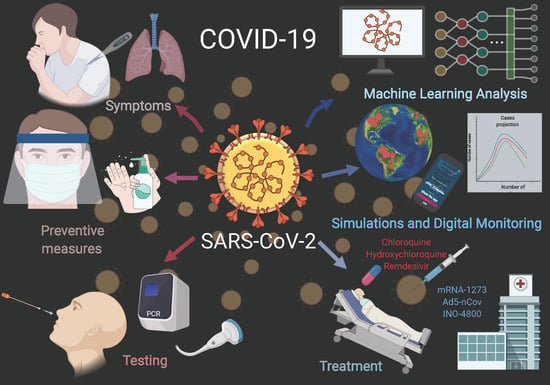
COVID-19 Diagnostics, Tools, and Prevention
Abstract
:1. Introduction
1.1. Coronaviruses
1.2. Immunity
1.3. Vaccines
2. COVID-19 Testing, Prevention Tools, and Tracking
2.1. Rapid Testing
2.2. Viral Transmission
2.3. Masks and Shields
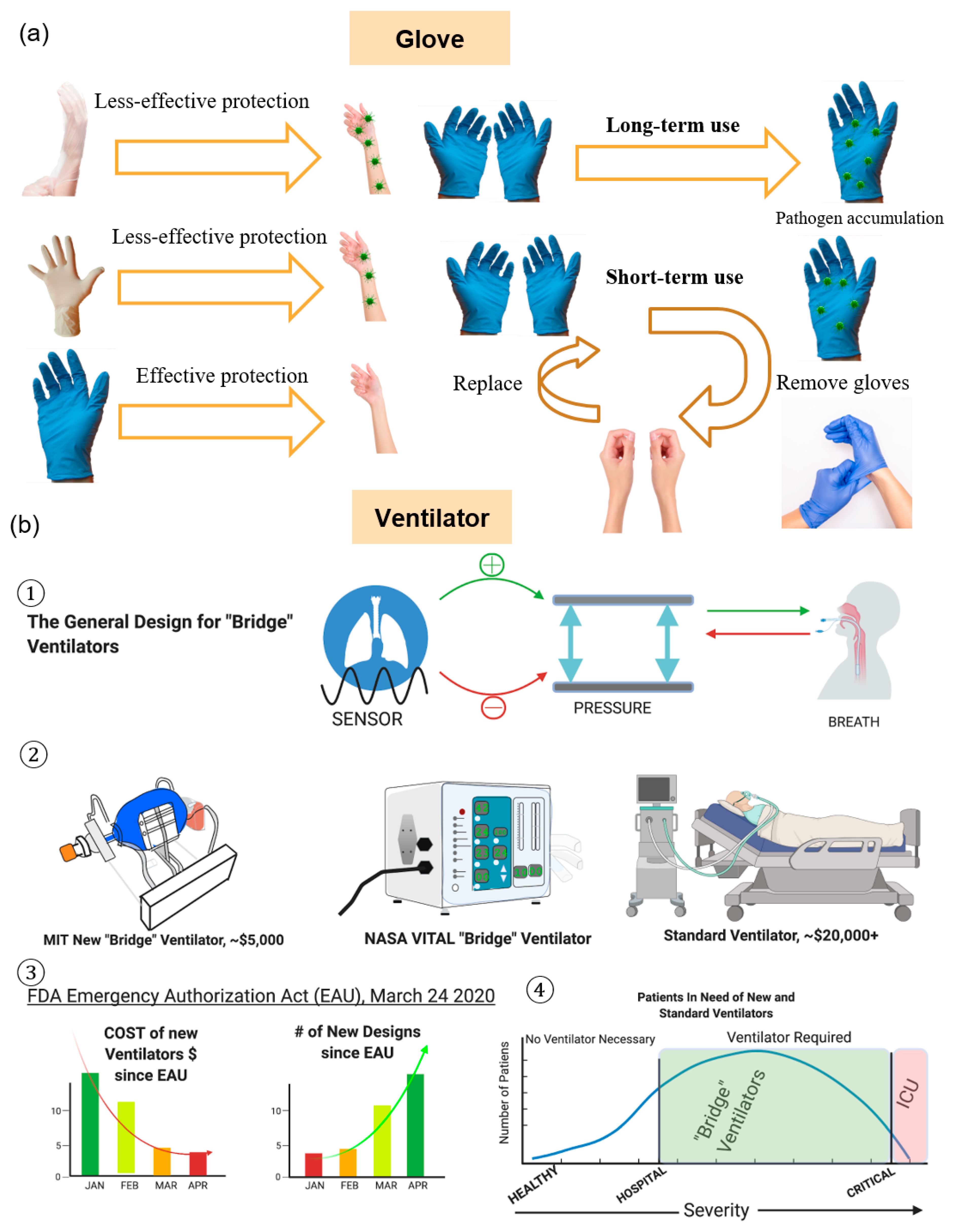
2.4. Gloves
2.5. Ventilators
2.6. Machine Learning Analysis of COVID-19
2.7. Pandemic Simulations
2.8. Digital Monitoring
3. Discussions
3.1. Vaccine Regulations
3.2. Testing Regulations
3.3. Immunity Ethics
3.4. Healthcare Impact
- Investing additional manpower, equipment, consumables, and other resources to ensure 100% preparedness for safety in the hospitals and eventual treatment of patients.
