Acoustic-Based Deep Learning Architectures for Lung Disease Diagnosis: A Comprehensive Overview
Abstract
:1. Introduction
1.1. An Overview and Motivations
- How diverse is deep learning’s usefulness in medical diagnosis?
- Will deep learning ever be able to take the place of doctors?
- Is deep learning still relevant, or will it be phased out?
1.2. Contributions and Review Structure
- This paper provides a wide assessment of the ideas and characteristics of deep learning being used in the realm of medical lung diagnosis.
- This paper describes the terms ‘‘breath’’, ‘‘respiratory’’, ‘‘lung sounds’’ ‘‘sound signal analysis’’, and ‘‘acoustic-based classifier’’.
- This survey presents a classification for diagnosis methods of lung disease in the respiratory system and highlights the use of auscultation systems.
- A respiratory system sound diagnosis framework is also displayed, which provides a general understanding of the inquiry of respiratory system diagnosis.
- This work makes a significant addition by presenting a complete assessment of current research on augmentation techniques for background auscultation of the respiratory system.
- This review highlights the role of deep learning CNN integration in enhancing lung auscultation screening.
- It also makes numerous recommendations for future study opportunities.
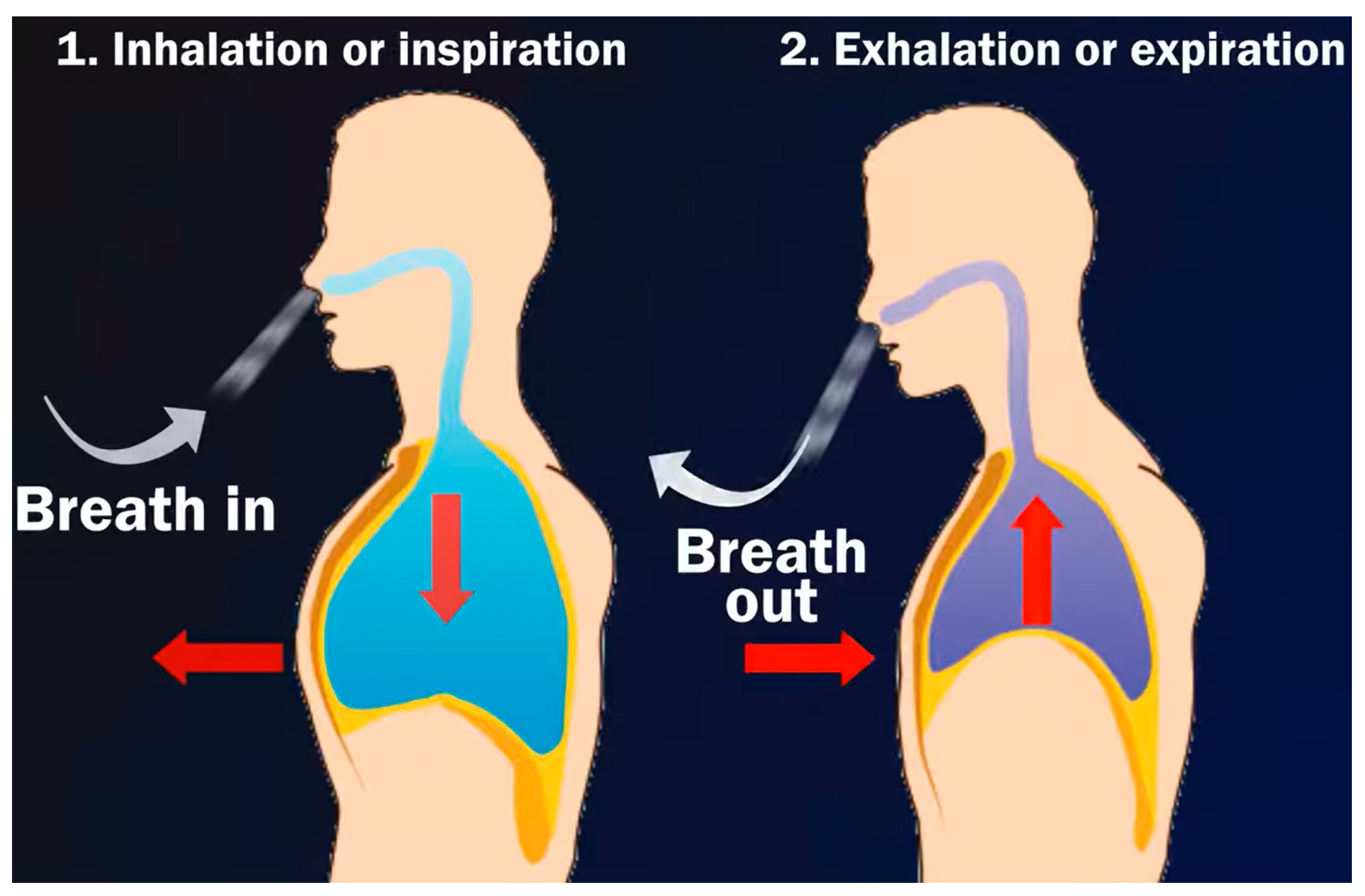
2. Lung Sound Waveforms
2.1. The Regular Lung Sound
- Vesicular breath or normal lung sound: The sound is more high-pitched during inhalation than exhalation, and more intense; it is also continuous, rustling in quality, low-pitched, and soft.
- Bronchial sound breathing: The sound is high-pitched, hollow, and loud. However, it could be a sign of a health problem if a doctor hears bronchial breaths outside the trachea.
- Normal tracheal breath sound: It is high-pitched, harsh, and very loud.
2.2. The Wheezing Lung Sound
- Squawks: A squawk is a momentary wheeze that happens while breathing in.
- Wheezes with numerous notes are called polyphonic wheezes, and they happen during exhalation. The pitch of them may also rise as exhalation nears its conclusion.
- Monophonic wheezes can last for a long time or happen during both phases of respiration. They can also have a constant or variable frequency.
2.3. Crackles Sound
2.4. Rhonchi Sound
2.5. Stridorand Pleural Rub Sounds
- A high-pitched sound called stridor forms in the upper airway. The sound is caused by air squeezing through a constricted portion of the upper respiratory system.
