The Study of Nanosized Silicate-Substituted Hydroxyapatites Co-Doped with Sr2+ and Zn2+ Ions Related to Their Influence on Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Nanosilicate-Substituted Strontium-Hydroxyapatite Powders
2.2. Characterization
2.3. Antimicrobial Activity
2.4. Antibiofilm Activity
2.5. Cell Cultures
2.5.1. Nanoparticles Stock Preparation
2.5.2. MTT Assay
2.6. Hemolytic Activity
2.7. Ames Test
3. Results
3.1. Structural and Morphology Analysis
3.2. SEM-EDS Analysis
3.3. Fourier-Transformed Infrared Spectroscopy
3.4. Antibacterial Activity
3.5. Antibiofilm Activity
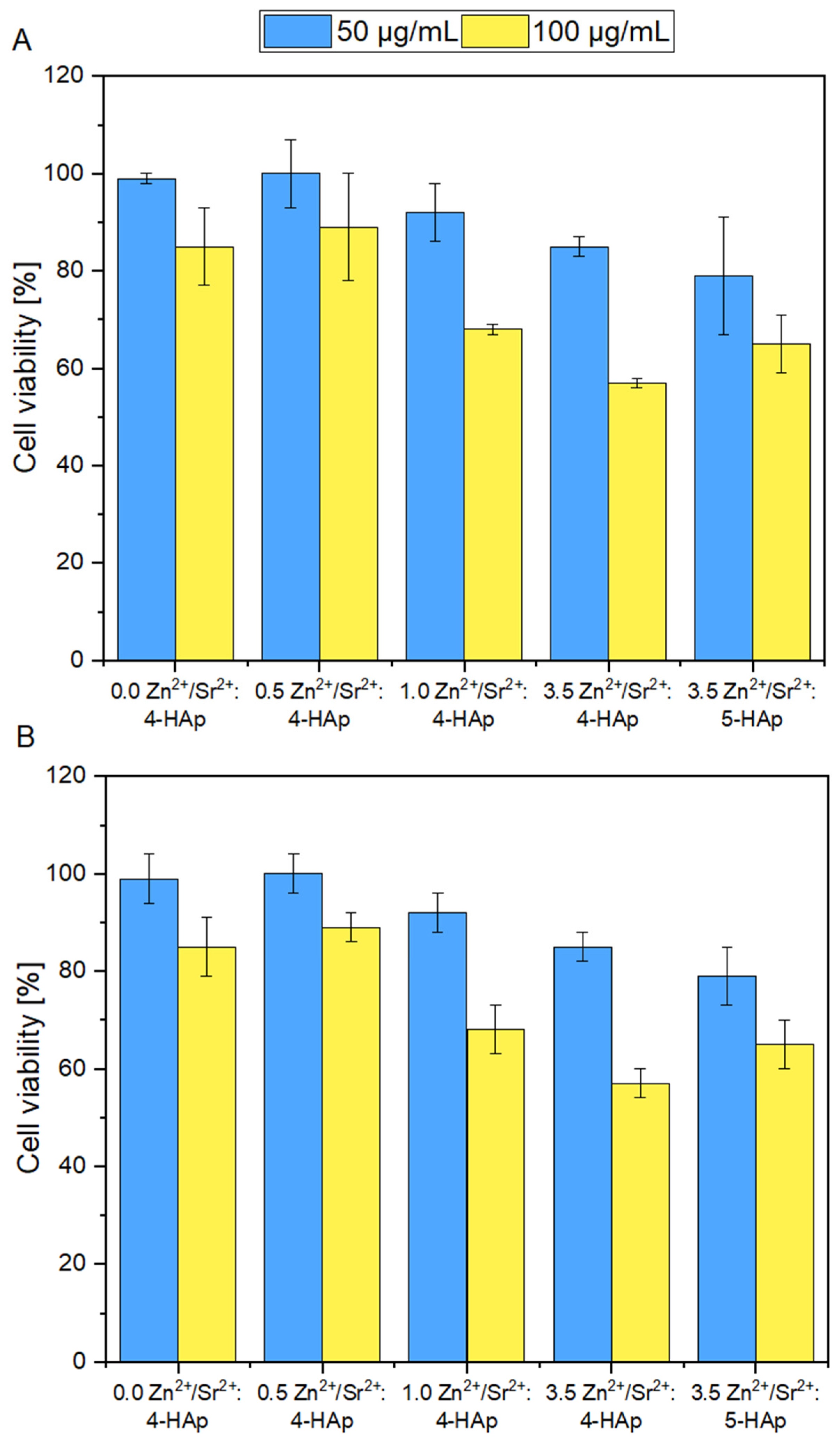
3.6. Cytotoxicity Evaluation
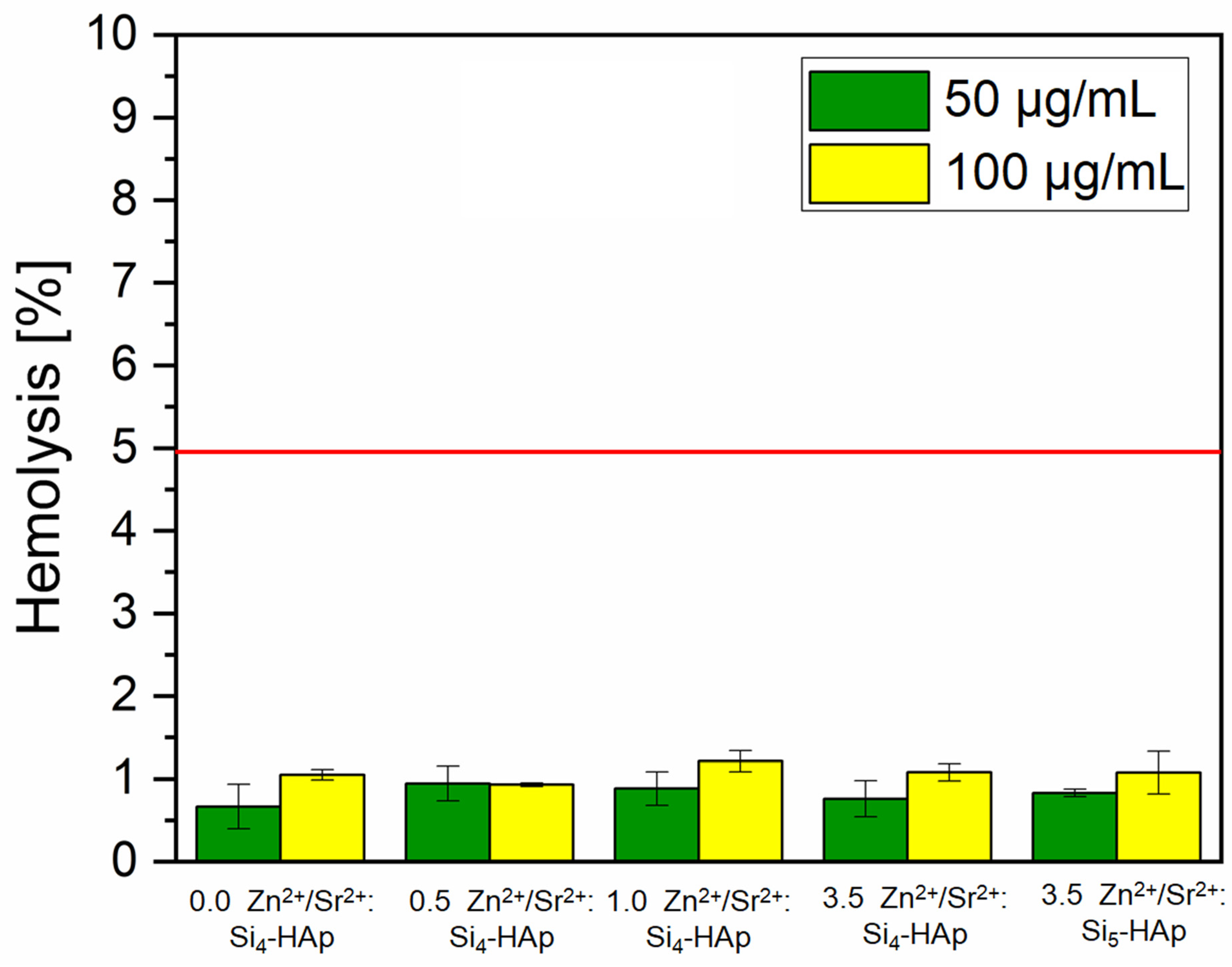
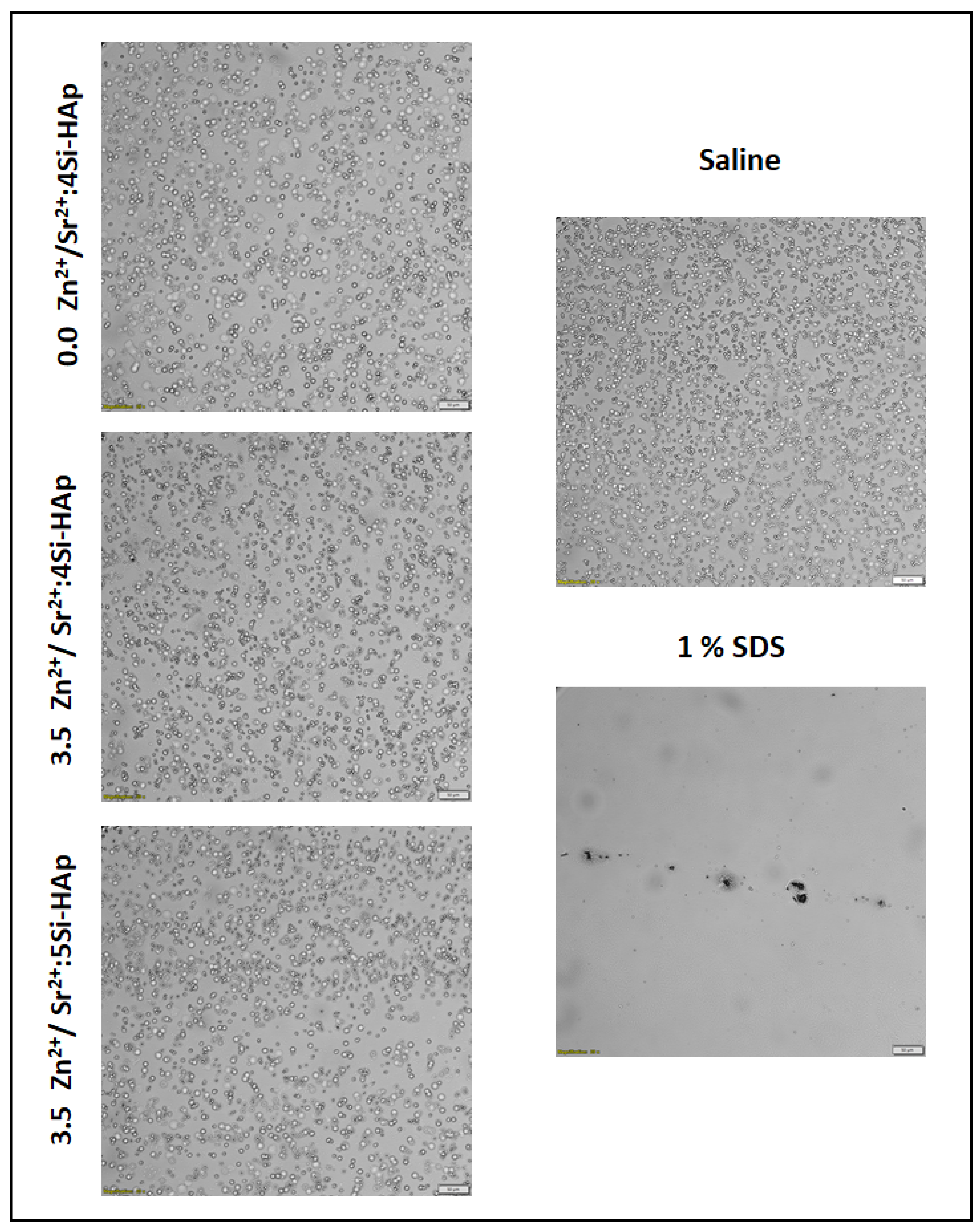
3.7. Hemolysis
3.8. Ames Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoff, J.C.; Akin, E.W. Microbial Resistance to Disinfectants: Mechanisms and Significance. Environ. Health Perspect. 1986, 69, 7–13. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.; Krishnan, R.; Tezel, U.; Pavlostathis, S.; Konstantinidis, K. Widely Used Benzalkonium Chloride Disinfectants Can Promote Antibiotic Resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef] [Green Version]
- Bakht, M.; Alizadeh, S.A.; Rahimi, S.; Kazemzadeh Anari, R.; Rostamani, M.; Javadi, A.; Peymani, A.; Marashi, S.M.A.; Nikkhahi, F. Phenotype and Genetic Determination of Resistance to Common Disinfectants among Biofilm-Producing and Non-Producing Pseudomonas Aeruginosa Strains from Clinical Specimens in Iran. BMC Microbiol. 2022, 22, 124. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sehar, S.; Koop, L.; Wong, Y.K.; Ahmed, S.; Siddiqui, K.S.; Manefield, M. Influence of Calcium in Extracellular DNA Mediated Bacterial Aggregation and Biofilm Formation. PLoS ONE 2014, 9, e91935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their in Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Klasen, H.J. Historical Review of the Use of Silver in the Treatment of Burns. I. Early Uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Terzioğlu, E.; Arslan, M.; Balaban, B.G.; Çakar, Z.P. Microbial Silver Resistance Mechanisms: Recent Developments. World J. Microbiol. Biotechnol. 2022, 38, 158. [Google Scholar] [CrossRef]