- Experiencing a sharp drop in the Outdoor Patient Department (OPD) patients, elective surgeries, and international patients.
3.5. Pharmaceutical Response
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Cruz, C.S.D.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Madhav, N.; Oppenheim, B.; Gallivan, M.; Mulembakani, P.; Rubin, E.; Wolfe, N. Pandemics: Risks, Impacts, and Mitigation. In Disease Control Priorities: Improving Health and Reducing Poverty; Jamison, D.T., Gelband, H., Horton, S., Jha, P., Laxminarayan, R., Mock, C.N., Nugent, R., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; ISBN 978-1-4648-0527-1. [Google Scholar]
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 16, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, V.C.C.; Lau, S.K.P.; Woo, P.C.Y.; Yuen, K.-Y. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection. Clin. Microbiol. Rev. 2007, 20, 660–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, N.; Shaib, H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 2019, 9, 35–42. [Google Scholar] [CrossRef]
- Fine, P.; Eames, K.; Heymann, D.L. “Herd Immunity”: A Rough Guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef]
- Mallory, M.L.; Lindesmith, L.C.; Baric, R.S. Vaccination-induced herd immunity: Successes and challenges. J. Allergy Clin. Immunol. 2018, 142, 64–66. [Google Scholar] [CrossRef] [Green Version]
- Weitz, J.S.; Beckett, S.J.; Coenen, A.R.; Demory, D.; Dominguez-Mirazo, M.; Dushoff, J.; Leung, C.-Y.; Li, G.; Măgălie, A.; Park, S.W.; et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat. Med. 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Touret, F.; De Lamballerie, X. Of chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef]
- Mahévas, M.; Tran, V.-T.; Roumier, M.; Chabrol, A.; Paule, R.; Guillaud, C.; Fois, E.; Lepeule, R.; Szwebel, T.-A.; Lescure, F.-X.; et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data. BMJ 2020, 369. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The Use of Antimalarial Drugs against Viral Infection. Microorganisms 2020, 8, 85. [Google Scholar] [CrossRef] [Green Version]
- Vausselin, T.; Calland, N.; Belouzard, S.; Descamps, V.; Douam, F.; Helle, F.; Francois, C.; Lavillette, D.; Duverlie, G.; Wahid, A.; et al. The antimalarial ferroquine is an inhibitor of hepatitis C virus. Hepatology 2013, 58, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.; Kalyesubula, I.; Bagenda, D.; Myers, C.; Olness, K. Effect of chloroquine on human immunodeficiency virus (HIV) vertical transmission. Afr. Health Sci. 2003, 3, 61–67. [Google Scholar] [PubMed]
- Clinical Trial Launches to Evaluate Antimalarial Drugs for COVID-19 Treatment. Available online: https://medicine.wustl.edu/news/clinical-trial-launches-to-evaluate-antimalarial-drugs-for-covid-19-treatment/ (accessed on 12 May 2020).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef] [Green Version]
- Delang, L.; Abdelnabi, R.; Neyts, J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 2018, 153, 85–94. [Google Scholar] [CrossRef]
- Guedj, J.; Piorkowski, G.; Jacquot, F.; Madelain, V.; Nguyen, T.H.T.; Rodallec, A.; Günther, S.; Carbonnelle, C.; Mentré, F.; Raoul, H.; et al. Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques. PLoS Med. 2018, 15, e1002535. [Google Scholar] [CrossRef] [Green Version]
- Chinese Clinical Trial Register (ChiCTR)-The World Health Organization International Clinical Trials Registered Organization Registered Platform. Available online: http://www.chictr.org.cn/showprojen.aspx?proj=49042 (accessed on 12 June 2020).
- Stanford University. A Phase 2 Randomized, Double Blinded, Placebo Controlled Study of Oral Favipiravir Compared to Current Standard of Care in Subjects With Mild or Asymptomatic COVID-19; ClinicalTrials.gov: Bethesda, ML, USA, 2020.
- Fujifilm Pharmaceuticals U.S.A., Inc. Open Label, Randomized, Controlled Phase 2 Proof-of-Concept Study of the Use of Favipiravir v. Standard of Care in Hospitalized Subjects With COVID-19; ClinicalTrials.gov: Bethesda, ML, USA, 2020.