- The rubbing and cracking sound known as "pleural rub" is caused by irritated pleural surfaces rubbing against one another.
3. Survey Methodology
3.1. The Commonly Considered Dataset in the Literate
3.2. Sound-Based Lung Disease Classification Workflow
3.3. General Methodology Diagram
3.4. Preprocessing
3.5. Deep Learning Algorithms
3.6. Wavelet Transform
- As the input, a lung sound recording folder is used. Lung sounds are a combination of lung sounds and noise (signal interference).
- As a signal, sounds can be played and written.
- The lung sounds are then examined by the scheme, saved in the data, and divided into an array of type bytes.
- The data array is transformed into a double-sized array.
- Repeatedly decomposing array data according to the chosen degree of disintegration creates two ranges, every half of the duration of the data range. The initial array is known as a low-pass filter, while the second span is known as a high-pass filter.
- Wavelet transform is applied to the coefficients in each array.
- In the data array, both arrays are reconstructed, with a low-pass filter at the beginning and a high-pass filter at the end.
- The data array is processed via a threshold, creating respiratory sound signal noise and two arrays.
- Repeat restoration as many times as the stage of restoration is set to each array.
- In the data array, the order of the preceding half-high-pass filter and half-low-pass filter is reversed, with a discontinuous high-pass filter low-pass filter for every array.
- Each array’s wavelet transform parameters are re-performed.
- The data array is then transformed from a double-sized array to a byte-sized array. The acoustic format and folder names that have been specified are functional to the information.
- A signal (data) of a breathing sound set is restructured to a breathing sound folder, and a data noise array is restructured to a noise beam.
3.7. Signal-to-Noise Ratio
3.8. Extracting Features
4. Lung Sound Characteristics and Types
Studies Review Lung Nodule Screening
5. Existing Literature Gaps
- Dataset selection: because the whole model is built on it, obtaining and maintaining a noise-free database is crucial. The training data must be properly preprocessed.
- Algorithm choice: it is significant to grasp the study’s function. A variety of algorithms may be tried to see which ones produce results that are closest to the objective.
- Feature extraction strategies: it is also an important task in the development of successful models. It is effective when high model accuracy is required, as well as optimum feature selection, which aids in the creation of redundant data throughout each data analysis cycle.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Ro, T.; Zhu, Z. Emotion Recognition with Audio, Video, EEG, and EMG: A Dataset and Baseline Approaches. IEEE Access 2022, 10, 13229–13242. [Google Scholar] [CrossRef]
- Sengupta, N.; Sahidullah, M.; Saha, G. Lung sound classification using cepstral-based statistical features. Comput. Biol. Med. 2016, 75, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Callier, P.; Sandel, O. Introduction to Artificial Intelligence. Actual. Pharm. 2021, 60, 18–20. [Google Scholar] [CrossRef]
- Chawla, J.; Walia, N.K. Artificial Intelligence based Techniques in Respiratory Healthcare Services: A Review. In Proceedings of the 3rd International Conference on Computing, Analytics and Networks (ICAN), Punjab, India, 18–19 November 2022. [Google Scholar] [CrossRef]
- Ghrabli, S.; Elgendi, M.; Menon, C. Challenges and Opportunities of Deep Learning for Cough-Based COVID-19 Diagnosis: A Scoping Review. Diagnostics 2022, 12, 2142. [Google Scholar] [CrossRef] [PubMed]
- Lung Sounds: Types and Their Causes and Treatment Options. Available online: https://www.medicalnewstoday.com/articles/lung-sounds (accessed on 18 April 2023).
- Bardou, D.; Zhang, K.; Ahmad, S.M. Lung sounds classification using convolutional neural networks. Artif. Intell. Med. 2018, 88, 58–69. [Google Scholar] [CrossRef]
- Rajkumar, S.; Sathesh, K.; Goyal, N.K. Neural network-based design and evaluation of performance metrics using adaptive line enhancer with adaptive algorithms for auscultation analysis. Neural Comput. Appl. 2020, 32, 15131–15153. [Google Scholar] [CrossRef]
- Sathesh, K.; Rajkumar, S.; Goyal, N.K. Least Mean Square (LMS) based neural design and metric evaluation for auscultation signal separation. Biomed. Signal Process. Control 2020, 59, 101784. [Google Scholar] [CrossRef]
- Borrelli, P.; Ly, J.; Kaboteh, R.; Ulén, J.; Enqvist, O.; Trägårdh, E.; Edenbrandt, L. AI-based detection of lung lesions in [18F]FDG PET-CT from lung cancer patients. EJNMMI Phys. 2021, 8, 3635. [Google Scholar] [CrossRef]
- Jeong, O.; Ryu, S.Y.; Park, Y.K. The value of preoperative lung spirometry test for predicting the operative risk in patients undergoing gastric cancer surgery. J. Korean Surg. Soc. 2013, 84, 18–26. [Google Scholar] [CrossRef]
- Petmezas, G.; Cheimariotis, G.A.; Stefanopoulos, L.; Rocha, B.; Paiva, R.P.; Katsaggelos, A.K.; Maglaveras, N. Automated Lung Sound Classification Using a Hybrid CNN-LSTM Network and Focal Loss Function. Sensors 2022, 22, 1232. [Google Scholar] [CrossRef]
- Romero Gómez, A.F.; Orjuela-Cañón, A.D. Respiratory Sounds Classification employing a Multi-label Approach. In Proceedings of the 2021 IEEE Colombian Conference on Applications of Computational Intelligence, ColCACI, Cali, Colombia, 26–28 May 2021. [Google Scholar] [CrossRef]
- Nguyen, T.; Pernkopf, F. Crackle Detection in Lung Sounds Using Transfer Learning and Multi-Input Convolutional Neural Networks. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 5796–5799. [Google Scholar] [CrossRef]
- Asatani, N.; Kamiya, T.; Mabu, S.; Kido, S. Classification of Respiratory Sounds by Generated Image and Improved CRNN. In Proceedings of the 21st International Conference on Control, Automation and Systems (ICCAS), Jeju, Republic of Korea, 12–15 October 2021. [Google Scholar] [CrossRef]
- Gómez, A.F.R.; Orjuela-Cañón, A.D. Multilabel and Multiclass Approaches Comparison for Respiratory Sounds Classification. In Proceedings of the Communications in Computer and Information Science, Cali, Colombia, 27–29 July 2022. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, J.; Liu, J.; Kuang, H.; Wu, F.; Wang, J. ARSC-Net: Adventitious Respiratory Sound Classification Network Using Parallel Paths with Channel-Spatial Attention. In Proceedings of the EEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 9–12 December 2021. [Google Scholar] [CrossRef]
- Naqvi, S.Z.H.; Choudhry, M.A. An automated system for classification of chronic obstructive pulmonary disease and pneumonia patients using lung sound analysis. Sensors 2020, 20, 6512. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, L.; Hassanin, O.; Fraiwan, M.; Khassawneh, B.; Ibnian, A.M.; Alkhodari, M. Automatic identification of respiratory diseases from stethoscopic lung sound signals using ensemble classifiers. Biocybern. Biomed. Eng. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- ICBHI 2017 Challenge|ICBHI Challenge. (n.d.). Available online: https://bhichallenge.med.auth.gr/ICBHI_2017_Challenge (accessed on 24 June 2022).