- Poole, K. At the Nexus of Antibiotics and Metals: The Impact of Cu and Zn on Antibiotic Activity and Resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A.; et al. Synergistic Antimicrobial Effects of Silver/Transition-Metal Combinatorial Treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef]
- Vaidya, M.; McBain, A.J.; Banks, C.E.; Whitehead, K.A. Single and Combined Antimicrobial Efficacies for Nine Metal Ion Solutions against Klebsiella Pneumoniae, Acinetobacter Baumannii and Enterococcus Faecium. Int. Biodeterior. Biodegrad. 2019, 141, 39–43. [Google Scholar] [CrossRef]
- Vaidya, M.Y.; McBain, A.J.; Butler, J.A.; Banks, C.E.; Whitehead, K.A. Antimicrobial Efficacy and Synergy of Metal Ions against Enterococcus Faecium, Klebsiella Pneumoniae and Acinetobacter Baumannii in Planktonic and Biofilm Phenotypes. Sci. Rep. 2017, 7, 5911. [Google Scholar] [CrossRef] [Green Version]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, B.; Alshemary, A.Z.; Evis, Z. Co-Doped Hydroxyapatites as Potential Materials for Biomedical Applications. Microchem. J. 2019, 144, 443–453. [Google Scholar] [CrossRef]
- Pormohammad, A.; Turner, R.J. Silver Antibacterial Synergism Activities with Eight Other Metal(Loid)-Based Antimicrobials against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Damien, E.; Revell, P.A. Coralline Hydroxyapatite Bone Graft Substitute: A Review of Experimental Studies and Biomedical Applications. J. Appl. Biomater. Biomech. 2004, 2, 65–73. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Rózycka, D. Substituted Hydroxyapatites with Antibacterial Properties. Biomed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.; Tang, S.; Uskokovic, V. Biological and Medical Applications of Materials and Interfaces Calcium Phosphate Nanoparticles as Intrinsic Inorganic Antimicrobials: The Antibacterial Effect Calcium Phosphate Nanoparticles as Intrinsic Inorganic Antimicrobials: The Antibacterial Effe. ACS Appl. Mater. Interfaces 2018, 10, 34013–34028. [Google Scholar] [CrossRef]