- Varghese, F.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- U.S. Food & Drugs. FDA Letter to Stakeholders: Do Not Use Ivermectin Intended for Animals as Treatment for COVID-19 in Humans; FDA: Rockville, MD, USA, 2020. [Google Scholar]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Román, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Moderna’s Work on a Potential Vaccine against COVID-19|Moderna, Inc. Available online: https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19 (accessed on 12 May 2020).
- CanSino Biologics’ Ad5-nCoV the First COVID-19 Vaccine to Phase II Clinical Trials. Available online: https://www.trialsitenews.com/cansino-biologics-ad5-ncov-the-first-covid-19-vaccine-to-phase-ii-clinical-trials/ (accessed on 12 May 2020).
- INOVIO. Completes Enrollment in the Phase 1 U.S. Trial of INO-4800 for COVID-19 DNA Vaccine. Interim Results Expected in June. Available online: http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Completes-Enrollment-in-the-Phase-1-US-Trial-of-INO-4800-for-COVID-19-DNA-Vaccine-Interim-Results-Expected-in-June/default.aspx (accessed on 12 May 2020).
- COVID-19 Vaccine Candidates Show Progress Amid Challenges. Available online: https://www.scienceboard.net/index.aspx?sec=sup&sub=imm&pag=dis&ItemID=677 (accessed on 12 May 2020).
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2019, 94, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Antibody-Dependent Enhancement of SARS Coronavirus Infection and Its Role in the Pathogenesis of SARS. Available online: https://www.hkmj.org/abstracts/v22n3%20Suppl%204/25.htm (accessed on 13 June 2020).
- Weingartl, H.; Czub, M.; Czub, S.; Neufeld, J.; Marszal, P.; Gren, J.; Smith, G.; Jones, S.; Proulx, R.; Deschambault, Y.; et al. Immunization with Modified Vaccinia Virus Ankara-Based Recombinant Vaccine against Severe Acute Respiratory Syndrome Is Associated with Enhanced Hepatitis in Ferrets. J. Virol. 2004, 78, 12672–12676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houser, K.V.; Broadbent, A.J.; Gretebeck, L.; Vogel, L.; Lamirande, E.W.; Sutton, T.; Bock, K.W.; Minai, M.; Orandle, M.; Moore, I.N.; et al. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog. 2017, 13, e1006565. [Google Scholar] [CrossRef] [Green Version]
- Tetro, J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020, 22, 72–73. [Google Scholar] [CrossRef]
- Roche’s Cobas SARS-CoV-2 Test to Detect Novel Coronavirus Receives FDA Emergency Use Authorization and Is Available in Markets Accepting the CE Mark. Available online: https://www.roche.com/media/releases/med-cor-2020-03-13.htm (accessed on 12 May 2020).
- Xpert®® Xpress SARS-CoV-2 Has Received FDA Emergency Use Authorization. Available online: https://www.cepheid.com/coronavirus (accessed on 12 May 2020).
- Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes- 27 March 2020. Available online: https://abbott.mediaroom.com/2020-03-27-Abbott-Launches-Molecular-Point-of-Care-Test-to-Detect-Novel-Coronavirus-in-as-Little-as-Five-Minutes (accessed on 12 May 2020).
- Radiological Findings from 81 Patients with COVID-19 Pneumonia in Wuhan, China: A Descriptive Study-The Lancet Infectious Diseases. Available online: https://www.thelancet.com/article/S1473-3099(20)30086-4/fulltext (accessed on 12 May 2020).
- LabCorp, Quest Expand COVID-19 Antibody Testing Nationwide. Available online: https://www.medtechdive.com/news/labcorp-quest-expand-covid-19-antibody-testing-nationwide/576884/ (accessed on 12 May 2020).
- Corrales-Aguilar, E.; Trilling, M.; Reinhard, H.; Falcone, V.; Zimmermann, A.; Adams, O.; Santibanez, S.; Hengel, H. Highly individual patterns of virus-immune IgG effector responses in humans. Med. Microbiol. Immunol. 2016, 205, 409–424. [Google Scholar] [CrossRef] [Green Version]
- Research from University of Washington Demonstrates High Performance of Abbott’s SARS-CoV-2 Antibody Blood Test-8 May 2020. Available online: https://abbott.mediaroom.com/2020-05-08-Research-from-University-of-Washington-Demonstrates-High-Performance-of-Abbotts-SARS-CoV-2-Antibody-Blood-Test (accessed on 12 May 2020).
- UW Starts Testing People with COVID Antibody Test That Boasts Nearly 100% Accuracy. Available online: https://www.kiro7.com/news/local/uw-starts-testing-people-with-covid-antibody-test-that-boasts-nearly-100-accuracy/RFCEDOCPVJEWPMYKUVSEVRRPYQ/ (accessed on 12 May 2020).
- Abbott Launches Third COVID-19 Test, A Laboratory-Based Antibody Blood Test That Will Ship in the U.S. Starting Tomorrow-15 April 2020. Available online: https://abbott.mediaroom.com/2020-04-15-Abbott-Launches-Third-COVID-19-Test-a-Laboratory-Based-Antibody-Blood-Test-That-Will-Ship-in-the-U-S-Starting-Tomorrow (accessed on 12 May 2020).