- Hsu, F.S.; Huang, S.R.; Huang, C.W.; Huang, C.J.; Cheng, Y.R.; Chen, C.C.; Hsiao, J.; Chen, C.W.; Chen, L.C.; Lai, Y.C.; et al. Benchmarking of eight recurrent neural network variants for breath phase and adventitious sound detection on a selfdeveloped open-access lung sound database-HF_Lung_V1. PLoS ONE 2021, 16, e0254134. [Google Scholar] [CrossRef] [PubMed]
- Altan, G.; Kutlu, Y.; Garbi, Y.; Pekmezci, A.Ö.; Nural, S. Multimedia Respiratory Database (RespiratoryDatabase@TR): Auscultation Sounds and Chest X-rays. Nat. Eng. Sci. 2017, 2, 59–72. [Google Scholar] [CrossRef]
- Altan, D.; Kutlu, Y. RespiratoryDatabase@TR (COPD Severity Analysis). 2020, 1. Available online: https://data.mendeley.com/datasets/p9z4h98s6j/1 (accessed on 27 March 2023).
- Baghel, N.; Nangia, V.; Dutta, M.K. ALSD-Net: Automatic lung sounds diagnosis network from pulmonary signals. Neural Comput. Appl. 2021, 33, 17103–17118. [Google Scholar] [CrossRef]
- Emmanouilidou, D.; McCollum, E.D.; Park, D.E.; Elhilali, M. Computerized Lung Sound Screening for Pediatric Auscultation in Noisy Field Environments. IEEE Trans. Biomed. Eng. 2018, 65, 1564–1574. [Google Scholar] [CrossRef]
- Oweis, R.J.; Abdulhay, E.W.; Khayal, A.; Awad, A. An alternative respiratory sounds classification system utilizing artificial neural networks. Biomed. J. 2015, 38. [Google Scholar] [CrossRef]
- Bahoura, M. Pattern recognition methods applied to respiratory sounds classification into normal and wheeze classes. Comput. Biol. Med. 2009, 89, 824–843. [Google Scholar] [CrossRef] [PubMed]
- The, R.A.L.E. Repository. Available online: http://www.rale.ca/ (accessed on 24 June 2022).
- Adhi Pramono, R.X.; Imtiaz, S.A.; Rodriguez-Villegas, E. Evaluation of features for classification of wheezes and normal respiratory sounds. PLoS ONE 2019, 14, e0213659. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.R.A.L.E. Lung Sounds 3.0. J. Hosp. Palliat. Nurs. 2003, 5, 139–141. [Google Scholar] [CrossRef]
- Respiratory Sounds Classification|CS 7651—Machine Learning (Team 7). (n.d.). Available online: https://fereshtehshah.github.io/Respiratory_Disorders (accessed on 24 June 2022).
- Palaniappan, R.; Sundaraj, K.; Sundaraj, S. A comparative study of the svm and k-nn machine learning algorithms for the diagnosis of respiratory pathologies using pulmonary acoustic signals. BMC Bioinform. 2014, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.; Jhamb, R. Classification of Lung Sounds and Disease Prediction using Dense CNN Network. Int. J. Eng. Adv. Technol. 2021, 11, 195–198. [Google Scholar] [CrossRef]
- Respiratory Sound Database|Kaggle. Available online: https://www.kaggle.com/datasets/vbookshelf/respiratory-sound-database (accessed on 25 June 2022).