- Sobierajska, P.; Nowak, N.; Rewak-Soroczynska, J.; Targonska, S.; Lewińska, A.; Grosman, L.; Wiglusz, R.J. Investigation of Topography Effect on Antibacterial Properties and Biocompatibility of Nanohydroxyapatites Activated with Zinc and Copper Ions: In Vitro Study of Colloids, Hydrogel Scaffolds and Pellets. Mater. Sci. Eng. C 2021, 134, 112547. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Applications. Nat. Publ. Gr. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural Properties and Antifungal Activity against Candida Albicans Biofilm of Different Composite Layers Based on Ag / Zn Doped. Polymers 2016, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Rewak-Soroczynska, J.; Dorotkiewicz-Jach, A.; Drulis-Kawa, Z. Culture Media Composition Influences the Antibacterial Effect of Silver, Cupric, and Zinc Ions against Pseudomonas aeruginosa. Biomolecules 2022, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Webster, T.J.; Massa-schlueter, E.A.; Smith, J.L.; Slamovich, E.B. Osteoblast Response to Hydroxyapatite Doped with Divalent and Trivalent Cations. Biomaterials 2004, 25, 2111–2121. [Google Scholar] [CrossRef]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current Status and Perspectives of Zinc-Based Absorbable Alloys for Biomedical Applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Zinc Stimulates Osteoblastogenesis and Suppresses Osteoclastogenesis by Antagonizing NF-ΚB Activation. Mol. Cell. Biochem. 2011, 355, 179–186. [Google Scholar] [CrossRef]
- Aina, V.; Bergandi, L.; Lusvardi, G.; Malavasi, G.; Imrie, F.E.; Gibson, I.R.; Cerrato, G.; Ghigo, D. Sr-Containing Hydroxyapatite: Morphologies of HA Crystals and Bioactivity on Osteoblast Cells. Mater. Sci. Eng. C 2013, 33, 1132–1142. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G. Strontium-Substituted Hydroxyapatite Coatings Synthesized by Pulsed-Laser Deposition: In Vitro Osteoblast and Osteoclast Response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lee, Y.T.; Huang, T.C.; Lin, G.S.; Chen, Y.W.; Lee, B.S.; Tung, K.L. In Vitro Bioactivity and Antibacterial Activity of Strontium-, Magnesium-, and Zinc-Multidoped Hydroxyapatite Porous Coatings Applied via Atmospheric Plasma Spraying. ACS Appl. Bio Mater. 2021, 4, 2523–2533. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Kolawole, S.K.; Liu, H.; Zhang, J.; Ren, L.; Yang, K. Synthesis, Characterization and in Vitro Evaluation of Zinc and Strontium Binary Doped Hydroxyapatite for Biomedical Application. Ceram. Int. 2020, 46, 14448–14459. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, P.N.; Shi, Z.; Neoh, K.G.; Ho, B.; Tay, B.Y. The Effects of Silver, Silicon-Containing Apatite towards Bacteria and Cell Responses. Biomed. Mater. 2014, 9, 015010. [Google Scholar] [CrossRef]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered Silica: Properties, Applications and Toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.J.; Seong, N.W.; So, B.J.; Seo, H.S.; Kim, J.H.; Hong, J.S.; Park, M.K.; Kim, M.S.; Kim, Y.R.; Cho, K.B.; et al. Evaluation of Silica Nanoparticle Toxicity after Topical Exposure for 90 Days. Int. J. Nanomed. 2014, 9, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Yoshioka, Y.; Takahashi, H.; Misato, K.; Mori, T.; Hirai, T.; Nagano, K.; Abe, Y.; Mukai, Y.; Kamada, H.; et al. Intestinal Absorption and Biological Effects of Orally Administered Amorphous Silica Particles. Nanoscale Res. Lett. 2014, 9, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciola, C.R.; Campoccia, D. Implant Infections: Adhesion, Biofilm Formation and Immune Evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Guan, R.; Johnson, I.; Cui, T.; Zhao, T.; Zhao, Z.; Li, X.; Liu, H. Alloy and in Vitro Evaluation of Degradation, Hemolysis, and Cytotoxicity. J. Biomed. Mater. Res. Part A 2012, 100, 999–1015. [Google Scholar] [CrossRef]
- Ames, B.N.; Durston, W.E.; Yamasaki, E.; Lee, F.D. Carcinogens are mutagens: A simple test system combining liver homogenates for activation and bacteria for detection. Proc. Natl. Acad. Sci. USA 1973, 70, 2281–2285. [Google Scholar] [CrossRef] [Green Version]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439804087. [Google Scholar]
- Fleet, M.E.; Liu, X.; Pan, Y. Site Preference of Rare Earth Elements in Hydroxyapatite [Ca10(PO4)6(OH)2]. J. Solid State Chem. 2000, 149, 391–398. [Google Scholar] [CrossRef]
- Zawisza, K.; Sobierajska, P.; Renaudin, G.; Nedelec, J.M.; Wiglusz, R.J. Effects of Crystalline Growth on Structural and Luminescence Properties of Ca(10-3: X)Eu2 x(PO4)6F2nanoparticles Fabricated by Using a Microwave Driven Hydrothermal Process. CrystEngComm 2017, 19, 6936–6949. [Google Scholar] [CrossRef]
- Barick, K.C.; Aslam, M.; Dravid, V.P.; Bahadur, D. Self-Aggregation and Assembly of Size-Tunable Transition Metal Doped ZnO Nanocrystals. J. Phys. Chem. C 2008, 112, 15163–15170. [Google Scholar] [CrossRef]
