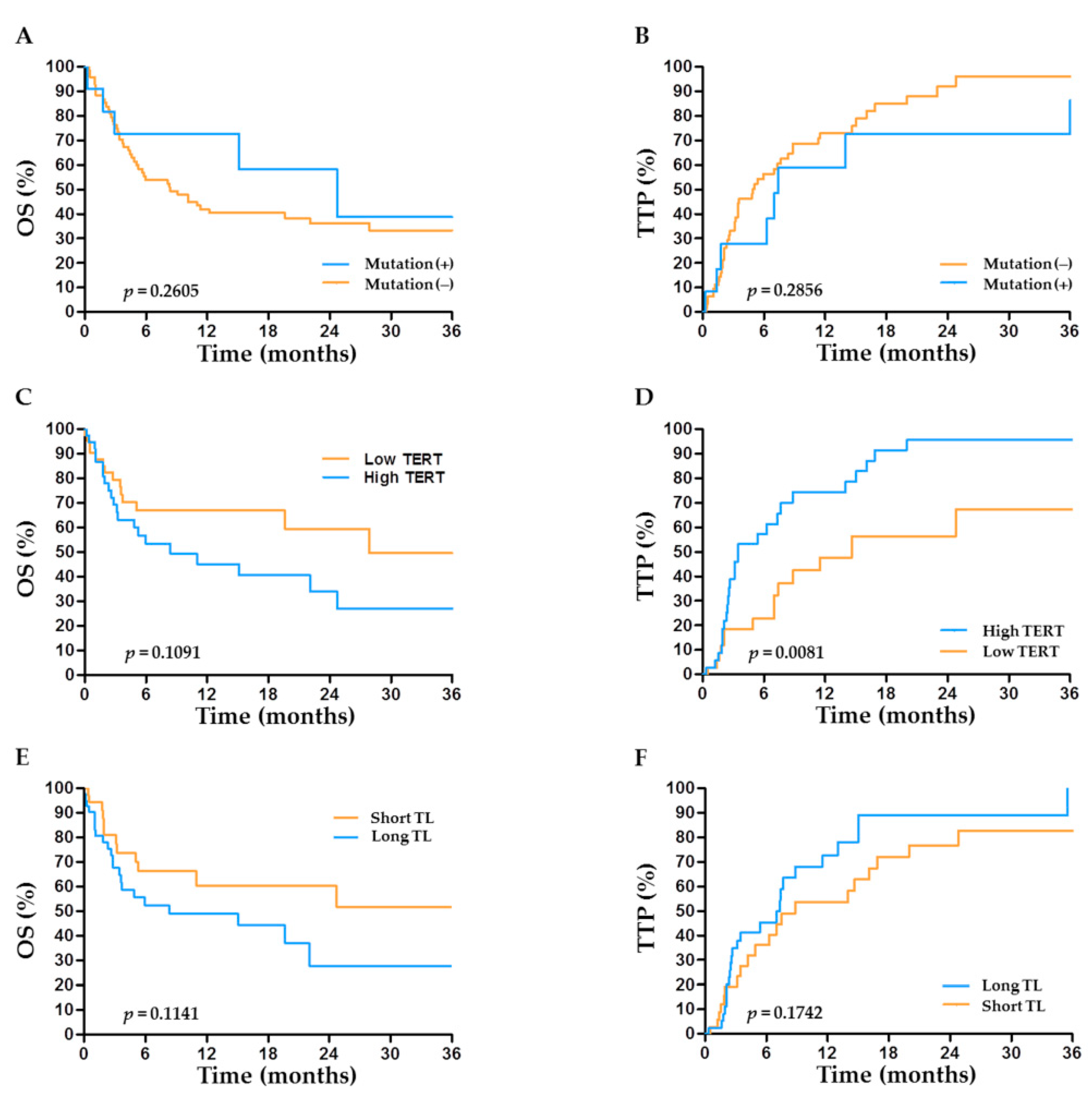
2.6. TERT Abnormalities and Outcome after TACE
3. Discussion
4. Materials and Methods
4.1. Patients and Treatment
4.2. Protein–Protein Interaction Methods
4.3. TERT Promoter Mutation
4.4. Quantitative Real-Time PCR Analysis of TERT Gene Expression and Shelterin Complex
4.5. Telomere Length Measurement
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBsAg | hepatitis B surface antigen |
| HBV | hepatitis B virus |
| CHB | chronic hepatitis B |
| HCC | hepatocellular carcinoma |
| NGS | next generation sequencing |
| OBI | occult HBV infection |
| PCR | polymerase chain reaction |
| TERT | telomerase reverse transcriptase |
| WGS | whole-genome sequencing |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S. The mutational landscape of hepatocellular carcinoma. Clin. Mol. Hepatol. 2015, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.-C.; Llovet, J.M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015, 149, 1226–1239.e1224. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-X.; Yang, C.-M.; Li, M.-G.; Tu, H. Telomerase reverse transcriptase promoter mutations in hepatocellular carcinogenesis. Hepatoma Res. 2019, 2019. [Google Scholar] [CrossRef]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.-C.; Ningarhari, M.; Rebouissou, S.; Zucman-Rossi, J. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 15, 544–558. [Google Scholar] [CrossRef]
- Huang, D.-S.; Wang, Z.; He, X.-J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.-Y.; Wang, W.; Jiang, X.-T. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976. [Google Scholar] [CrossRef]
- Okamoto, K.; Seimiya, H. Revisiting telomere shortening in cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef]
- In der Stroth, L.; Tharehalli, U.; Günes, C.; Lechel, A. Telomeres and Telomerase in the Development of Liver Cancer. Cancers 2020, 12, 2048. [Google Scholar] [CrossRef]
- Kawai-Kitahata, F.; Asahina, Y.; Tanaka, S.; Kakinuma, S.; Murakawa, M.; Nitta, S.; Watanabe, T.; Otani, S.; Taniguchi, M.; Goto, F. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J. Gastroenterol. 2016, 51, 473–486. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, T.I.; Jang, S.Y.; Park, S.Y.; Park, W.-J.; Jung, S.-J.; Lee, J.-H. Clinicopathological characteristics of TERT promoter mutation and telomere length in hepatocellular carcinoma. Medicine 2017, 96, e5766. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.I.; Choi, C.; Ha, S.Y.; Park, C.-K.; Kang, S.Y.; Joh, J.-W.; Paik, S.W.; Kim, S.; Kim, M.; Jung, S.H. Clinical importance of TERT overexpression in hepatocellular carcinoma treated with curative surgical resection in HBV endemic area. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ako, S.; Nouso, K.; Kinugasa, H.; Matsushita, H.; Terasawa, H.; Adachi, T.; Wada, N.; Takeuchi, Y.; Mandai, M.; Onishi, H.; et al. Human Telomerase Reverse Transcriptase Gene Promoter Mutation in Serum of Patients with Hepatocellular Carcinoma. Oncology 2020, 98, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Kang, W.; Wong, S.H.; Wang, M.; Zhou, Y.; Fang, X.; Zhang, X.; Yang, H.; Wong, C.H. Genomic analysis of liver cancer unveils novel driver genes and distinct prognostic features. Theranostics 2018, 8, 1740. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Calderaro, J.; Di Tommaso, L.; Balabaud, C.; Zafrani, E.S.; Bioulac-Sage, P.; Roncalli, M.; Zucman-Rossi, J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology 2014, 60, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Pinyol, R.; Tovar, V.; Llovet, J.M. TERT promoter mutations: Gatekeeper and driver of hepatocellular carcinoma. J. Hepatol. 2014, 61, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Low, K.C.; Tergaonkar, V. Telomerase: Central regulator of all of the hallmarks of cancer. Trends Biochem. Sci. 2013, 38, 426–434. [Google Scholar] [CrossRef]
- Artandi, S.E.; Chang, S.; Lee, S.-L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645. [Google Scholar] [CrossRef]
- El Idrissi, M.; Hervieu, V.; Merle, P.; Mortreux, F.; Wattel, E. Cause-specific telomere factors deregulation in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2013, 32, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoo, J.E.; Cho, J.Y.; Oh, B.K.; Yoon, Y.S.; Han, H.S.; Lee, H.S.; Jang, J.J.; Jeong, S.H.; Kim, J.W.; et al. Telomere length, TERT and shelterin complex proteins in hepatocellular carcinomas expressing “stemness”-related markers. J. Hepatol. 2013, 59, 746–752. [Google Scholar] [CrossRef]
- Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association–National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver 2019, 13, 227–299. [CrossRef] [PubMed]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Park, I.J.; Yu, Y.S.; Mustafa, B.; Park, J.Y.; Seo, Y.B.; Kim, G.D.; Kim, J.; Kim, C.M.; Noh, H.D.; Hong, S.M.; et al. A Nine-Gene Signature for Predicting the Response to Preoperative Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer. Cancers 2020, 12, 800. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.; Dhillon, V.; Thomas, P.; Fenech, M. A quantitative real-time PCR method for absolute telomere length. Biotechniques 2008, 44, 807–809. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All Patients (n = 205) | Surgical Group (n = 121) | TACE Group (n = 84) |
|---|---|---|---|
| Sex | |||
| Male | 165 (80.5) | 89 (73.5) | 76 (90.5) |
| Female | 40 (19.5) | 32 (26.4) | 8 (9.5) |
| Age (years) | 60.0 ± 11.7 | 58.1 ± 11.6 | 64.5 ± 11.9 |
| Cause of liver disease | |||
| HBV | 138 (67.3) | 85 (70.2) | 53 (63.1) |
| HCV | 16 (7.8) | 13 (10.7) | 3 (3.6) |
| Non-viral | 51 (24.9) | 23 (19.0) | 28 (33.3) |
| AST (IU/L) | 45 (29.5−94.5) | 45 (30−94.3) | 42 (29−91) |
| ALT (IU/L) | 34 (23−68.5) | 33.5 (23−69) | 33 (22−65) |
| Child−Pugh class | |||
| A | 167 (81.5) | 111 (91.7) | 56 (66.7) |
| B/C | 38 (18.5) | 10 (8.3) | 28 (33.3) |
| Tumor size (cm) | 6.5 ± 4.9 | 4.9 ± 4.5 | 8.8 ± 4.9 |
| Tumor number | |||
| Single | 111 (54.1) | 88 (72.7) | 23 (27.4) |
| Multifocal | 94 (45.9) | 33 (27.3) | 61 (72.6) |
| α-fetoprotein (ng/mL) | 50.3 (5.5−800.8) | 48.4 (5.4−699.1) | 57.4 (5.6−881.4) |
| mUICC stage | |||
| I | 13 (6.3) | 9 (7.4) | 4 (4.8) |
| II | 80 (39.0) | 66 (54.5) | 14 (16.7) |
| III | 49 (23.9) | 29 (24.0) | 20 (23.8) |
| IV | 63 (30.7) | 17 (14.0) | 46 (54.8) |
| Variables | Overall Survival | Recurrence | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| p | HR (95% CI) | p | p | HR (95% CI) | p | |
| Male sex | 0.423 | 0.694 | ||||
| Age > 60 years | 0.319 | 0.047 | 5.07 (1.67−15.15) | 0.004 | ||
| Cause of liver disease | 0.133 | 0.144 | ||||
| AST > 90 U/L | 0.353 | 0.473 | ||||
| ALT > 60 U/L | 0.357 | 0.595 | ||||
| Child−Pugh class B/C | 0.095 | 1.02 (0.94−1.09) | 0.616 | 0.120 | ||
| Tumor size > 5 cm | <0.001 | 7.97 (3.13−20.24) | <0.001 | <0.001 | 1.81 (0.63−5.18) | 0.263 |
| Tumor multiplicity | 0.075 | 1.56 (0.43−5.65) | 0.491 | 0.019 | 1.85 (0.44−7.71) | 0.398 |
| α-fetoprotein | 0.317 | <0.001 | 1.54 (0.56−4.23) | 0.396 | ||
| Tumor stage (mUICC) | <0.001 | 2.46 (1.45−4.16) | 0.001 | <0.001 | 2.71 (1.48−4.96) | 0.001 |