- Içer, S.; Gengeç, Ş. Classification and analysis of non-stationary characteristics of crackle and rhonchus lung adventitious sounds. Digit. Signal Process. A Rev. J. 2014, 28, 18–27. [Google Scholar] [CrossRef]
- Maria, A.; Jeyaseelan, A.S. Development of Optimal Feature Selection and Deep Learning Toward Hungry Stomach Detection Using Audio Signals. J. Control. Autom. Electr. Syst. 2021, 32, 853–874. [Google Scholar] [CrossRef]
- De Benito-Gorron, D.; Ramos, D.; Toledano, D.T. A Multi-Resolution CRNN-Based Approach for Semi-Supervised Sound Event Detection in DCASE 2020 Challenge. IEEE Access 2021, 9, 89029–89042. [Google Scholar] [CrossRef]
- Gerhard, D. Audio Signal Classification: History and Current Techniques; Department of Computer Science, University of Regina: Regina, Canada, 2003. [Google Scholar]
- Sharma, G.; Umapathy, K.; Krishnan, S. Trends in audio signal feature extraction methods. Appl. Acoust. 2020, 158, 107020. [Google Scholar] [CrossRef]
- Ayvaz, U.; Gürüler, H.; Khan, F.; Ahmed, N.; Whangbo, T.; Bobomirzaevich, A.A. Automatic Speaker Recognition Using Mel-Frequency Cepstral Coefficients Through Machine Learning. Comput. Mater. Contin. 2022, 71, 5511–5521. [Google Scholar] [CrossRef]
- Ajibola Alim, S.; Khair Alang Rashid, N. Some Commonly Used Speech Feature Extraction Algorithms. In From Natural to Artificial Intelligence—Algorithms and Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Rizal, A.; Hidayat, R.; Nugroho, H.A. Comparison of discrete wavelet transform and wavelet packet decomposition for the lung sound classification. Far East J. Electron. Commun. 2017, 25, 1065–1078. [Google Scholar] [CrossRef]
- Sreejyothi, S.; Renjini, A.; Raj, V.; Swapna, M.N.S.; Sankararaman, S.I. Unwrapping the phase portrait features of adventitious crackle for auscultation and classification: A machine learning approach. J. Biol. Phys. 2021, 47, 103–115. [Google Scholar] [CrossRef]
- Naqvi, S.Z.H.; Arooj, M.; Aziz, S.; Khan, M.U.; Choudhary, M.A.; Ul Hassan, M.N. Spectral Analysis of Lungs sounds for Classification of Asthma and Pneumonia Wheezing. In Proceedings of the International Conference on Electrical, Communication, and Computer Engineering (ICECCE), Istanbul, Turkey, 12–13 June 2020. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, W.T.; Tan, T.H.; Chang, C.C.; Chang, Y.J. Using K-nearest neighbor classification to diagnose abnormal lung sounds. Sensors 2015, 15, 13132–13158. [Google Scholar] [CrossRef] [PubMed]
- Neili, Z.; Fezari, M.; Redjati, A. ELM and K-nn machine learning in classification of Breath sounds signals. Int. J. Electr. Comput. Eng. 2020, 10, 3528–3536. [Google Scholar] [CrossRef]
- Haider, N.S.; Singh, B.K.; Periyasamy, R.; Behera, A.K. Respiratory Sound Based Classification of Chronic Obstructive Pulmonary Disease: A Risk Stratification Approach in Machine Learning Paradigm. J. Med. Syst. 2019, 43, 255. [Google Scholar] [CrossRef]
- Taspinar, Y.S.; Koklu, M.; Altin, M. Identification of the english accent spoken in different countries by the k-nearest neighbor method. Int. J. Intell. Syst. Appl. Eng. 2020, 8, 191–194. [Google Scholar] [CrossRef]
- Demir, F.; Sengur, A.; Bajaj, V. Convolutional neural networks based efficient approach for classification of lung diseases. Health Inf. Sci. Syst. 2020, 8, 4. [Google Scholar] [CrossRef]
- Falah, A.H.; Jondri, J. Lung sounds classification using stacked autoencoder and support vector machine. In Proceedings of the 7th International Conference on Information and Communication Technology (ICoICT), Kuala Lumpur, Malaysia, 24–26 July 2019. [Google Scholar] [CrossRef]
- Rizal, A.; Priharti, W.; Rahmawati, D.; Mukhtar, H. Classification of Pulmonary Crackle and Normal Lung Sound Using Spectrogram and Support Vector Machine. J. Biomim. Biomater. Biomed. Eng. 2022, 55, 143–153. [Google Scholar] [CrossRef]
- Azmy, M.M. Classification of lung sounds based on linear prediction cepstral coefficients and support vector machine. In Proceedings of the IEEE Jordan Conference on Applied Electrical Engineering and Computing Technologies (AEECT), Amman, Jordan, 3–5 November 2015. [Google Scholar] [CrossRef]
- Phani Sudershan, C.; Narayana Rao, S.V.N. Classification of crackle sounds using support vector machine. Mater. Today Proc. 2020, 81. [Google Scholar] [CrossRef]
- Mayorga, P.; Ibarra, D.; Zeljkovic, V.; Druzgalski, C. Quartiles and Mel Frequency Cepstral Coefficients vectors in Hidden Markov-Gaussian Mixture Models classification of merged heart sounds and lung sounds signals. In Proceedings of the International Conference on High Performance Computing & Simulation (HPCS), Amsterdam, The Netherlands, 20–24 July 2015. [Google Scholar] [CrossRef]
- Maruf, S.O.; Azhar, M.U.; Khawaja, S.G.; Akram, M.U. Crackle separation and classification from normal Respiratory sounds using Gaussian Mixture Model. In Proceedings of the IEEE 10th International Conference on Industrial and Information Systems (ICIIS), Peradeniya, Sri Lanka, 18–20 December 2015. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, X.; Pei, Z.; Li, M.; Li, J. Triple-Classification of Respiratory Sounds Using Optimized S-Transform and Deep Residual Networks. IEEE Access 2019, 7, 32845–32852. [Google Scholar] [CrossRef]
- Das, N.; Topalovic, M.; Janssens, W. Artificial intelligence in diagnosis of obstructive lung disease. Curr. Opin. Pulm. Med. 2018, 24, 117–123. [Google Scholar] [CrossRef]
- Foeady, A.Z.; Riqmawatin, S.R.; Novitasari, D.C.R. Lung cancer classification based on CT scan image by applying FCM segmentation and neural network technique. Telkomnika 2021, 19, 1284–1290. [Google Scholar] [CrossRef]
- Kim, Y.; Hyon, Y.K.; Jung, S.S.; Lee, S.; Yoo, G.; Chung, C.; Ha, T. Respiratory sound classification for crackles, wheezes, and rhonchi in the clinical field using deep learning. Sci. Rep. 2021, 11, 17186. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Cai, M.; Shi, Y.; Ren, S.; Xu, W.; Gao, W.; Luo, Z.; Reinhardt, J.M. A Novel Method for Automatic Identification of Breathing State. Sci. Rep. 2019, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. In Proceedings of the 3rd International Conference on Learning Representations, ICLR 2015—Conference Track Proceedings, San Diego, CA, USA, 7–9 May 2015. [Google Scholar]
- ul Hassan, M. VGG16—Convolutional Network for Classification and Detection. Neurohive. 2018. Available online: https://neurohive.io/en/popular-networks/vgg16/ (accessed on 27 March 2023).
