The Predictive Value of Lung Ultrasound Score on Hemodynamically Significant Patent Ductus Arteriosus among Neonates ≤25 Weeks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Hemodynamically Significant PDA
2.3. LU and LUS
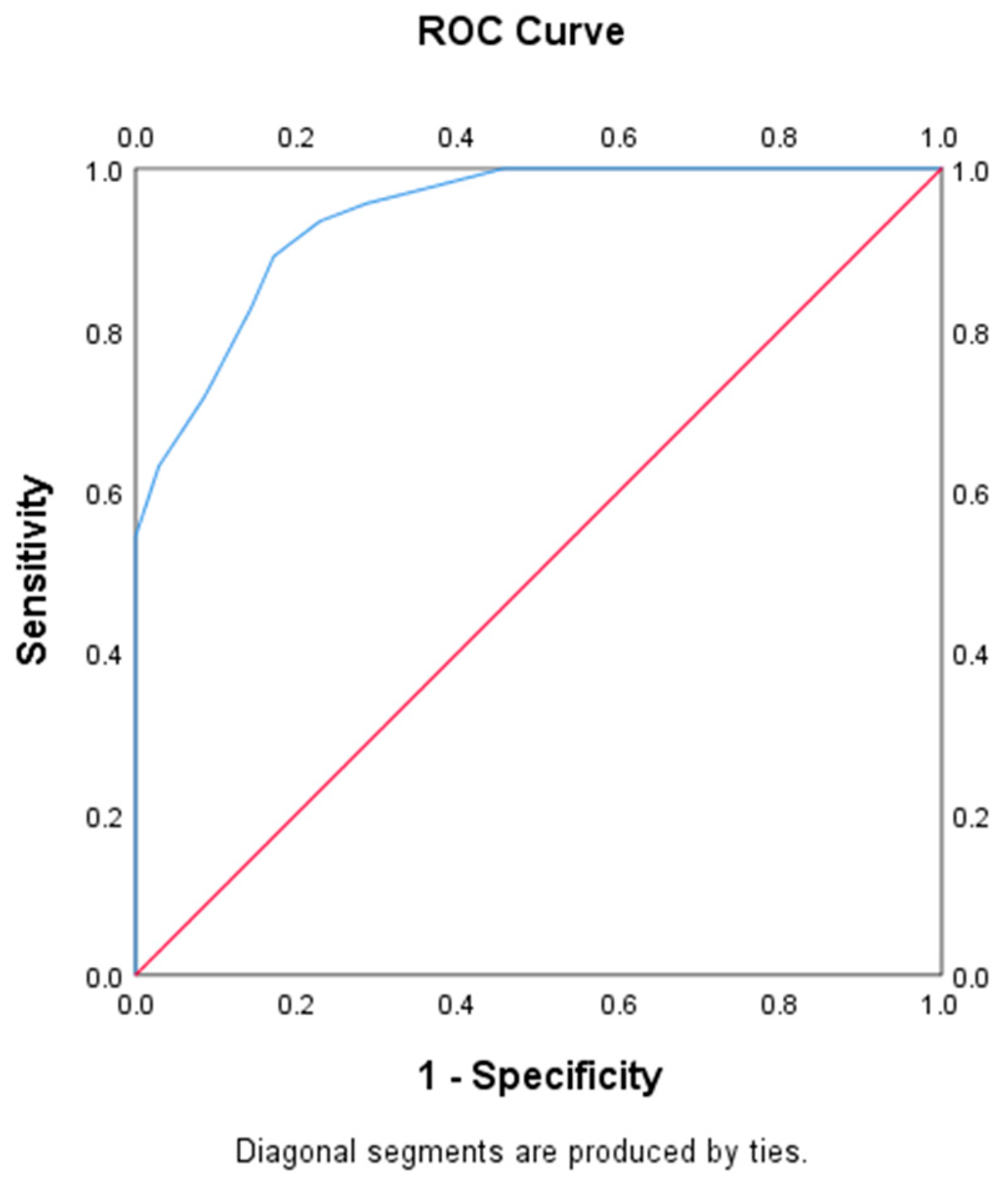
2.4. Sample Size and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gill, A.W. Postnatal cardiovascular adaptation. Arch. Dis. Child.-Fetal Neonatal Ed. 2019, 104, F220–F224. [Google Scholar] [CrossRef] [PubMed]
- Sellmer, A.; Bjerre, J.V.; Schmidt, M.R.; McNamara, P.J.; Hjortdal, V.E.; Høst, B.; Bech, B.H.; Henriksen, T.B. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F505–F510. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.; Hansmann, G. Patent ductus arteriosus of the preterm infant. Pediatrics 2010, 125, 1020–1030. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Yoon, S.J.; Han, J.; Song, I.G.; Lim, J.; Shin, J.E.; Eun, H.S.; Park, K.I.; Park, M.S.; Lee, S.M. Patent ductus arteriosus treatment trends and associated morbidities in neonates. Sci. Rep. 2021, 11, 10689. [Google Scholar] [CrossRef] [PubMed]
- Othman, H.F.; Linfield, D.T.; Mohamed, M.A.; Aly, H. Ligation of patent ductus arteriosus in very low birth weight premature infants. Pediatr. Neonatol. 2020, 61, 399–405. [Google Scholar] [CrossRef]
- Clyman, R.I.; Chan, C.Y.; Mauray, F.; Chen, Y.Q.; Cox, W.; Seidner, S.R.; Lord, E.M.; Weiss, H.; Waleh, N.; Evans, S.M.; et al. Permanent anatomic closure of the ductus arteriosus in newborn baboons: The roles of postnatal constriction, hypoxia, and gestation. Pediatr. Res. 1999, 45, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Hamrick, S.E.; Sallmon, H.; Rose, A.T.; Porras, D.; Shelton, E.L.; Reese, J.; Hansmann, G. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics 2020, 146, e20201209. [Google Scholar] [CrossRef]
- Clyman, R.I. Patent ductus arteriosus, its treatments, and the risks of pulmonary morbidity. Semin. Perinatol. 2018, 42, 235–242. [Google Scholar] [CrossRef]
- Singh, Y.; Tissot, C.; Fraga, M.V.; Yousef, N.; Cortes, R.G.; Lopez, J.; Sanchez-De-Toledo, J.; Brierley, J.; Colunga, J.M.; Raffaj, D.; et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 2020, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Brusa, G.; Savoia, M.; Vergine, M.; Bon, A.; Copetti, R.; Cattarossi, L. Neonatal Lung Sonography: Interobserver Agreement between Physician Interpreters with Varying Levels of Experience. J. Ultrasound Med. 2015, 34, 1549–1554. [Google Scholar] [CrossRef]
- Brat, R.; Yousef, N.; Klifa, R.; Reynaud, S.; Aguilera, S.S.; De Luca, D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated with Continuous Positive Airway Pressure. JAMA Pediatr. 2015, 169, e151797. [Google Scholar] [CrossRef] [Green Version]