- Boal, A.K.; Ilhan, F.; Derouchey, J.E.; Thurn-Albrecht, T.; Russell, T.P.; Rotello, V.M. Self-Assembly of Nanoparticles into Structured Spherical and Network Aggregates. Nature 2000, 404, 746–748. [Google Scholar] [CrossRef]
- Lee, B.S.; Lin, H.P.; Chan, J.C.C.; Wang, W.C.; Hung, P.H.; Tsai, Y.H.; Lee, Y.L. A Novel Sol-Gel-Derived Calcium Silicate Cement with Short Setting Time for Application in Endodontic Repair of Perforations. Int. J. Nanomed. 2018, 13, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouard, N.; Caurant, D.; Majérus, O.; Dussossoy, J.L.; Loiseau, P.; Grygiel, C.; Peuget, S. External Irradiation with Heavy Ions of Neodymium Silicate Apatite Ceramics and Glass-Ceramics. J. Nucl. Mater. 2019, 516, 11–29. [Google Scholar] [CrossRef]
- Sobierajska, P.; Pazik, R.; Zawisza, K.; Renaudin, G.; Nedelec, J.-M.; Wiglusz, R.J. Effect of Lithium Substitution on the Charge Compensation, Structural and Luminescence Properties of Nanocrystalline Ca10(PO4)6F2 Activated with Eu3+ Ions. CrystEngComm 2016, 18, 3447–3455. [Google Scholar] [CrossRef]
- Targonska, S.; Wiglusz, R.J.; Szyszka, K.; Rewak-Soroczynska, J. New Approach to Spectroscopic and Structural Studies of the Nano-Sized Silicate-Substituted Hydroxyapatite Doped with Eu3+ Ions. Dalt. Trans. 2019, 48, 8303–8316. [Google Scholar] [CrossRef]
- Williams, R.L.; Hadley, M.J.; Jiang, P.J.; Rowson, N.A.; Mendes, P.M.; Rappoport, J.Z.; Grover, L.M. Thiol Modification of Silicon-Substituted Hydroxyapatite Nanocrystals Facilitates Fluorescent Labelling and Visualisation of Cellular Internalisation. J. Mater. Chem. B 2013, 1, 4370–4378. [Google Scholar] [CrossRef] [Green Version]
- Clegg, S.; Murphy, C.N. Epidemiology and Virulence of Klebsiella Pneumoniae. In Urinary Tract Infections: Molecular Pathogenesis and Clinical Management; John Wiley & Sons.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef] [Green Version]
- Van Laar, T.A.; Chen, T.; You, T.; Leung, P. Sublethal Concentrations of Carbapenems Alter Cell Morphology and Genomic Expression of Klebsiella Pneumoniae Biofilms. Antimicrob. Agents Chemother. 2015, 59, 1707–1717. [Google Scholar] [CrossRef]