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Alex Net. Adv. Neural Inf. Process. Syst. 2012. [Google Scholar]
- Zhang, C.; Benz, P.; Argaw, D.M.; Lee, S.; Kim, J.; Rameau, F.; Bazin, J.C.; Kweon, I.S. ResNet or DenseNet? Introducing dense shortcuts to ResNet. In Proceedings of the IEEE Winter Conference on Applications of Computer Vision (WACV), Waikoloa, HI, USA, 3–8 January 2021. [Google Scholar]
- Punn, N.S.; Agarwal, S. Inception U-Net Architecture for Semantic Segmentation to Identify Nuclei in Microscopy Cell Images. ACM Trans. Multimed. Comput. Commun. Appl. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Das, D.; Santosh, K.C.; Pal, U. Truncated inception net: COVID-19 outbreak screening using chest X-rays. Phys. Eng. Sci. Med. 2020, 43, 915–925. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Coleman, S.; Kerr, D. DENSE-INception U-net for medical image segmentation. Comput. Methods Programs Biomed. 2020, 192, 105395. [Google Scholar] [CrossRef]
- Li, J.; Yu, Z.L.; Gu, Z.; Liu, H.; Li, Y. Dilated-inception net: Multi-scale feature aggregation for cardiac right ventricle segmentation. IEEE Trans. Biomed. Eng. 2019, 66, 3499–3508. [Google Scholar] [CrossRef]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Lecun, Y.; Bottou, L.; Bengio, Y.; Ha, P. LeNet. Proc. IEEE 1998, 86. [Google Scholar]
- Wu, H.; Song, Q.; Jin, G. Underwater acoustic signal analysis: Preprocessing and classification by deep learning. Neural Netw. World 2020, 30, 85–96. [Google Scholar] [CrossRef]
- Bermejo, D.; Ledesma-Carbayo, M.J.; Jose-Estepar, R.S. Emphysema detection and classification using a multi-scale deep Convolutional Neural Network. Int. J. Comput. Assist. Radiol. Surg. 2017, 2018, 519–522. [Google Scholar]
- Zhao, Z.; Yang, Z.; Luo, L.; Zhang, Y.; Wang, L.; Lin, H.; Wang, J. ML-CNN: A novel deep learning based disease named entity recognition architecture. In Proceedings of the IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Shenzhen, China, 15–18 December 2016; p. 794. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Cai, M.; Zhang, X.D. A lung sound category recognition method based on wavelet decomposition and BP neural network. Int. J. Biol. Sci. 2019, 15, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Peng, Y. Neural classification of lung sounds using wavelet packet coefficients energy. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: London, UK, 2006. [Google Scholar] [CrossRef]
- Syahputra, M.F.; Situmeang, S.I.G.; Rahmat, R.F.; Budiarto, R. Noise reduction in breath sound files using wavelet transform based filter. In Proceedings of the International Conference on Electrical Engineering, Computer Science and Informatics (EECSI), Semarang, Indonesia, 26–25 November 2016. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.M.; Zhao, Y.H.; Dong, L. Feature extraction and classification of lung sounds based on wavelet packet multiscale analysis. Jisuanji Xuebao Chin. J. Comput. 2006, 29, 769. [Google Scholar]
- Shaish, H.; Ahmed, F.S.; Lederer, D.; D’Souza, B.; Armenta, P.; Salvatore, M.; Saqi, A.; Huang, S.; Jambawalikar, S.; Mutasa, S. Deep Learning of Computed Tomography Virtual Wedge Resection for Prediction of Histologic Usual Interstitial Pneumonitis. Ann. Am. Thorac. Soc. 2021, 18, 51–59. [Google Scholar] [CrossRef]
- Wu, Y.C.; Han, C.C.; Chang, C.S.; Chang, F.L.; Chen, S.F.; Shieh, T.Y.; Chen, H.M.; Lin, J.Y. Development of an Electronic Stethoscope and a Classification Algorithm for Cardiopulmonary Sounds. Sensors 2022, 22, 4263. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.H.M.; Malik, N.A.; Sidek, K.A. Distinctive features for normal and crackles respiratory sounds using cepstral coefficients. Bull. Electr. Eng. Inform. 2019, 8, 875–881. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Z.; Dou, Y.; Zhou, J. Whispered Speech Conversion Based on the Inversion of Mel Frequency Cepstral Coefficient Features. Algorithms 2022, 15, 68. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sadegh Mohammadi, H.R. Robust features fusion for text independent speaker verification enhancement in noisy environments. In Proceedings of the Iranian Conference on Electrical Engineering (ICEE), Tehran, Iran, 2–4 May 2017. [Google Scholar] [CrossRef]
- Sovijärvi, A.R.A.; Vanderschoot, J.; Earis, J.E. Standardization of computerized respiratory sound analysis. Eur. Respir. Rev. 2000, 10, 585. [Google Scholar]
- Liu, Z.; Yao, C.; Yu, H.; Wu, T. Deep reinforcement learning with its application for lung cancer detection in medical Internet of Things. Futur. Gener. Comput. Syst. 2019, 97, 1–9. [Google Scholar] [CrossRef]
- Gu, D.; Liu, G.; Xue, Z. On the performance of lung nodule detection, segmentation and classification. Comput. Med. Imaging Graph. 2021, 89, 101886. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Goh, V. What can artificial intelligence teach us about the molecular mechanisms underlying disease? Eur. J. Nucl. Med. Mol. Imaging 2019, 1, 28–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chen, Y.P.P. Image based computer aided diagnosis system for cancer detection. Expert Syst. Appl. 2015, 42, 5356–5365. [Google Scholar] [CrossRef]
- Riquelme, D.; Akhloufi, M. Deep Learning for Lung Cancer Nodules Detection and Classification in CT Scans. AI 2020, 1, 28–67. [Google Scholar] [CrossRef]
