Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients
Abstract
:1. Introduction
2. Materials and Methods
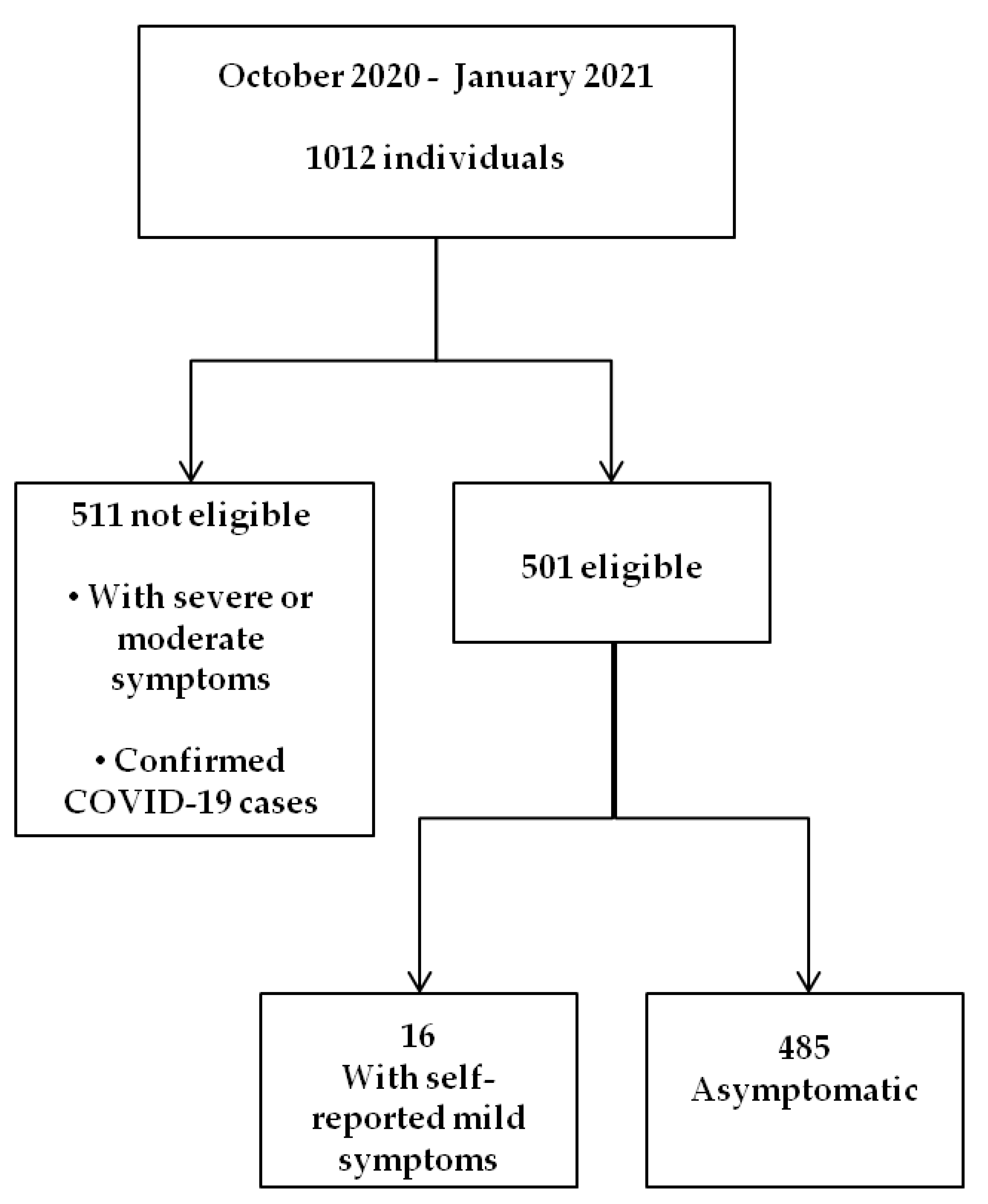
2.1. Population Study and Informed Consent
2.2. Sample Collection
2.3. Study Design
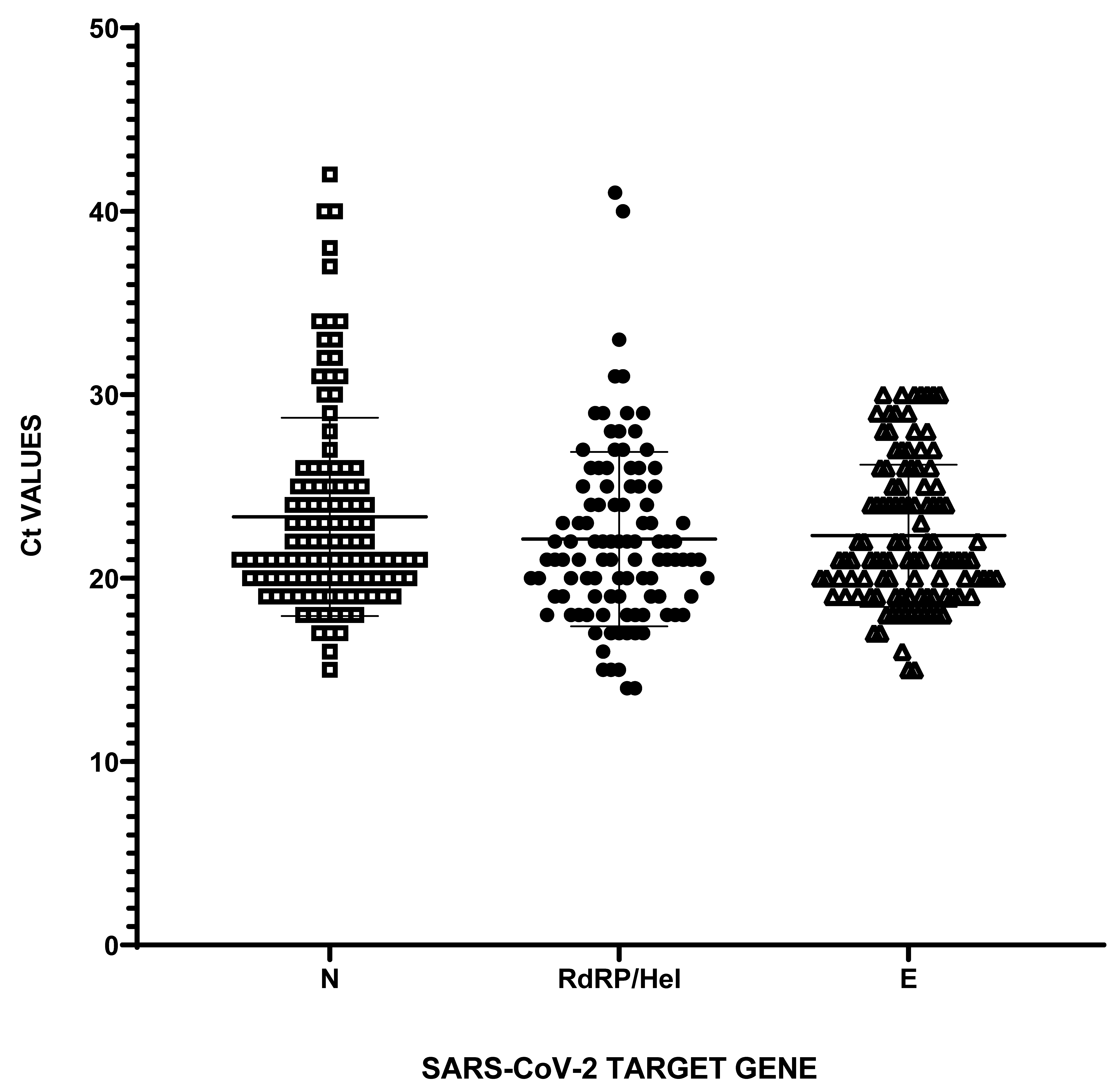
2.4. Sample Preparation and RT-qPCR
2.5. Ag-RDT
2.6. Recombinant Protein Production and Purification
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Mardian, Y.; Kosasih, H.; Karyana, M.; Neal, A.; Lau, C.-Y. Review of Current COVID-19 Diagnostics and Opportunities for Further Development. Front. Med. 2021, 8, 615099. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance. 27 May 2020 (who.int). Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 30 December 2021).
- World Health Organization. Diagnostic Testing for SARS-CoV-2: Interim Guidance, 11 September 2020 (who.int). Available online: https://apps.who.int/iris/handle/10665/334254 (accessed on 30 December 2021).
- Centers for Disease Control and Prevention, CDC. Overview of Testing for SARS-CoV-2 (COVID-19). 17 March 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (accessed on 30 December 2021).
- Stephen, M.; Hahn, M.D. Commissioner of Food and Drugs. Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus That Causes COVID-19 in Patients. 9 May 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-antigen-test-help-rapid-detection-virus-causes (accessed on 30 December 2021).