| TERT promoter mutation | 0.029 | 4.24 (1.75−10.26) | 0.001 | 0.162 | 2.98 (1.01−8.33) | 0.048 |
| TERT expression | 0.913 | 0.362 | ||||
| Telomere length | 0.446 | 0.208 | ||||
| Variables | Overall Survival | Time to Progression | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| p | HR (95% CI) | p | p | HR (95% CI) | p | |
| Male sex | 0.420 | 0.958 | ||||
| Age > 60 years | 0.575 | 0.800 | ||||
| Cause of liver disease | 0.150 | 0.291 | ||||
| AST > 88 U/L | 0.042 | 1.55 (0.49−3.12) | 0.644 | 0.067 | 1.44 (0.72−2.90) | 0.300 |
| ALT > 57 U/L | 0.119 | 0.283 | ||||
| Child−Pugh class B/C | <0.001 | 4.55 (1.96−10.55) | 0.001 | 0.087 | 1.27 (0.54−2.96) | 0.576 |
| Tumor size > 5 cm | 0.001 | 2.68 (0.83−8.67) | 0.099 | 0.003 | 2.11 (0.96−4.62) | 0.061 |
| Tumor multiplicity | 0.469 | 0.102 | ||||
| α-fetoprotein | 0.003 | 2.67 (1.01−7.05) | 0.047 | 0.061 | 1.07 (0.51−2.23) | 0.855 |
| Tumor stage (mUICC) | <0.001 | 1.98 (1.02−3.84) | 0.043 | <0.001 | 1.70 (1.11−2.60) | 0.013 |
| TERT promoter mutation | 0.181 | 0.330 | ||||
| TERT expression | 0.028 | 1.58 (0.67−3.70) | 0.289 | 0.010 | 2.06 (1.06−4.01) | 0.033 |
| Telomere length | 0.137 | 0.277 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-W.; Kim, J.-S.; Kim, H.-S.; Tak, K.-Y.; Lee, S.-K.; Nam, H.-C.; Sung, P.-S.; Kim, C.-M.; Park, J.-Y.; Bae, S.-H.; et al. Significance of TERT Genetic Alterations and Telomere Length in Hepatocellular Carcinoma. Cancers 2021, 13, 2160. https://doi.org/10.3390/cancers13092160
Jang J-W, Kim J-S, Kim H-S, Tak K-Y, Lee S-K, Nam H-C, Sung P-S, Kim C-M, Park J-Y, Bae S-H, et al. Significance of TERT Genetic Alterations and Telomere Length in Hepatocellular Carcinoma. Cancers. 2021; 13(9):2160. https://doi.org/10.3390/cancers13092160
Chicago/Turabian StyleJang, Jeong-Won, Jin-Seoub Kim, Hye-Seon Kim, Kwon-Yong Tak, Soon-Kyu Lee, Hee-Chul Nam, Pil-Soo Sung, Chang-Min Kim, Jin-Young Park, Si-Hyun Bae, and et al. 2021. "Significance of TERT Genetic Alterations and Telomere Length in Hepatocellular Carcinoma" Cancers 13, no. 9: 2160. https://doi.org/10.3390/cancers13092160
APA StyleJang, J.-W., Kim, J.-S., Kim, H.-S., Tak, K.-Y., Lee, S.-K., Nam, H.-C., Sung, P.-S., Kim, C.-M., Park, J.-Y., Bae, S.-H., Choi, J.-Y., & Yoon, S.-K. (2021). Significance of TERT Genetic Alterations and Telomere Length in Hepatocellular Carcinoma. Cancers, 13(9), 2160. https://doi.org/10.3390/cancers13092160