- Binczyk, F.; Prazuch, W.; Bozek, P.; Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1186. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Dong, L.T. Artificial intelligence tools for refining lung cancer screening. J. Clin. Med. 2020, 9, 3860. [Google Scholar] [CrossRef]
- Wu, G.; Jochems, A.; Refaee, T.; Ibrahim, A.; Yan, C.; Sanduleanu, S.; Woodruff, H.C.; Lambin, P. Structural and functional radiomics for lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3961–3974. [Google Scholar] [CrossRef]
- Demir, F.; Ismael, A.M.; Sengur, A. Classification of Lung Sounds with CNN Model Using Parallel Pooling Structure. IEEE Access 2020, 8, 105376–105383. [Google Scholar] [CrossRef]
- Serbes, G.; Ulukaya, S.; Kahya, Y.P. An automated lung sound preprocessing and classification system based onspectral analysis methods. IFMBE Proc. 2018, 66, 45–49. [Google Scholar] [CrossRef]
- Sen, I.; Saraclar, M.; Kahya, Y.P. A Comparison of SVM and GMM-Based Classifier Configurations for Diagnostic Classification of Pulmonary Sounds. IEEE Trans. Biomed. Eng. 2015, 62, 1768–1776. [Google Scholar] [CrossRef]
- Saraiva, A.A.; Santos, D.B.S.; Francisco, A.A.; Moura Sousa, J.V.; Fonseca Ferreira, N.M.; Soares, S.; Valente, A. Classification of respiratory sounds with convolutional neural network. In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies—BIOINFORMATICS, Valletta, Malta, 24–26 February 2020. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Song, M.; Parada-Cabaleiro, E.; Schuller, B.W. Adventitious respiratory classification using attentive residual neural networks. Proc. Interspeech 2020, 2912–2916. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Yu, Q.; Zhang, Y.; Li, Y.; Zhao, J.; Wang, G. Lungbrn: A smart digital stethoscope for detecting respiratory disease using bi-resnet deep learning algorithm. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019. [Google Scholar] [CrossRef]
- Pham, L.; Phan, H.; Palaniappan, R.; Mertins, A.; McLoughlin, I. CNN-MoE Based Framework for Classification of Respiratory Anomalies and Lung Disease Detection. IEEE J. Biomed. Health Inform. 2021, 25, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Gairola, S.; Tom, F.; Kwatra, N.; Jain, M. RespireNet: A Deep Neural Network for Accurately Detecting Abnormal Lung Sounds in Limited Data Setting. In Proceedings of the 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021. [Google Scholar] [CrossRef]
- Liu, R.; Cai, S.; Zhang, K.; Hu, N. Detection of Adventitious Respiratory Sounds based on Convolutional Neural Network. In Proceedings of the International Conference on Intelligent Informatics and Biomedical Sciences (ICIIBMS), Shangai, China, 21–24 November 2019. [Google Scholar] [CrossRef]
- Acharya, J.; Basu, A. Deep Neural Network for Respiratory Sound Classification in Wearable Devices Enabled by Patient Specific Model Tuning. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Altan, G.; Kutlu, Y.; Pekmezci, A.Ö.; Nural, S. Deep learning with 3D-second order difference plot on respiratory sounds. Biomed. Signal Process. Control 2018, 45, 58–69. [Google Scholar] [CrossRef]
- Kochetov, K.; Putin, E.; Balashov, M.; Filchenkov, A.; Shalyto, A. Noise masking recurrent neural network for respiratory sound classification. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics, Proceedings of the 27th International Conference on Artificial Neural Networks, Rhodes, Greece, 4–7 October 2018; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Minami, K.; Lu, H.; Kim, H.; Mabu, S.; Hirano, Y.; Kido, S. Automatic Classification of Large-Scale Respiratory Sound Dataset Based on Convolutional Neural Network. In Proceedings of the International Conference on Control, Automation and Systems, Jeju, Republic of Korea, 15–18 October 2019. [Google Scholar] [CrossRef]
- Chambres, G.; Hanna, P.; Desainte-Catherine, M. Automatic detection of patient with respiratory diseases using lung sound analysis. In Proceedings of the International Workshop on Content-Based Multimedia Indexing, La Rochelle, France, 4–6 September 2018. [Google Scholar]
- Jakovljević, N.; Lončar-Turukalo, T. Hidden Markov model based respiratory sound classification. In Proceedings of the IFMBE, La Rochelle, France, 4–6 September 2018. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.; Li, Y. LungRN+NL: An improved adventitious lung sound classification using non-local block resnet neural network with mixup data augmentation. Proc. Interspeech 2020, 2902–2906. [Google Scholar] [CrossRef]
- Nguyen, T.; Pernkopf, F. Lung Sound Classification Using Co-tuning and Stochastic Normalization. IEEE Trans. Biomed. Eng. 2022, 69, 2872–2882. [Google Scholar] [CrossRef]
- Aykanat, M.; Kılıç, Ö.; Kurt, B.; Saryal, S. Classification of lung sounds using convolutional neural networks. Eurasip J. Image Video Process. 2017, 2017, 65. [Google Scholar] [CrossRef]
- Chamberlain, D.; Kodgule, R.; Ganelin, D.; Miglani, V.; Fletcher, R.R. Application of semi-supervised deep learning to lung sound analysis. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar] [CrossRef]
- Behzadi-khormouji, H.; Rostami, H.; Salehi, S.; Derakhshande-Rishehri, T.; Masoumi, M.; Salemi, S.; Keshavarz, A.; Gholamrezanezhad, A.; Assadi, M.; Batouli, A. Deep learning, reusable and problem-based architectures for detection of consolidation on chest X-ray images. Comput. Methods Programs Biomed. 2020, 185, 105162. [Google Scholar] [CrossRef]