- Deotale, V.S.; Attal, R.; Joshi, S.; Bankar, N. Correlation Between Biofilm Formation and Highly Drug Resistant Uropathogens (Hdru). Int. J. Curr. Res. Rev. 2015, 7, 61–65. [Google Scholar]
- Aditya, N.P.; Chimote, G.; Gunalan, K.; Banerjee, R.; Patankar, S.; Madhusudhan, B. Curcuminoids-Loaded Liposomes in Combination with Arteether Protects against Plasmodium Berghei Infection in Mice. Exp. Parasitol. 2012, 131, 292–299. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial Infections and Their Control Strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Ronde-oustau, C.; Lustig, S.; Dupieux, C.; Ferry, T. Implant-Associated ESBL- Klebsiella Pneumonia Producing Small Colony Variant Bone and Joint Infection in a Healthy 40-Year-Old Man. Case Rep. 2017, 1, 2016–2017. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, N.D.; Balu, R.; Sampath Kumar, T.S. Strontium-Substituted Calcium Deficient Hydroxyapatite Nanoparticles: Synthesis, Characterization, and Antibacterial Properties. J. Am. Ceram. Soc. 2012, 95, 2700–2708. [Google Scholar] [CrossRef]
- Karetsi, V.A.; Banti, C.N.; Kourkoumelis, N.; Papachristodoulou, C.; Stalikas, C.D. Antibiotics An E Ffi Cient Disinfectant, Composite Material {SLS@[Zn3(CitH)2]} as Ingredient for Development of Sterilized and Non Infectious Contact Lens. Antibiotics 2019, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewasthale, S.; Mani, I.; Vasdev, K. Microbial Biofilm: Current Challenges in Health Care Industry. J. Appl. Biotechnol. Bioeng. 2018, 5, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.M.; Johani, K.; Melo, D.S.; Lopes, L.K.O.; Lopes Lima, L.K.O.; Tipple, A.F.V.; Hu, H.; Vickery, K. Biofilm Contamination of High-Touched Surfaces in Intensive Care Units: Epidemiology and Potential Impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Kos, M.; Junka, A.; Smutnicka, D.; Szymczyk, P.; Gluza, K.; Bartoszewicz, M. Bisphosphonates Enhance Bacterial Adhesion and Biofilm Formation on Bone Hydroxyapatite. J. Cranio-Maxillofac. Surg. 2015, 43, 863–869. [Google Scholar] [CrossRef]
- Jaffar, N.; Miyazaki, T.; Maeda, T. Biofilm Formation of Periodontal Pathogens on Hydroxyapatite Surfaces: Implications for Periodontium Damage. J. Biomed. Mater. Res. Part A 2016, 104, 2873–2880. [Google Scholar] [CrossRef]