- Zong, H.-F.; Guo, G.; Liu, J.; Bao, L.-L.; Yang, C.-Z. Using lung ultrasound to quantitatively evaluate pulmonary water content. Pediatr. Pulmonol. 2020, 55, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Migliaro, F.; Corsini, I.; Meneghin, F.; Dolce, P.; Pierri, L.; Perri, A.; Aversa, S.; Nobile, S.; Lama, S.; et al. Lung Ultrasound Score Progress in Neonatal Respiratory Distress Syndrome. Pediatrics 2021, 147, e2020030528. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.; Tana, M.; Riccardi, R.; Iannotta, R.; Giordano, L.; Rubortone, S.A.; Priolo, F.; Di Molfetta, D.V.; Zecca, E.; Vento, G. Neonatal lung ultrasonography score after surfactant in preterm infants: A prospective observational study. Pediatr. Pulmonol. 2020, 55, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ojembarrena, A.; Serna-Guerediaga, I.; Aldecoa-Bilbao, V.; Gregorio-Hernández, R.; Alonso-Quintela, P.; Concheiro-Guisán, A.; Ramos-Rodríguez, A.; Heras-Martín, M.d.L.; Rodeño-Fernández, L.; Oulego-Erroz, I. The Predictive Value of Lung Ultrasound Scores in Developing Bronchopulmonary Dysplasia: A Prospective Multicenter Diagnostic Accuracy Study. Chest 2021, 160, 1006–1016. [Google Scholar] [CrossRef]
- Singh, Y.; Fraisse, A.; Erdeve, O.; Atasay, B. Echocardiographic Diagnosis and Hemodynamic Evaluation of Patent Ductus Arteriosus in Extremely Low Gestational Age Newborn (ELGAN) Infants. Front. Pediatr. 2020, 8, 573627. [Google Scholar] [CrossRef]
- van Laere, D.; van Overmeire, B.; Gupta, S.; El-Khuffash, A.; Savoia, M.; McNamara, P.J.; Schwarz, C.E.; de Boode, W.P.; on behalf of the European Special Interest Group ‘Neonatologist Performed Echocardiography’ (NPE). Application of Neonatologist Performed Echocardiography in the assessment of a patent ductus arteriosus. Pediatr. Res. 2018, 84, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Weisz, D.E.; Martins, F.F.; Nield, L.E.; El-Khuffash, A.; Jain, A.; McNamara, P.J. Acetaminophen to avoid surgical ligation in extremely low gestational age neonates with persistent hemodynamically significant patent ductus arteriosus. J. Perinatol. 2016, 36, 649–653. [Google Scholar] [CrossRef]
- Weisz, D.E.; Mirea, L.; Resende, M.H.F.; Ly, L.; Church, P.T.; Kelly, E.; Kim, S.J.; Jain, A.; McNamara, P.J.; Shah, P.S. Outcomes of Surgical Ligation after Unsuccessful Pharmacotherapy for Patent Ductus Arteriosus in Neonates Born Extremely Preterm. J. Pediatr. 2018, 195, 292–296.e3. [Google Scholar] [CrossRef]
- Liu, J.; Guo, G.; Kurepa, D.; Volpicelli, G.; Sorantin, E.; Lovrenski, J.; Alonso-Ojembarrena, A.; Hsieh, K.-S.; Lodha, A.; Yeh, T.F.; et al. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J. Matern.-Fetal Neonatal Med. 2022, 35, 1003–1016. [Google Scholar] [CrossRef]