- Brümmer, L.E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; Pollock, N.R.; et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLOS Med. 2021, 18, e1003735. [Google Scholar] [CrossRef]
- Rasmussen, A.L.; Popescu, S.V. SARS-CoV-2 transmission without symptoms. Science 2021, 371, 1206–1207. [Google Scholar] [CrossRef]
- Schwartz, K.L.; McGeer, A.J.; Bogoch, I.I. Rapid antigen screening of asymptomatic people as a public health tool to combat COVID-19. Can. Med. Assoc. J. 2021, 193, E449–E452. [Google Scholar] [CrossRef]
- Candel, F.J.; Barreiro, P.; Román, J.S.; Abanades, J.C.; Barba, R.; Barberán, J.; Bibiano, C.; Canora, J.; Cantón, R.; Calvo, C.; et al. Recommendations for use of antigenic tests in the diagnosis of acute SARS-CoV-2 infection in the second pandemic wave: Attitude in different clinical settings. Rev. Española Quimioter. 2020, 33, 466–484. [Google Scholar] [CrossRef]
- National Institute of Health. COVID-19 Treatment Guidelines. 21 April 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 30 December 2021).
- Polvere, I.; Silvestri, E.; Sabatino, L.; Giacco, A.; Iervolino, S.; Peluso, T.; Guida, R.; Zerillo, L.; Varricchio, R.; D’Andrea, S.; et al. Sample-Pooling Strategy for SARS-CoV-2 Detection among Students and Staff of the University of Sannio. Diagnostics 2021, 11, 1166. [Google Scholar] [CrossRef]
- Vessichelli, M.; Ferravante, A.; Zotti, T.; Reale, C.; Scudiero, I.; Picariello, G.; Vito, P.; Stilo, R. Neuroepithelial Transforming Gene 1 (Net1) Binds to Caspase Activation and Recruitment Domain (CARD)- and Membrane-associated Guanylate Kinase-like Domain-containing (CARMA) Proteins and Regulates Nuclear Factor κB Activation. J. Biol. Chem. 2012, 287, 13722–13730. [Google Scholar] [CrossRef] [Green Version]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Target Product Profiles for Priority Diagnostics to Support Response to the COVID-19 Pandemic v.1.0. 28 September 2020. Available online: https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1 (accessed on 30 December 2021).
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling up COVID-19 rapid antigen tests: Promises and challenges. Lancet Infect. Dis. 2021, 21, E290–E295. [Google Scholar] [CrossRef]
- Lee, R.A.; Herigon, J.C.; Benedetti, A.; Pollock, N.R.; Denkinger, C.M. Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: A Systematic Review and Meta-analysis. J. Clin. Microbiol. 2021, 59, e02881–20. [Google Scholar] [CrossRef] [PubMed]
- Hasanoglu, I.; Korukluoglu, G.; Asilturk, D.; Cosgun, Y.; Kalem, A.K.; Altas, A.B.; Kayaaslan, B.; Eser, F.; Kuzucu, E.A.; Guner, R. Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection 2021, 49, 117–126. [Google Scholar] [CrossRef]
- Shah, S.; Singhal, T.; Davar, N.; Thakkar, P. No correlation between Ct values and severity of disease or mortality in patients with COVID 19 disease. Indian J. Med. Microbiol. 2021, 39, 116–117. [Google Scholar] [CrossRef]
- Han, M.S.; Byun, J.-H.; Cho, Y.; Rim, J.H. RT-PCR for SARS-CoV-2: Quantitative versus qualitative. Lancet Infect. Dis. 2021, 21, 165. [Google Scholar] [CrossRef]
- Peña, M.; Ampuero, M.; Garcés, C.; Gaggero, A.; García, P.; Velasquez, M.S.; Luza, R.; Alvarez, P.; Paredes, F.; Acevedo, J.; et al. Performance of SARS-CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int. J. Infect. Dis. 2021, 107, 201–204. [Google Scholar] [CrossRef]
- Boum, Y.; Fai, K.N.; Nikolay, B.; Mboringong, A.B.; Bebell, L.M.; Ndifon, M.; Abbah, A.; Essaka, R.; Eteki, L.; Luquero, F.; et al. Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: A clinical, prospective, diagnostic accuracy study. Lancet Infect. Dis. 2021, 21, 1089–1096. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Pfeifer, N.; Ciccariello, L.; Sibilio, S.; Tezza, G.; Ausserhofer, D. Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: A preliminary report. J. Infect. 2021, 82, e14–e16. [Google Scholar] [CrossRef]
- Cubas-Atienzar, A.I.; Kontogianni, K.; Edwards, T.; Wooding, D.; Buist, K.; Thompson, C.R.; Williams, C.T.; Patterson, E.I.; Hughes, G.L.; Baldwin, L.; et al. Limit of detection in different matrices of 19 commercially available rapid antigen tests for the detection of SARS-CoV-2. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Geneva: Foundation for Innovative New Diagnostics. FIND Evaluation of Joysbio (Tianjin) Biotechnology Co., Ltd. SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold). External Report Version 1.0. 11 February 2021. Available online: https://www.finddx.org/sarscov2-eval-antigen/ (accessed on 30 December 2021).