- Qiu, K.; Zhao, X.J.; Wan, C.X.; Zhao, C.S.; Chen, Y.W. Effect of Strontium Ions on the Growth of ROS17/2.8 Cells on Porous Calcium Polyphosphate Scaffolds. Biomaterials 2006, 27, 1277–1286. [Google Scholar] [CrossRef]
- Ni, G.X.; Yao, Z.P.; Huang, G.T.; Liu, W.G.; Lu, W.W. The Effect of Strontium Incorporation in Hydroxyapatite on Osteoblasts in Vitro. J. Mater. Sci. Mater. Med. 2011, 22, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-Edged Effects and Mechanisms of Zn2+ Microenvironments on Osteogenic Activity of BMSCs: Osteogenic Differentiation or Apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, Solubility, and Cytocompatibility of Zinc-Releasing Calcium Phosphate Ceramics. J. Biomed. Mater. Res. 2000, 50, 178–183. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-Substituted Hydroxyapatite: A Biomaterial with Enhanced Bioactivity and Antibacterial Properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of Silica Nanoparticles Depends on Size, Dose, and Cell Type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Ahamed, M. Silica Nanoparticles-Induced Cytotoxicity, Oxidative Stress and Apoptosis in Cultured A431 and A549 Cells. Hum. Exp. Toxicol. 2013, 32, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Manivasagam, G.; Kumar, P.; Ambasta, R.K. Cellular Toxicity of Mesoporous Silica Nanoparticle in SHSY5Y and BMMNCs Cell. Pharm. Nanotechnol. 2018, 6, 245–252. [Google Scholar] [CrossRef]
- Tank, K.P.; Chudasama, K.S.; Thaker, V.S.; Joshi, M.J. Pure and Zinc Doped Nano-Hydroxyapatite: Synthesis, Characterization, Antimicrobial and Hemolytic Studies. J. Cryst. Growth 2014, 401, 474–479. [Google Scholar] [CrossRef]
- Rozainah, N.; Abdul, N.; Nadhirah, A.; Sazri, M.; Yee, C.Y.; Luddin, N.; Ponnuraj, K.T. Genotoxicity Evaluation of Locally Produced Nano-Hydroxyapatite-Silica: An In Vitro Study Using the Bacterial Reverse Mutation Test. Dent. Med. Res. 2019, 7, 12–15. [Google Scholar] [CrossRef]
- Jantová, S.; Theiszová, M.; Letašiová, S.; Birošová, L.; Palou, T.M. In Vitro Effects of Fluor-Hydroxyapatite, Fluorapatite and Hydroxyapatite on Colony Formation, DNA Damage and Mutagenicity. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2008, 652, 139–144. [Google Scholar] [CrossRef] [PubMed]

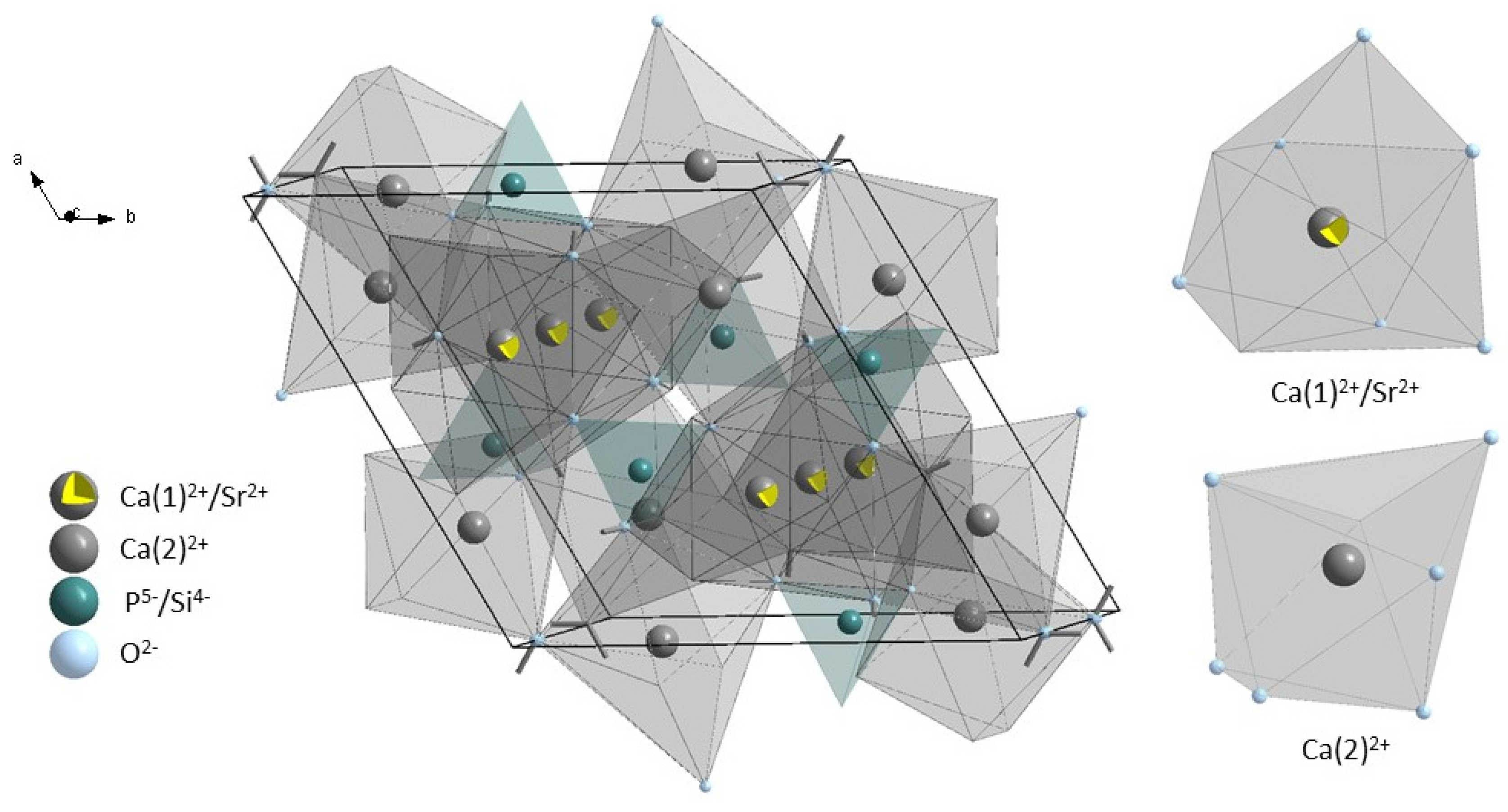

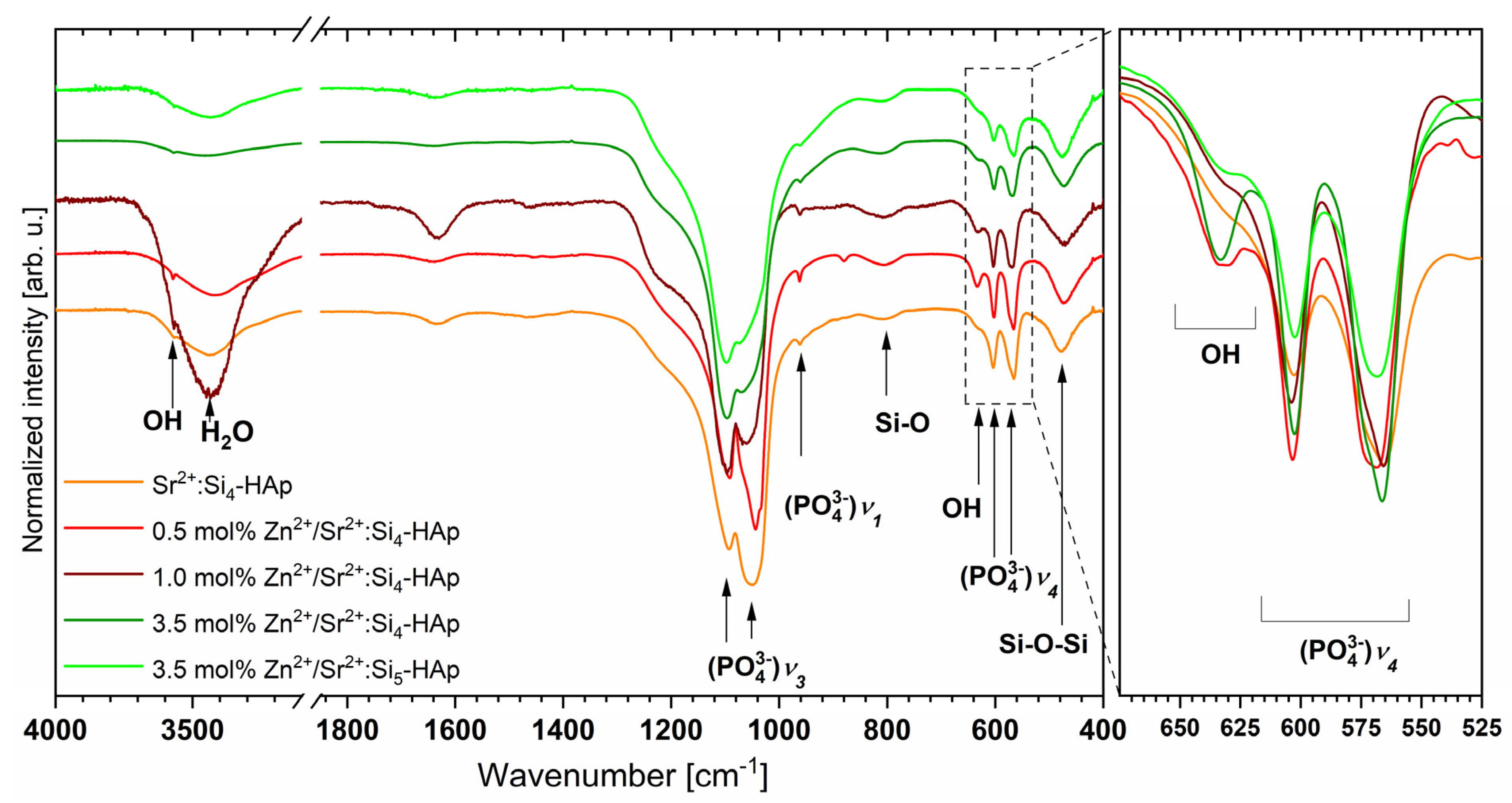