- Abbas, Q. Lung-Deep: A Computerized Tool for Detection of Lung Nodule Patterns using Deep Learning Algorithms Detection of Lung Nodules Patterns. Int. J. Adv. Comput. Sci. Appl. 2017, 8, 27894647. [Google Scholar] [CrossRef]
- Jang, S.; Song, H.; Shin, Y.J.; Kim, J.; Kim, J.; Lee, K.W.; Lee, S.S.; Lee, W.; Lee, S.; Lee, K.H. Deep Learning–based Automatic Detection Algorithm for Reducing Overlooked Lung Cancers on Chest Radiographs. Radiology 2020, 296, 652–661. [Google Scholar] [CrossRef]
- Lee, J.H.; Sun, H.Y.; Park, S.; Kim, H.; Hwang, E.J.; Goo, J.M.; Park, C.M. Performance of a deep learning algorithm compared with radiologic interpretation for lung cancer detection on chest radiographs in a health screening population. Radiology 2020, 297, 687–696. [Google Scholar] [CrossRef]
- Brain, G. TensorFlow Hub; TensorFlow: Mountain View, CA, USA, 2021. [Google Scholar]
- Mang, L.D.; Canadas-Quesada, F.J.; Carabias-Orti, J.J.; Combarro, E.F.; Ranilla, J. Cochleogram-based adventitious sounds classification using convolutional neural networks. Biomed. Signal Process. Control 2023, 82, 104555. [Google Scholar] [CrossRef]
- Borwankar, S.; Verma, J.P.; Jain, R.; Nayyar, A. Improvise approach for respiratory pathologies classification with multilayer convolutional neural networks. Multimed. Tools Appl. 2022, 81, 39185–39205. [Google Scholar] [CrossRef] [PubMed]
- Varghese, G.; Mathan, M.; Kumar, S.V.; Joseph, J.; Raphel, F.; George, R. Automated Detection and Classification of Lung Sound Using 3-D Printed Stethoscope. In Proceedings of the 2022 IEEE 6th Conference on Information and Communication Technology, CICT 2022, Gwalior, India, 18–20 November 2022. [Google Scholar] [CrossRef]














| Ref. | Discussion Lung Diseases | Literature Focus | Images/Sound | Highlighting Literature Gaps | Proposing a Solution | Published Date |
|---|---|---|---|---|---|---|
| [4] | Limited | Emerging artificial intelligence methods with respiratory healthcare domain applications | Images+ sound | No | No | 2022 |
| [5] | Limited to COVID-19 | Deep learning for cough audio sample-based COVID-19 diagnosis | Sound | Yes | Yes | 2022 |
| This work | Extensive with most common diseases | Lung disease recognition based on sound signal analysis with machine learning | Images + sound | Yes | Yes | 2023 |
| Search Query | Database | Initial Search | After Remove Repeated Ones | Exclude Based on Title, Abstract | Not Providing Sufficient Info. | Final Selection |
|---|---|---|---|---|---|---|
| Title includes (audio, sound or acoustic) and (lung and/or respiratory), and (deep learning, machine learning, artificial intelligence) | IEEE Xplore | 45 | 32 | 4 | 0 | 28 |
| Web of science | 58 | 49 | 12 | 2 | 35 | |
| Title, abstract, and keywords include (audio, sound or acoustic) and (lung and/or respiratory), and (deep leaning, machine learning, artificial intelligence) | Scopus | 76 | 79 | 14 | 7 | 58 |
| Total | 179 | 160 | 30 | 9 | 121 | |
| Dataset Name | Description | Used by | Source |
|---|---|---|---|
| Respiratory Sounds Dataset (RSD) ICBHI 2017 | Regular sound signals in addition to three kinds of adventitious respiratory sound signals: wheezes, crackles, and a combination between wheezes and crackles. | [12,13,14,15,16,17,18,19] | [20] |
| HF_Lung_V1 | Comprises 9765 lung sound audio files (each lasting 15 s), 18,349 exhalation labels, 34,095 inhalation labels, 15,600 irregular adventitious sounds’ classes, and 13,883 regular adventitious sound classes (including, 4740 rhonchus classes, 8458 wheeze classes, and 686 stridor classes). | [21] | [21] |
| Respiratory-Database@TR | Each patient has 12-channel lung sounds. Short-term recordings, multi-channel analysis, 5 COPD (chronic obstructive lung disease) severity levels (COPD4, COPD3, COPD2, COPD1, COPD0) (At least 17 s). | [22] | [23] |
| Own Generated Database | The lung sounds were captured using an e-stethoscope and an amplifier linked to a laptop. An e-stethoscope with a chest piece that is touched by the patient and a microphone-based recording sound signals with a 44,100 Hz sampling rate that is attached to signal amplifiers are used in this setup. The amplifier kits extend the signal range to about (70–2000 Hz) with respiratory sounds (with frequency controller and control amplifier) when associated with an earphone (to listen to live records) and a PC. | [24] | [24] |
| Own Generated Database | Data are separated into two types: sub-interval set, which includes complete patient set, which comprises all patients’ measures and is classed as abnormal or normal, counting all patients’ sub-interval measurements of any duration. It has around 255 h of measured lung sound signals. | [25] | [25] |
| Own Generated Database | RSs non-stationary data collection with 28 separate patient records. For training and testing, two distinct sets of signals were employed. Except for crackles and wheezes, which were data from six patients each, each class in the training and test sets comprised two recordings from distinct patients. The sampling frequency of the recorded data was 44.1 kHz. | [26] | [26] |
| R.A.L.E. Repository | It is a collection of digital recordings of respiratory sounds in health and sickness. These are the breath sounds that physicians, nurses, respiratory therapists, and physical therapists hear using a stethoscope when they auscultate a patient’s chest. Try-R.A.L.E. Lung Sounds, which provides a vast collection of sound recordings and case presentations, as well as a quiz for self-assessment. | [27] | [28] |
