Combination of Two Rapid Ophthalmic Test Kits for Improved Diagnosis in Cases of Severe Binocular Conjunctivitis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Determination of Adenovirus from Conjunctival Sac and IgE in Tears
2.2.1. Capilia Adeno Eye®
2.2.2. Allerwatch® Tear IgE
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EKC | Epidemic keratoconjunctivitis |
| IC | Immunochromatography |
| EIA | Enzyme immunoassay |
| AKC | Atopic keratoconjunctivitis |
| VKC | vernal keratoconjunctivitis |
| PCR | Polymerase chain reaction |
References
- Chaberny, I.E.; Schnitzler, P.; Geiss, H.K.; Wendt, C. An outbreak of epidemic keratoconjunctivtis in a pediatric unit due to adenovirus type 8. Infect. Control Hosp. Epidemiol. 2003, 24, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Renard, G. Adenoviral keratoconjunctivitis. J. Fr. Ophtalmol. 2010, 33, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Aoki, K.; Ohguchi, T.; Ohgami, K.; Ohno, S.; Shiot, H.; Shrestha, J.K.; Upadhyay, M.P. Pathogenesis of infectious conjunctivitis in Nepal. J. Jpn. Ophthalmol. Soc. 2009, 113, 1088–1091. [Google Scholar]
- Uchio, E.; Aoki, K.; Saitoh, W.; Itoh, N.; Ohno, S. Rapid diagnosis of adenoviral conjunctivitis on conjunctival swabs by 10-min immunochromatography. Ophthalmology 1997, 104, 1294–1299. [Google Scholar] [CrossRef]
- Manners, T. Managing eye conditions in general practice. BMJ 1997, 315, 816–817. [Google Scholar] [CrossRef] [Green Version]
- Friedlaender, M.H. Conjunctivitis of allergic origin: Clinical presentation and differential diagnosis. Surv. Ophthalmol. 1993, 38, 105–114. [Google Scholar] [CrossRef]
- Abelson, M.B. A review of olopatadine for the treatment of ocular allergy. Exp. Opin. Pharmacother. 2004, 5, 1979–1994. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Amano, S.; Funatsu, H.; Arimoto, A.; Usui, T.; Ono, K.; Araie, M.; Okamoto, S. Allergens in Japanese patients with allergic conjunctivitis in autumn. Eye (Lond) 2005, 19, 995–999. [Google Scholar] [CrossRef] [Green Version]
- Liotet, S.; Warnet, V.N.; Arrata, M. Lacrimal immunoglobulin E and allergic conjunctivitis. Ophthalmologica 1983, 186, 31–34. [Google Scholar] [CrossRef]
- Sirbikaite, L.; Ehlers, N. The use of Lacrytest in ocular allergy. Acta Ophthalmol. Scand. 2007, 85, 117. [Google Scholar] [CrossRef]