- Homza, M.; Zelena, H.; Janosek, J.; Tomaskova, H.; Jezo, E.; Kloudova, A.; Mrazek, J.; Svagera, Z.; Prymula, R. Five Antigen Tests for SARS-CoV-2: Virus Viability Matters. Viruses 2021, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-Based Testing but Not Real-Time Polymerase Chain Reaction Correlates with Severe Acute Respiratory Syndrome Coronavirus 2 Viral Culture. Clin. Infect. Dis. 2021, 73, e2861–e2866. [Google Scholar] [CrossRef] [PubMed]
- Parvu, V.; Gary, D.S.; Mann, J.; Lin, Y.-C.; Mills, D.; Cooper, L.; Andrews, J.C.; Manabe, Y.C.; Pekosz, A.; Cooper, C.K. Factors that Influence the Reported Sensitivity of Rapid Antigen Testing for SARS-CoV-2. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Pray, I.W.; Ford, L.; Cole, D.; Lee, C.; Bigouette, J.P.; Abedi, G.R.; Bushman, D.; Delahoy, M.J.; Currie, D.; Cherney, B.; et al. Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses—Wisconsin, September–October 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Kissler, S.M.; Fauver, J.R.; Mack, C.; Olesen, S.W.; Tai, C.; Shiue, K.Y.; Kalinich, C.C.; Jednak, S.; Ott, I.M.; Vogels, C.B.F.; et al. Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health strategies. PLoS Biol. 2021, 19, e3001333. [Google Scholar] [CrossRef]
- Millioni, R.; Mortarino, C. Test Groups, Not Individuals: A Review of the Pooling Approaches for SARS-CoV-2 Diagnosis. Diagnostics 2021, 11, 68. [Google Scholar] [CrossRef]
- Weissleder, R.; Lee, H.; Ko, J.; Pittet, M.J. COVID-19 diagnostics in context. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Ciotti, M.; Benedetti, F.; Zella, D.; Angeletti, S.; Ciccozzi, M.; Bernardini, S. SARS-CoV-2 Infection and the COVID-19 Pandemic Emergency: The Importance of Diagnostic Methods. Chemotherapy 2021, 66, 17–23. [Google Scholar] [CrossRef]
- Polvere, I.; Parrella, A.; Casamassa, G.; D’Andrea, S.; Tizzano, A.; Cardinale, G.; Voccola, S.; Porcaro, P.; Stilo, R.; Vito, P.; et al. Seroprevalence of Anti-SARS-CoV-2 IgG and IgM among Adults over 65 Years Old in the South of Italy. Diagnostics 2021, 11, 483. [Google Scholar] [CrossRef]
- Polvere, I.; Voccola, S.; Cardinale, G.; Fumi, M.; Aquila, F.; Parrella, A.; Madera, J.R.; Stilo, R.; Vito, P.; Zotti, T. A peptide-based assay discriminates individual antibody response to SARS-CoV-2. Genes Dis. 2021, 9, 275–281. [Google Scholar] [CrossRef] [PubMed]


| N | RdRP/Hel | SARS-CoV-2 Infection |
|---|---|---|
| Ct > 40 or absent | Ct > 40 or absent | Not detected |
| Ct ≤ 40 | Ct > 40 or absent | Detected |
| Ct > 40 or absent | Ct ≤ 40 | Detected |
| Gender | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | No. | % | Female | % | Male | % | Symptoms | No. | % |
| 0–20 | 34 | 6.79 | 12 | 2.40 | 22 | 4.39 | Mild symptoms | 16 | 3.19 |
| 21–40 | 194 | 38.72 | 71 | 14.17 | 124 | 24.75 | Asymptomatic | 485 | 96.81 |
| 41–60 | 174 | 34.73 | 81 | 16.17 | 92 | 18.36 | TOTAL | 501 | 100.00 |
| 61–80 | 80 | 15.97 | 33 | 6.59 | 47 | 9.38 | |||
| 81–100 | 12 | 2.40 | 5 | 1.00 | 7 | 1.40 | |||