| R.A.L.E. Lung Sounds 3.0 | It includes five regular breathing recordings, four crackling recordings, and four wheeze recordings. To eliminate DC components, a first-order Butterworth high-pass filter with a cut-off frequency of 7.5 Hz was employed, followed by an eighth-order Butterworth low-pass filter with a cut-off frequency of 2.5 kHz to band restrict the signal. | [29] | [30] |
| Respiratory Sound Database | It was developed by two Portuguese and Greek research teams. It has 920 recordings. The duration of each recording varies. 126 patients were recorded, and each tape is documented. Annotations include the start and finish timings of each respiratory cycle, as well as if the cycle comprises wheeze and/or crackle. Wheezes and crackles are known as adventitious noises, and their presence is utilized by doctors to diagnose respiratory disorders. | [12,18,24,31,32,33] | [34] |
| Network | Ref. | Acronym | Year | Other Variants |
|---|---|---|---|---|
| VGG | [61,62] | Visual Geometry Group | 2014 | VGG-D1, VGG-V2, VGG-V1, VGG-B3, and VGG-B1 |
| Alex-Net | [63] | Krizhevsky, Alex | 2012 | Its architecture has sixty million parameters |
| ResNet | [64] | Residual Neural Networks | 2015 | An error rate of 3.6 percent |
| Inception Net | [65,66,67,68] | InceptionNet or GoogleNet | 2014 | With a 6.67 percent error rate, four million parameters |
| LeNet | [69,70,71] | Yann LeCun et al. | 1998 | It contains a full link layer, pooling layer, and convolutional layer. |
| M-CNN | [72] | Multi-scale CNN | 2017 | Several convolutional layers are stacked over the real vector to extract the higher-level features. |
| ML-CNN | [73] | Deep-learning-based disease NER architecture (ML-CNN) | 2017 | Lexicon feature, character-level, and word level. Embeddings are concatenated as input of the CNN model |
| Study | Method | Splitting Strategy | Performance | |||
|---|---|---|---|---|---|---|
| Specificity | Sensitivity | Accuracy | Score | |||
| Demir et al. [92] | VGG16 | 10-fold CV | - | - | 63.09% | - |
| Serbes et al. [93] | SVM | official 60/40 | - | - | 49.86% | - |
| Sen I, et al. [94] | GMM Classifier | - | 90% | 90% | 85.00% | - |
| Saraiva et al. [95] | CNN | random 70/30 | - | - | 74.3% | - |
| Yang et al. [96] | ResNet + SE + SA | official 60/40 | 81.25% | 17.84% | - | 49.55% |
| Ma et al. [97] | bi-ResNet | official 60/40 random 10-fold CV | 69.20% 80.06% | 31.12% 58.54% | 52.79% 67.44% | 50.16% 69.30% |
| Pham et al. [98] | CNN-MoE | official 60/40 random 5-fold CV | 68% 90% | 26% 68% | - | 47% 97% |
| Gairola et al. [99] official 60/40 | CNN | official 60/40 interpatient 80/20 | 72.3% 83.3% | 40.1% 53.7% | - | 56.2% 68.5% |
| Liu et al. [100] | CNN | random 75/25 | - | - | 81.62% | - |
| Acharya and Basu [101] | CNN-RNN | interpatient 80/20 | 84.14% | 48.63% | - | 66.38% |
| Allahwardiand Altan et al. [102] | Deep Belief Networks (DBN) | - | 93.65% 73.33% | 93.34% 67.22% | 95.84% 70.28% | |
| Kochetov et al. [103] | RNN | interpatient 5-fold CV | 73% | 58.4% | - | 65.7% |
| Minami et al. [104] | CNN | official 60/40 | 81% | 28% | - | 54% |
| Georgios Petmezas et al. [12] | CNN-LSTM with FL | Interpatient 10-fold CV LOOCV | 84.26% - | 52.78% 60.29% | 76.39% 74.57% | 68.52% - |
| Chambres et al. [105] | HMM SVM | official 60/40 | 56.69% 77.80% | 42.32% 48.90% | 49.50% 49.98% | 39.37% 49.86% |
| Oweis et al. [26] | ANN | - | 100% | 97.8% | 98.3% | - |
| Jakovljevi’c and Lonˇcar-Turukalo [106] | HMM | official 60/40 | - | - | - | 39.56% |
| Bahoura [27] | GMM | - | 92.8% | 43.7% | 80.00% | - |
| Emmanouilidou D et al. [25] | RBF SVM Classifier | - | 86.55 (±0.36) | 86.82 (±0.42) | 86.70% | - |
| Ma et al. [107] | ResNet + NL | official 60/40 interpatient 5-fold CV | 63.20% 64.73% | 41.32% 63.69% | - | 64.21% 52.26% |
| Nangia et al. [24] | CNN | - | - | - | 94.24% | 93.6% |
| Pramono RX et al. [29] | SVM | - | 83.86% | 82.06% | 87.18% | 82.67% |
| Nguyen and Pernkopf [108] | ResNet | official 60/40 official 60/40 | 79.34% 82.46% | 47.37% 37.24% | - 73.69% | 58.29% 64.92% |
| Bardou D et al. [7] | CNN | - | - | - | 95.56% | - |
| Aykanat M et al. [109] | ANN | - | 86% | 86% | 76.00% | - |
| Chamberlain et al. [110] | - | - | 0.56 | - | 86% Wheeze | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfayyih, A.H.; Sabry, A.H.; Jameel, S.M.; Sulaiman, N.; Raafat, S.M.; Humaidi, A.J.; Kubaiaisi, Y.M.A. Acoustic-Based Deep Learning Architectures for Lung Disease Diagnosis: A Comprehensive Overview. Diagnostics 2023, 13, 1748. https://doi.org/10.3390/diagnostics13101748
Sfayyih AH, Sabry AH, Jameel SM, Sulaiman N, Raafat SM, Humaidi AJ, Kubaiaisi YMA. Acoustic-Based Deep Learning Architectures for Lung Disease Diagnosis: A Comprehensive Overview. Diagnostics. 2023; 13(10):1748. https://doi.org/10.3390/diagnostics13101748
Chicago/Turabian StyleSfayyih, Alyaa Hamel, Ahmad H. Sabry, Shymaa Mohammed Jameel, Nasri Sulaiman, Safanah Mudheher Raafat, Amjad J. Humaidi, and Yasir Mahmood Al Kubaiaisi. 2023. "Acoustic-Based Deep Learning Architectures for Lung Disease Diagnosis: A Comprehensive Overview" Diagnostics 13, no. 10: 1748. https://doi.org/10.3390/diagnostics13101748
APA StyleSfayyih, A. H., Sabry, A. H., Jameel, S. M., Sulaiman, N., Raafat, S. M., Humaidi, A. J., & Kubaiaisi, Y. M. A. (2023). Acoustic-Based Deep Learning Architectures for Lung Disease Diagnosis: A Comprehensive Overview. Diagnostics, 13(10), 1748. https://doi.org/10.3390/diagnostics13101748