- Abelson, M.B.; Smith, L.; Chapin, M. Ocular allergic disease: Mechanisms, disease sub-types, treatment. Ocul. Surf. 2003, 1, 127–149. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS Dry Eye Workshop II Report. Executive Summary. Ocul. Surf. 2017, 5, 802–812. [Google Scholar] [CrossRef]
- Bielory, L.; Friedlaender, M.H. Allergic conjunctivitis. Immunol. Allergy Clin. N. Am. 2008, 28, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Akanuma, M.; Ohguchi, T.; Ohgami, K.; Asato, Y.; Ohashi, T.; Shoji, J.; Norome, K.; Hinokuma, R.; Aoki, K.; et al. Evaluation of a rapid diagnostic kit, Capilia Adeno® Eye, for adenoviral conjunctivitis. Jpn. J. Clin. Ophthalmol. 2008, 62, 71–74. [Google Scholar]
- Mimura, T.; Usui, T.; Mori, M.; Funatsu, H.; Noma, H.; Aixinjueluo, W.; Yamamoto, H.; Amano, S. Rapid immunochromatography of total tear immunoglobulin E in allergic conjunctivitis with Allerwatch. J. Investig. Allergol. Clin. Immunol. 2010, 20, 627–628. [Google Scholar] [PubMed]
- Guglielmetti, S.; Dart, J.K.; Calder, V. Atopic keratoconjunctivitis and atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 478–485. [Google Scholar] [CrossRef]
- Calder, V.L.; Jolly, G.; Hingorani, M.; Adamson, P.; Leonardi, A.; Secchi, A.G.; Buckley, R.J.; Lightman, S. Cytokine production and mRNA expression by conjunctival T-cell lines in chronic allergic eye disease. Clin. Exp. Allergy 1999, 29, 1214–1222. [Google Scholar] [CrossRef]
- Leonardi, A.; De Dominicis, C.; Motterle, L. Immunopathogenesis of ocular allergy: A schematic approach to different clinical entities. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 429–435. [Google Scholar] [CrossRef]
- Leonardi, A.; Battista, M.C.; Gismondi, M.; Fregona, I.A.; Secchi, A.G. Antigen sensitivity evaluated by tear-specific and serum-specific IgE, skin tests, and conjunctival and nasal provocation tests in patients with ocular allergic disease. Eye (Lond) 1993, 7 Pt 3, 461–464. [Google Scholar] [CrossRef]
- Fujishima, H.; Sahashi, N.; Shimazaki, J.; Tsubota, K. Allergic conjunctivitis caused by sugi (Cryptomeria japonica D. Don) pollen out of season. Asian Pac. J. Allergy Immunol. 1995, 13, 113–117. [Google Scholar]
- Mimura, T.; Usui, T.; Mori, M.; Funatsu, H.; Noma, H.; Yamamoto, H.; Aixinjueluo, W.; Amano, S. Relationship between total tear and serum IgE in allergic conjunctivitis. Int. Arch. Allergy Immunol. 2011, 154, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, M.H. Overview of ocular allergy treatment. Curr. Allergy Asthma Rep. 2001, 1, 375–379. [Google Scholar] [CrossRef] [PubMed]