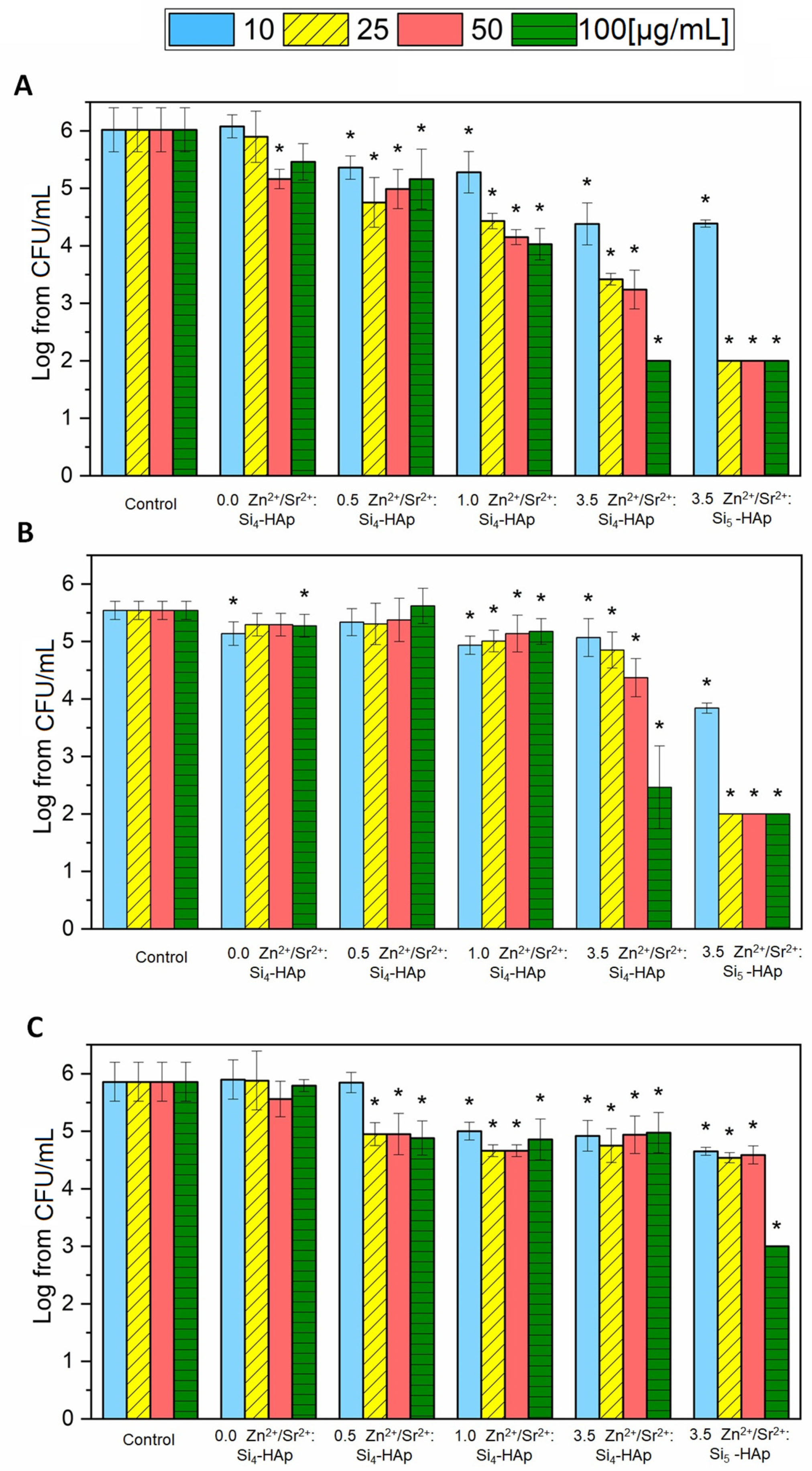
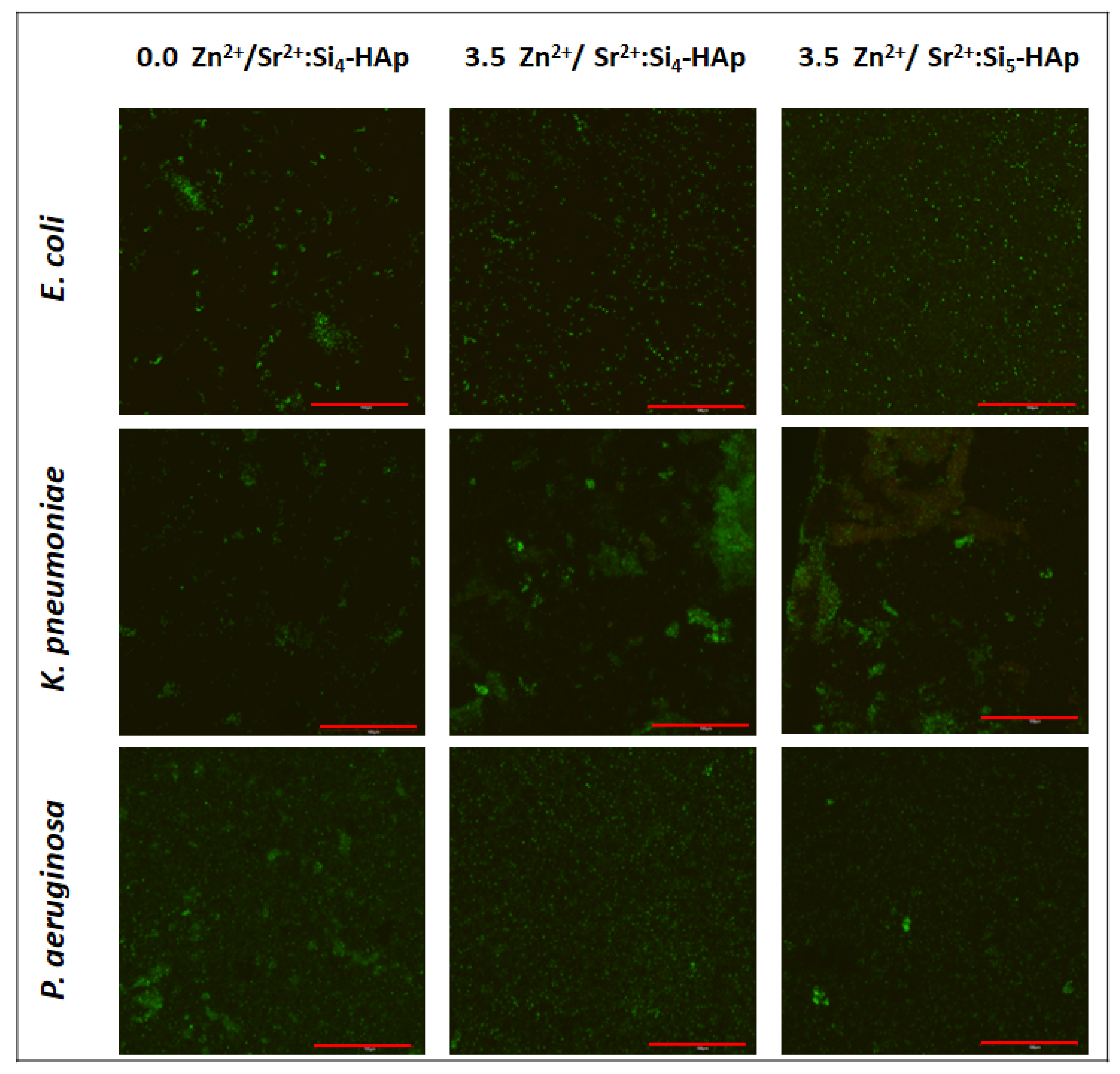
| Sample | Theoretical Chemical Formula | EDS Technique | ||
|---|---|---|---|---|
| n Sr2+ [mol%] | x Zn2+ [mol%] | y (SiO4)4− [mol%] | ||
| 0.0 mol% Zn2+/Sr2+:Si4-HAp | Ca9.8Sr0.2(PO4)2(SiO4)4(OH)2 | 0.19 | 0.00 | 3.40 |
| 0.5 mol%Zn2+/Sr2+:Si4-HAp | Ca9.3Sr0.2Zn0.5 (PO4)2(SiO4)4(OH)2 | 0.15 | 0.33 | 3.78 |
| 1.0 mol%Zn2+/Sr2+:Si4-HAp | Ca8.8Sr0.2Zn1 (PO4)2(SiO4)4(OH)2 | 0.13 | 1.12 | 3.64 |
| 3.5 mol%Zn2+/Sr2+:Si4-HAp | Ca6.3Sr0.2Zn3.5(PO4)2(SiO4)4(OH)2 | 0.17 | 2.49 | 3.79 |
| 3.5 mol%Zn2+/Sr2+:Si5-HAp | Ca6.3Sr0.2Zn3.5(PO4)(SiO4)5(OH)2 | 0.15 | 3.44 | 4.23 |
| Compound | Concentration [μg/mL] | S. Typhimurium TA98 | S. Typhimurium TA100 |
|---|---|---|---|
| Mutagenic Ratio (MR) | Mutagenic Ratio (MR) | ||
| Negative control | 1.00 | 1.00 | |
| Positive control | 13.20 | 9.14 | |
| 0.0 mol% Zn2+/Sr2+:Si4-HAp | 50 | 0.88 | 1.31 |
| 100 | 0.68 | 1.42 | |
| 0.5 mol% Zn2+/ Sr2+:Si4-HAp | 50 | 0.90 | 1.00 |
| 100 | 0.78 | 1.04 | |
| 1.0 mol% Zn2+/ Sr2+:Si4-HAp | 50 | 0.78 | 1.35 |
| 100 | 0.80 | 0.98 | |
| 3.5 mol% Zn2+/ Sr2+:Si4-HAp | 50 | 0.90 | 1.02 |
| 100 | 0.78 | 1.25 | |
| 3.5 mol% Zn2+/ Sr2+:Si5-HAp | 50 | 0.82 | 1.09 |
| 100 | 0.83 | 1.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rewak-Soroczynska, J.; Nowak, N.; Targonska, S.; Piecuch, A.; Wiglusz, R.J. The Study of Nanosized Silicate-Substituted Hydroxyapatites Co-Doped with Sr2+ and Zn2+ Ions Related to Their Influence on Biological Activities. Curr. Issues Mol. Biol. 2022, 44, 6229-6246. https://doi.org/10.3390/cimb44120425
Rewak-Soroczynska J, Nowak N, Targonska S, Piecuch A, Wiglusz RJ. The Study of Nanosized Silicate-Substituted Hydroxyapatites Co-Doped with Sr2+ and Zn2+ Ions Related to Their Influence on Biological Activities. Current Issues in Molecular Biology. 2022; 44(12):6229-6246. https://doi.org/10.3390/cimb44120425
Chicago/Turabian StyleRewak-Soroczynska, Justyna, Nicole Nowak, Sara Targonska, Agata Piecuch, and Rafal J. Wiglusz. 2022. "The Study of Nanosized Silicate-Substituted Hydroxyapatites Co-Doped with Sr2+ and Zn2+ Ions Related to Their Influence on Biological Activities" Current Issues in Molecular Biology 44, no. 12: 6229-6246. https://doi.org/10.3390/cimb44120425