- COVID-19. Antibody Testing|ARCpoint Labs. Available online: https://www.arcpointlabs.com/covid-19-antibody-testing/ (accessed on 12 May 2020).
- Antibody Tests Could Be Key to Reopening the Country. Here’s How They Work. Available online: https://www.usatoday.com/in-depth/news/2020/04/20/coronavirus-testing-how-antibody-tests-work-and-why-needed/2988440001/ (accessed on 12 May 2020).
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- COVID-19 and The Problem with Dental Aerosols. Available online: https://www.perioimplantadvisory.com/periodontics/oral-medicine-anesthetics-and-oral-systemic-connection/article/14173521/covid19-and-the-problem-with-dental-aerosols (accessed on 12 May 2020).
- Jordan, D.J.; Pritchard-Jones, R. Tying a surgical mask to prevent fogging. Ann. R. Coll. Surg. Engl. 2014, 96, 165. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jiang, A.; Gong, L.; Luo, L.; Guo, W.; Li, C.; Zheng, J.; Li, C.; Yang, B.; Zeng, J.; et al. Temperature significant change COVID-19 Transmission in 429 cities. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Caspi, G.; Shalit, U.; Kristensen, S.L.; Aronson, D.; Caspi, L.; Rossenberg, O.; Shina, A.; Caspi, O. Climate effect on COVID-19 spread rate: An online surveillance tool. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- In Response to COVID-19: A Technique to Improve the Viral Protection of a Dental Procedure Mask in Absence of an N95 Shield Respirator. Available online: https://www.perioimplantadvisory.com/periodontics/oral-medicine-anesthetics-and-oral-systemic-connection/article/14173091/in-response-to-covid19-a-technique-to-improve-the-viral-protection-of-a-dental-procedure-mask-in-absence-of-an-n95-shield-respirator (accessed on 12 May 2020).
- Kroo, L.; Kothari, A.; Hannebelle, M.; Herring, G.; Pollina, T.; Md, R.C.; Banavar, S.P.; Flaum, E.; Soto-Montoya, H.; Li, H.; et al. Pneumask: Modified Full-Face Snorkel Masks as Reusable Personal Protective Equipment for Hospital Personnel. medRxiv 2020. [Google Scholar] [CrossRef]
- Technologies & Start-ups—Duke OLV. Available online: https://olv.duke.edu/covid-19/technologies-and-startups/ (accessed on 12 May 2020).
- Efficiency of HEPA Filters. Available online: https://www.hamilton-medical.com/en_EG/E-Learning-and-Education/Knowledge-Base/Knowledge-Base-Detail~2020-03-18~Efficiency-of-HEPA-filters~d5358f88-753e-4644-91c6-5c7b862e941f~.html#DataTables_Table_0=od3 (accessed on 12 May 2020).
- CDC. Coronavirus Disease 2019 (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html (accessed on 12 May 2020).
- Good Samaritan Drug and Medical Supply Donation Act-American Legislative Exchange Council. Available online: https://www.alec.org/model-policy/good-samaritan-drug-and-medical-supply-donation-act/ (accessed on 12 May 2020).
- Center for Devices and Radiological Health. Surgical Masks-Premarket Notification [510(k)] Submissions. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/surgical-masks-premarket-notification-510k-submissions (accessed on 12 May 2020).
- Clifton, W.; Damon, A.; Martin, A.K. Considerations and Cautions for Three-Dimensional-Printed Personal Protective Equipment in the COVID-19 Crisis. 3D Print. Addit. Manuf. 2020, 7, 97–99. [Google Scholar] [CrossRef]
- Roberge, R.J. Face shields for infection control: A review. J. Occup. Environ. Hyg. 2016, 13, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W.G.; Noti, J.D.; Blachere, F.M.; Szalajda, J.V.; Beezhold, D. Efficacy of face shields against cough aerosol droplets from a cough simulator. J. Occup. Environ. Hyg. 2014, 11, 509–518. [Google Scholar] [CrossRef] [PubMed]
- UPDATED. 2nd April -From Design to Mass 3D Printing of Medical Shields in Three Days. Available online: https://blog.prusaprinters.org/from-design-to-mass-3d-printing-of-medical-shields-in-three-days/ (accessed on 12 May 2020).
- 3D printed face shields for medics and professionals. Prusa3D-3D Printers from Josef Průša. Available online: https://www.prusa3d.com/covid19/ (accessed on 12 May 2020).
- Face Shields—Rapid Response. Available online: https://pwp.gatech.edu/rapid-response/face-shields/ (accessed on 12 May 2020).
- MIT Initiates Mass Manufacture of Disposable Face Shields for Covid-19 Response. Available online: http://news.mit.edu/2020/face-shield-ppe-manufacture-covid-19-0331 (accessed on 12 May 2020).