| Adenoviral Keratoconjunctivitis | ||
| Advantages | Disadvantages | |
| Cytologic examination | rapid | quite limited for the clinic |
| (mononuclear cells) | auxiliary diagnosis | |
| Genetic testing | rapid | require the well-equipped laboratory |
| (e.g., nested-PCR, real-time PCR) | high sensitivity | |
| Test kit (e.g., Capilia Adeno Eye®) | rapid | limited accuracy |
| (virus antigen) | easy-to-perform | |
| Allergic Conjunctivitis | ||
| Advantages | Disadvantages | |
| Cytologic examination | rapid | quite limited for the clinic |
| (eosinophils) | confirmed diagnosis | |
| Serum testing | confirmed diagnosis | time-consuming |
| (allergen-specific serum IgE) | require the well-equipped laboratory | |
| Test kit (e.g., Allerwatch®) | rapid | limited accuracy |
| (total tear IgE) | easy-to-perform |
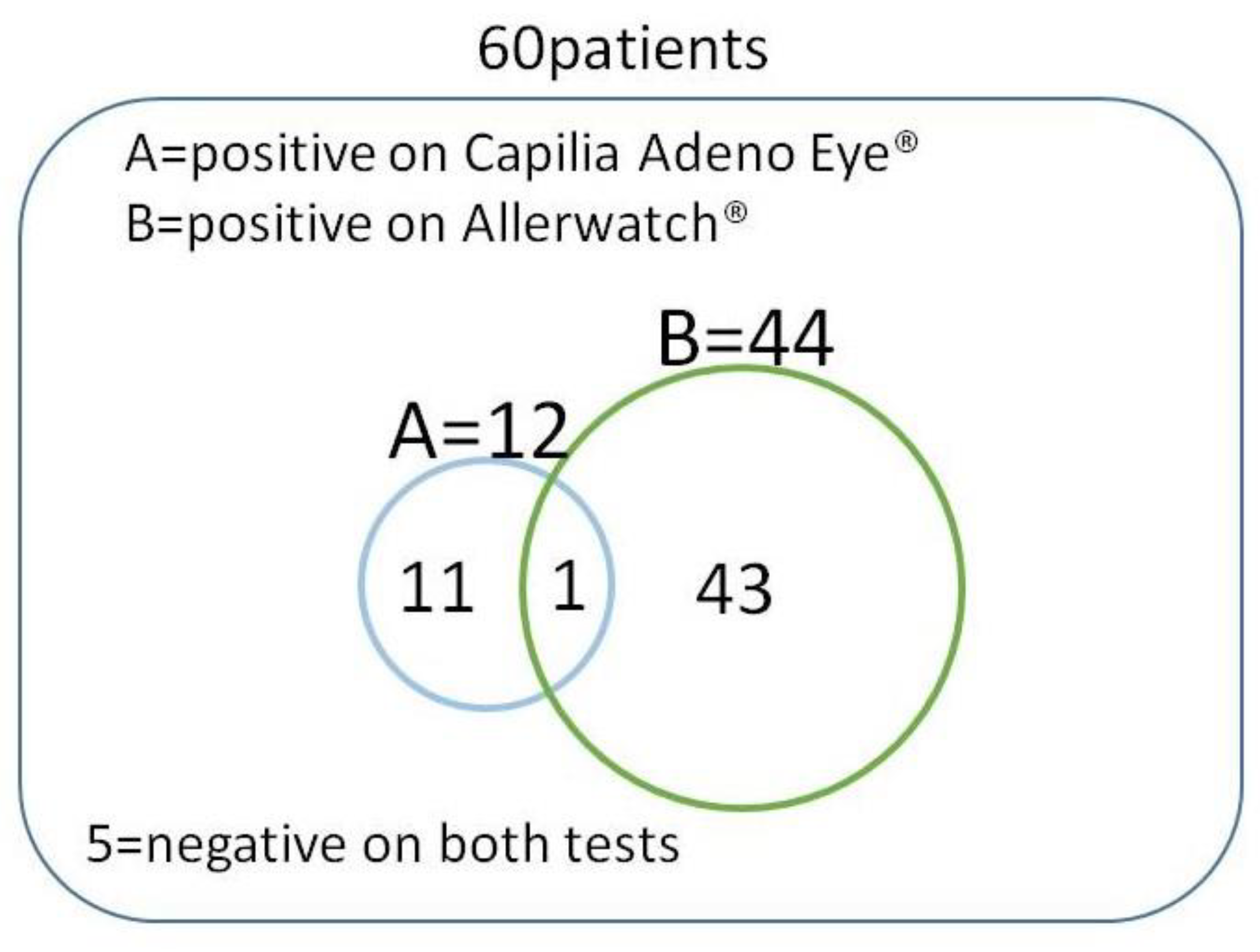
| Capilia Adeno Eye® | Allerwatch® | Capilia Adeno Eye® and/or Allerwatch® | |
|---|---|---|---|
| Positive | 12 | Grade2, 38 | 55 |
| Grade1, 6 | |||
| Negative | 48 | Grade0, 16 | 5 |
| Positivity Rate | 12/60 (20%) | 44/60 (73.3%) | 55/60 (91.7%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, A.; Kobayashi, F.; Yang, H.Y.; Fukagawa, K.; Yazu, H.; Fujishima, H. Combination of Two Rapid Ophthalmic Test Kits for Improved Diagnosis in Cases of Severe Binocular Conjunctivitis. Diagnostics 2020, 10, 109. https://doi.org/10.3390/diagnostics10020109
Kodama A, Kobayashi F, Yang HY, Fukagawa K, Yazu H, Fujishima H. Combination of Two Rapid Ophthalmic Test Kits for Improved Diagnosis in Cases of Severe Binocular Conjunctivitis. Diagnostics. 2020; 10(2):109. https://doi.org/10.3390/diagnostics10020109
Chicago/Turabian StyleKodama, Asako, Fumitaka Kobayashi, Hao Yung Yang, Kazumi Fukagawa, Hiroyuki Yazu, and Hiroshi Fujishima. 2020. "Combination of Two Rapid Ophthalmic Test Kits for Improved Diagnosis in Cases of Severe Binocular Conjunctivitis" Diagnostics 10, no. 2: 109. https://doi.org/10.3390/diagnostics10020109
APA StyleKodama, A., Kobayashi, F., Yang, H. Y., Fukagawa, K., Yazu, H., & Fujishima, H. (2020). Combination of Two Rapid Ophthalmic Test Kits for Improved Diagnosis in Cases of Severe Binocular Conjunctivitis. Diagnostics, 10(2), 109. https://doi.org/10.3390/diagnostics10020109