| N/A | 7 | 1.40 | 2 | 0.40 | 5 | 1.00 | |||
| TOTAL | 501 | 100.00 | 204 | 40.72 | 297 | 59.28 | |||
| Features | Overall (n = 501) | RT-qPCR Positive (n = 115) | RT-qPCR Negative (n = 386) | p-Value * | Ag-RDT Positive (n = 116) | Ag-RDT Negative (n = 385) | p-Value * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | % within the Same Group | No. | % | % within the Same Group | No. | % | % within the Same Group | No. | % | % within the Same Group | ||||
| Gender | Male | 297 | 59.28 | 65 | 12.97 | 21.89 | 232 | 59.28 | 78.11 | 0.5174 | 64 | 12.77 | 21.55 | 233 | 46.51 | 78.45 | 0.3324 |
| Female | 204 | 40.72 | 50 | 9.98 | 24.51 | 154 | 30.74 | 75.49 | 52 | 10.38 | 25.49 | 152 | 30.34 | 74.51 | |||
| Age Group | 0–20 | 34 | 6.79 | 8 | 1.60 | 23.53 | 26 | 5.19 | 76.47 | 0.8874 | 8 | 1.60 | 23.53 | 26 | 5.19 | 76.47 | 0.9588 |
| 21–40 | 194 | 38.72 | 41 | 8.18 | 21.13 | 153 | 30.54 | 78.87 | 42 | 8.38 | 21.65 | 152 | 30.34 | 78.35 | |||
| 41–60 | 174 | 34.73 | 43 | 8.78 | 24.71 | 131 | 26.15 | 75.29 | 44 | 8.78 | 25.29 | 130 | 25.95 | 74.71 | |||
| 61–80 | 80 | 15.97 | 18 | 3.59 | 22.50 | 62 | 12.38 | 77.50 | 18 | 3.59 | 22.50 | 62 | 12.38 | 77.50 | |||
| 81–100 | 12 | 2.40 | 4 | 0.60 | 33.33 | 8 | 1.60 | 66.67 | 3 | 0.60 | 25.00 | 9 | 1.80 | 75.00 | |||
| N/A | 7 | 1.40 | 1 | 0.20 | 14.29 | 6 | 1.20 | 85.71 | 1 | 0.20 | 14.29 | 6 | 1.20 | 85.71 | |||
| Symptoms | Mild symptoms | 16 | 3.19 | 4 | 0.80 | 25.00 | 12 | 2.39 | 75.00 | 0.7691 | 4 | 0.80 | 25.00 | 12 | 2.40 | 75.00 | 0.7709 |
| Asymptomatic | 485 | 96.81 | 111 | 22.16 | 22.89 | 374 | 74.65 | 77.11 | 112 | 22.36 | 23.09 | 373 | 74.45 | 76.91 | |||
| Ag-RDT results | Positive | 116 | 23.15 | 113 | 22.55 | 97.41 | 3 | 0.60 | 2.59 | <0.001 * | |||||||
| Negative | 385 | 76.85 | 2 | 0.40 | 0.52 | 383 | 76.85 | 99.48 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polvere, I.; Voccola, S.; D'Andrea, S.; Zerillo, L.; Varricchio, R.; Madera, J.R.; Stilo, R.; Vito, P.; Zotti, T. Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients. Diagnostics 2022, 12, 650. https://doi.org/10.3390/diagnostics12030650
Polvere I, Voccola S, D'Andrea S, Zerillo L, Varricchio R, Madera JR, Stilo R, Vito P, Zotti T. Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients. Diagnostics. 2022; 12(3):650. https://doi.org/10.3390/diagnostics12030650
Chicago/Turabian StylePolvere, Immacolata, Serena Voccola, Silvia D'Andrea, Lucrezia Zerillo, Romualdo Varricchio, Jessica Raffaella Madera, Romania Stilo, Pasquale Vito, and Tiziana Zotti. 2022. "Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients" Diagnostics 12, no. 3: 650. https://doi.org/10.3390/diagnostics12030650
APA StylePolvere, I., Voccola, S., D'Andrea, S., Zerillo, L., Varricchio, R., Madera, J. R., Stilo, R., Vito, P., & Zotti, T. (2022). Evaluation of FAST COVID-19 SARS-CoV-2 Antigen Rapid Test Kit for Detection of SARS-CoV-2 in Respiratory Samples from Mildly Symptomatic or Asymptomatic Patients. Diagnostics, 12(3), 650. https://doi.org/10.3390/diagnostics12030650