- HappyShield. Available online: https://happyshield.github.io/en/ (accessed on 12 May 2020).
- Covid-19: We Help You! Available online: https://www.umdasch.com/en/company/news-and-press/covid-19-we-help-you (accessed on 12 May 2020).
- GLASSAFE-Aviointeriors. Available online: http://aviointeriors.it/2020/press/glassafe/ (accessed on 12 May 2020).
- JANUS SEAT. Available online: http://aviointeriors.it/2020/press/janus-seat/ (accessed on 12 May 2020).
- CDC Communities, Schools, Workplaces, & Events. Available online: https://www.cdc.gov/coronavirus/2019-ncov/community/reopen-guidance.html (accessed on 12 May 2020).
- Banned Devices; Powdered Surgeon’s Gloves, Powdered Patient Examination Gloves, and Absorbable Powder for Lubricating a Surgeon’s Glove. Available online: https://www.federalregister.gov/documents/2016/12/19/2016-30382/banned-devices-powdered-surgeons-gloves-powdered-patient-examination-gloves-and-absorbable-powder (accessed on 12 May 2020).
- Beitler, J.R.; Malhotra, A.; Thompson, B.T. Ventilator-induced Lung Injury. Clin. Chest Med. 2016, 37, 633–646. [Google Scholar] [CrossRef]
- University, S. Stanford Student Helps Design Ventilator for COVID-19 Patients in Peru. Available online: https://news.stanford.edu/2020/04/23/stanford-student-helps-design-ventilator-covid-19-patients-peru/ (accessed on 12 May 2020).
- MIT E-VENT|Emergency Ventilator Design Toolbox. Available online: https://e-vent.mit.edu/ (accessed on 12 May 2020).
- MIT-Based Team Works on Rapid Deployment of Open-Source, Low-Cost Ventilator. Available online: http://news.mit.edu/2020/ventilator-covid-deployment-open-source-low-cost-0326 (accessed on 12 May 2020).
- Simple, Low-Cost Ventilator Builds on Available Resuscitation Bags. Available online: https://news.gatech.edu/2020/04/06/simple-low-cost-ventilator-builds-available-resuscitation-bags (accessed on 12 May 2020).
- Greicius, T. NASA Develops COVID-19 Prototype Ventilator in 37 Days. Available online: http://www.nasa.gov/feature/jpl/nasa-develops-covid-19-prototype-ventilator-in-37-days (accessed on 12 May 2020).
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052. [Google Scholar] [CrossRef] [PubMed]
- AlphaFold: Using AI for Scientific Discovery. Available online: /blog/article/AlphaFold-Using-AI-for-scientific-discovery (accessed on 7 May 2020).
- Zhavoronkov, A. Artificial Intelligence for Drug Discovery, Biomarker Development, and Generation of Novel Chemistry. Mol. Pharm. 2018, 15, 4311–4313. [Google Scholar] [CrossRef] [Green Version]
- A Data-Driven Drug Repositioning Framework Discovered a Potential Therapeutic Agent Targeting COVID-19|BioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2020.03.11.986836v1 (accessed on 7 May 2020).
- Yan, L.; Zhang, H.-T.; Xiao, Y.; Wang, M.; Sun, C.; Liang, J.; Li, S.; Zhang, M.; Guo, Y.; Xiao, Y.; et al. Prediction of criticality in patients with severe Covid-19 infection using three clinical features: A machine learning-based prognostic model with clinical data in Wuhan. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Deng, X.; Fu, Q.; Zhou, Q.; Feng, J.; Ma, H.; Liu, W.; Wang, X. Deep Learning-based Detection for COVID-19 from Chest CT using Weak Label. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, R.; Barbastathis, G. Quantifying the effect of quarantine control in Covid-19 infectious spread using machine learning. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Ge, Q.; Li, S.; Jin, L.; Xiong, M. Artificial Intelligence Forecasting of Covid-19 in China. arXiv 2020, arXiv:2002.07112. [q-bio]. [Google Scholar]
- Bershteyn, A.; Gerardin, J.; Bridenbecker, D.; Lorton, C.W.; Bloedow, J.; Baker, R.S.; Chabot-Couture, G.; Chen, Y.; Fischle, T.; Frey, K.; et al. Implementation and applications of EMOD, an individual-based multi-disease modeling platform. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- These Simulations Show How to Flatten the Coronavirus Growth Curve. Available online: https://www.washingtonpost.com/graphics/2020/world/corona-simulator/ (accessed on 12 May 2020).
- Agent-Based Simulation of COVID-19 Health and Economical Effects. Available online: https://towardsdatascience.com/agent-based-simulation-of-covid-19-health-and-economical-effects-6aa4ae0ff397 (accessed on 12 May 2020).
- COVID-19 HPC Consortium. Available online: https://covid19-hpc-consortium.org/ (accessed on 12 May 2020).
- Smith, M.; Smith, J.C. Repurposing Therapeutics for COVID-19: Supercomputer-Based Docking to the SARS-CoV-2 Viral Spike Protein and Viral Spike Protein-Human ACE2 Interface. ChemRxiv 2020. [Google Scholar] [CrossRef]
- PostEra|COVID-19. Available online: https://covid.postera.ai/covid (accessed on 12 May 2020).
- Folding@home—Fighting Disease with a World Wide distributed super computer. Available online: https://foldingathome.org/ (accessed on 12 May 2020).
- Cruz, M.A.; Frederick, T.E.; Singh, S.; Vithani, N.; Zimmerman, M.I.; Porter, J.R.; Moeder, K.E.; Amarasinghe, G.K.; Bowman, G.R. Discovery of a cryptic allosteric site in Ebola’s ‘undruggable’ VP35 protein using simulations and experiments. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Rosetta’s role in fighting coronavirus—Institute for Protein Design. Available online: https://www.ipd.uw.edu/2020/02/rosettas-role-in-fighting-coronavirus/ (accessed on 12 May 2020).
- Cho, H.; Ippolito, D.; Yu, Y.W. Contact Tracing Mobile Apps for COVID-19: Privacy Considerations and Related Trade-offs. arXiv 2020, arXiv:2003.11511. [cs]. [Google Scholar]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.G.; Fraser, C. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020, 368, eabb6936. [Google Scholar] [CrossRef] [Green Version]
- Ting, D.; Carin, L.; Dzau, V.; Wong, T.Y. Digital technology and COVID-19. Nat. Med. 2020, 26, 459–461. [Google Scholar] [CrossRef] [Green Version]
- Raskar, R.; Schunemann, I.; Barbar, R.; Vilcans, K.; Gray, J.; Vepakomma, P.; Kapa, S.; Nuzzo, A.; Gupta, R.; Berke, A.; et al. Apps Gone Rogue: Maintaining Personal Privacy in an Epidemic. arXiv 2020, arXiv:2003.08567. [cs]. [Google Scholar]
- Maghdid, H.S.; Ghafoor, K.Z.; Sadiq, A.S.; Curran, K.; Rabie, K. A Novel AI-enabled Framework to Diagnose Coronavirus COVID 19 using Smartphone Embedded Sensors: Design Study. arXiv 2020, arXiv:2003.07434. [cs, q-bio]. [Google Scholar]
- Rao, A.S.R.S.; Vazquez, J.A. Identification of COVID-19 can be quicker through artificial intelligence framework using a mobile phone–based survey when cities and towns are under quarantine. Infect. Control. Hosp. Epidemiol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healthy Together|COVID-19. Available online: https://www.healthytogether.io (accessed on 12 June 2020).
- Australian Government Department of Health of COVIDSafe App. Available online: https://www.health.gov.au/resources/apps-and-tools/covidsafe-app (accessed on 12 June 2020).
- COVID Symptom Study-Help Slow the Spread of COVID-19. Available online: https://covid.joinzoe.com/us (accessed on 12 May 2020).
- HowWeFeel-Join the Global Movement. Available online: https://howwefeel.org/ (accessed on 12 May 2020).
- Boston, 677 Huntington Avenue; Ma 02115 +1495-1000 Surveys, Apps to Track COVID-19. Available online: https://www.hsph.harvard.edu/coronavirus/surveys-apps-to-track-covid-19/ (accessed on 12 May 2020).
- Chan, J.; Foster, D.; Gollakota, S.; Horvitz, E.; Jaeger, J.; Kakade, S.; Kohno, T.; Langford, J.; Larson, J.; Singanamalla, S.; et al. PACT: Privacy Sensitive Protocols and Mechanisms for Mobile Contact Tracing. arXiv 2020, arXiv:2004.03544. [cs]. [Google Scholar]
- TraceTogether. Available online: https://www.tracetogether.gov.sg/ (accessed on 12 May 2020).
- CovidSafe. Available online: https://covidsafe.cs.washington.edu/ (accessed on 12 May 2020).
- PACT: Private Automated Contact Tracing. Available online: https://pact.mit.edu/ (accessed on 12 May 2020).
- Lampos, V.; Moura, S.; Yom-Tov, E.; Cox, I.J.; McKendry, R.; Edelstein, M. Tracking COVID-19 using online search. arXiv 2020, arXiv:2004.07183v2. [cs.CY]. [Google Scholar]
- El-Aziz, T.M.A.; Stockand, J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020, 83, 104327. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Tracker|RAPS. Available online: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker?feed=Regulatory-Focus?utm_source=Facebook&utm_medium=social&utm_campaign=Regulatory-Focus (accessed on 12 May 2020).
- Padron-Regalado, E. Vaccines for SARS-CoV-2: Lessons from Other Coronavirus Strains. Infect. Dis. Ther. 2020, 9, 255–274. [Google Scholar] [CrossRef] [Green Version]
- AstraZeneca and Oxford University Announce Landmark Agreement for COVID-19 Vaccine. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html (accessed on 12 May 2020).
- April 30, CBS EVENING NEWS; 2020; Am, 7:07 Oxford Scientists Say a Vaccine May Be Widely Available by September. Available online: https://www.cbsnews.com/news/coronavirus-vaccine-september-oxford-university/ (accessed on 12 May 2020).
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Administrator, J. Website Global Progress on COVID-19 Serology-Based Testing. Available online: https://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html (accessed on 12 May 2020).
- The Latest on Coronavirus Testing: New Methods, Accuracy, and Availability-GoodRx. Available online: https://www.goodrx.com/blog/coronavirus-covid-19-testing-updates-methods-cost-availability/ (accessed on 12 May 2020).
- Phelan, A.L. COVID-19 immunity passports and vaccination certificates: Scientific, equitable, and legal challenges. Lancet 2020, 395, 1595–1598. [Google Scholar] [CrossRef]
- Lancet, T. COVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- NIH to Launch Public-Private Partnership to Speed COVID-19 Vaccine and Treatment Options. Available online: https://www.nih.gov/news-events/news-releases/nih-launch-public-private-partnership-speed-covid-19-vaccine-treatment-options (accessed on 12 May 2020).
- An Update on Abbott’s Work on COVID-19 Testing. Available online: https://www.abbott.com/corpnewsroom/product-and-innovation/an-update-on-abbotts-work-on-COVID-19-testing.html (accessed on 12 May 2020).
- AbbVie’s Update on COVID-19 and the Coronavirus Crisis. Available online: https://www.abbvie.com/coronavirus.html (accessed on 12 May 2020).
- Can Therapeutic Antibodies Offer Hope for COVID-19 Patients? Available online: https://www.amgen.com/media/featured-news/2020/04/can-therapeutic-antibodies-offer-hope-for-covid-19-patients/ (accessed on 12 May 2020).
- COVID-19 Information Hub. Available online: https://www.astrazeneca.com/media-centre/articles/2020/covid-19-information-hub.html (accessed on 12 May 2020).
- Pfizer and BioNTech Dose First Participants in the U.S. as Part of Global COVID-19 mRNA Vaccine Development Program|Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_and_biontech_dose_first_participants_in_the_u_s_as_part_of_global_covid_19_mrna_vaccine_development_program (accessed on 12 May 2020).
- Updated Statement: Bristol Myers Squibb on Coronavirus (COVID-19)|BMS Newsroom. Available online: https://news.bms.com/press-release/corporatefinancial-news/statement-bristol-myers-squibb-2019-novel-coronavirus (accessed on 12 May 2020).
- Lilly Begins Clinical Testing of Therapies for COVID-19. Available online: https://investor.lilly.com/news-releases/news-release-details/lilly-begins-clinical-testing-therapies-covid-19 (accessed on 12 May 2020).
- Evotec Statement on COVID-19-Evotec. Available online: https://www.evotec.com/en/invest/news--announcements/p/evotec-statement-on-covid-19-5911 (accessed on 12 May 2020).
- Gilead Announces Results from Phase 3 Trial of Investigational Antiviral Remdesivir in Patients with Severe COVID-19. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2020/4/gilead-announces-results-from-phase-3-trial-of-investigational-antiviral-remdesivir-in-patients-with-severe-covid-19 (accessed on 12 May 2020).
- Sanofi and GSK to Join Forces in Unprecedented Vaccine Collaboration to Fight COVID-19|GSK. Available online: https://www.gsk.com/en-gb/media/press-releases/sanofi-and-gsk-to-join-forces-in-unprecedented-vaccine-collaboration-to-fight-covid-19/ (accessed on 12 May 2020).
- Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19; Landmark New Partnership with U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use|Johnson & Johnson. Available online: https://www.jnj.com/johnson-johnson-announces-a-lead-vaccine-candidate-for-covid-19-landmark-new-partnership-with-u-s-department-of-health-human-services-and-commitment-to-supply-one-billion-vaccines-worldwide-for-emergency-pandemic-use (accessed on 12 May 2020).
- Home-KSQ Therapeutics. Available online: https://ksqtx.com/#discovery (accessed on 12 May 2020).
- Merck and Institute for Systems Biology Collaborate to Define Molecular Mechanisms of SARS-CoV-2 Infection and Identify Potential Prognostic Biomarkers|Merck Newsroom Home. Available online: https://www.mrknewsroom.com/news-release/research-and-development-news/merck-and-institute-systems-biology-collaborate-define-mo (accessed on 12 May 2020).
- NIH Clinical Trial of Investigational Vaccine for COVID-19 Begins. Available online: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed on 12 May 2020).
- Novartis Announces Plan to Initiate Clinical Trial of Canakinumab for Patients with COVID-19 Pneumonia. Available online: https://www.novartis.com/news/novartis-announces-plan-initiate-clinical-trial-canakinumab-patients-covid-19-pneumonia (accessed on 12 May 2020).
- Lab Diagnostics Systems, Instruments, Assays, and Tests|Roche Diagnostics USA. Available online: https://diagnostics.roche.com/us/en/home.html (accessed on 12 May 2020).
- COVID-19: Working Together to Go Faster for Patients. Available online: https://www.takeda.com/newsroom/featured-topics/working-together-to-go-faster-for-patients/ (accessed on 12 May 2020).
- Vir Biotechnology Proceeding with Two Clinical Development Candidates for COVID-19. Available online: https://investors.vir.bio/news-releases/news-release-details/vir-biotechnology-proceeding-two-clinical-development-candidates (accessed on 12 May 2020).






| Company | Field of COVID-19 Work | Description of Work |
|---|---|---|
| Abbott | Diagnostics | Working on molecular (m2000 and ID NOW) and antibody tests [126] |
| Abbvie | Treatment | Adopting its antiretroviral therapy of HIV (Lopinavir/Ritonavir) for COVID-19 [127] |
| Amgen and Adaptive Biotechnologies | Diagnostics and Treatment | Researching neutralizing antibodies to prevent and treat COVID-19 [128] |
| AstraZeneca | Diagnostics, Prevention, and Treatment | Collaborating with different university labs to manufacture COVID-19 diagnostics, treatment, and vaccines [129] |
| BioNTech and Pfizer | Prevention | Collaborating on a COVID-19 vaccine [130] |
| Bristol Myers Squibb | Treatment | Collaborating with the community to accelerate therapies via potential molecule screening and testing current medications [131] |
| Eli Lilly | Treatment | Adopting its rheumatoid arthritis therapy (Baricitinib) and other antibody therapies for COVID-19 [132] |
| Evotec | Diagnostics and Treatment | Collaborating with the community to accelerate therapies via potential antibody screening [133] |
| Gilead | Treatment | Adopting its antiviral therapy (Remdesivir) for COVID-19 [134] |
| GlaxoSmithKline and Sanofi (GSK) | Prevention | Collaborating on a COVID-19 vaccine using GSK’s pandemic adjuvant technology and Sanofi’s S-protein COVID-19 antigen [135] |
| Johnson & Johnson and Janssen | Prevention | Collaborating on a COVID-19 vaccine using Janssen’s AdVac® and PER.C6® technology [136] |
| KSQ Therapeutics | Treatment | Using its CRISPRomics® to discover targets for the current COVID-19 treatments [137] |
| Merck | Prevention and Treatment | Researching the molecular mechanism of COVID-19 to identify potential vaccine and medicine targets for COVID-19 [138] |
| Moderna | Prevention | Collaborating on a COVID-19 vaccine using mRNA technology [139] |
| Novartis | Prevention | Adopting its autoimmune disease therapy (Canakinumab) for COVID-19 [140] |
| Roche | Diagnostics and Treatment | Researching COVID-19 antibody tests [141] |
| Takeda, CSL Behring, Biotest, BPL, LFB, and Octapharma | Treatment | Adopting hyperimmune globulin for COVID-19 treatment [142] |
| Vir Biotechnology | Diagnostics and Treatment | Researching neutralizing antibodies to prevent and treat COVID-19 [143] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allam, M.; Cai, S.; Ganesh, S.; Venkatesan, M.; Doodhwala, S.; Song, Z.; Hu, T.; Kumar, A.; Heit, J.; Study Group, C.-1.; et al. COVID-19 Diagnostics, Tools, and Prevention. Diagnostics 2020, 10, 409. https://doi.org/10.3390/diagnostics10060409
Allam M, Cai S, Ganesh S, Venkatesan M, Doodhwala S, Song Z, Hu T, Kumar A, Heit J, Study Group C-1, et al. COVID-19 Diagnostics, Tools, and Prevention. Diagnostics. 2020; 10(6):409. https://doi.org/10.3390/diagnostics10060409
Chicago/Turabian StyleAllam, Mayar, Shuangyi Cai, Shambavi Ganesh, Mythreye Venkatesan, Saurabh Doodhwala, Zexing Song, Thomas Hu, Aditi Kumar, Jeremy Heit, COVID-19 Study Group, and et al. 2020. "COVID-19 Diagnostics, Tools, and Prevention" Diagnostics 10, no. 6: 409. https://doi.org/10.3390/diagnostics10060409
APA StyleAllam, M., Cai, S., Ganesh, S., Venkatesan, M., Doodhwala, S., Song, Z., Hu, T., Kumar, A., Heit, J., Study Group, C.-1., & Coskun, A. F. (2020). COVID-19 Diagnostics, Tools, and Prevention. Diagnostics, 10(6), 409. https://doi.org/10.3390/diagnostics10060409