- Dani, C.; Lista, G.; Bianchi, S.; Mosca, F.; Schena, F.; Ramenghi, L.; Zecca, E.; Vento, G.; Poggi, C.; Leonardi, V.; et al. Intravenous paracetamol in comparison with ibuprofen for the treatment of patent ductus arteriosus in preterm infants: A randomized controlled trial. Eur. J. Pediatr. 2021, 180, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.I.; Chang, Y.S.; Kim, J.; Choi, J.H.; Ahn, S.Y.; Park, W.S. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23–28 weeks of gestation. PLoS ONE 2019, 14, e0212256. [Google Scholar] [CrossRef] [PubMed]
- Benitz, W.E.; Committee on Fetus and Newborn; Watterberg, K.L.; Aucott, S.; Cummings, J.J.; Eichenwald, E.C.; Goldsmith, J.; Poindexter, B.B.; Puopolo, K.; Stewart, D.L.; et al. Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2016, 137, e20153730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambonie, G.; Rozé, J.-C.; Marchand-Martin, L.; Marret, S.; Durrmeyer, X.; Torchin, H.; Ancel, P.-Y. Neurodevelopment at 5 Years of Age According to Early Screening for Patent Ductus Arteriosus in Extremely Preterm Infants. JAMA 2022, 328, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Aichhorn, L.; Küng, E.; Habrina, L.; Werther, T.; Berger, A.; Urlesberger, B.; Schwaberger, B. The Role of Lung Ultrasound in the Management of the Critically Ill Neonate—A Narrative Review and Practical Guide. Children 2021, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Oulego-Erroz, I.; Alonso-Quintela, P.; Terroba-Seara, S.; Jiménez-González, A.; Rodríguez-Blanco, S. Early assessment of lung aeration using an ultrasound score as a biomarker of developing bronchopulmonary dysplasia: A prospective observational study. J. Perinatol. 2021, 41, 62–68. [Google Scholar] [CrossRef]
- El Amrousy, D.; Elgendy, M.; Eltomey, M.; Elmashad, A.E. Value of lung ultrasonography to predict weaning success in ventilated neonates. Pediatr. Pulmonol. 2020, 55, 2452–2456. [Google Scholar] [CrossRef]
- Zong, H.; Huang, Z.; Zhao, J.; Lin, B.; Fu, Y.; Lin, Y.; Huang, P.; Sun, H.; Yang, C. The Value of Lung Ultrasound Score in Neonatology. Front. Pediatr. 2022, 10, 791664. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, X.-M.; Niu, L.; Ni, W.-X.; Zhang, Z.-Q. Lung Ultrasound Score Predicts the Extravascular Lung Water Content in Low-Birth-Weight Neonates with Patent Ductus Arteriosus. Med. Sci. Monit. 2020, 26, e921671. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N. Role of chest ultrasound in neonatal lung disease: A review of current evidences. J. Matern.-Fetal Neonatal Med. 2019, 32, 310–316. [Google Scholar] [CrossRef]
- Bland, R.D.; Nielson, D.W. Developmental changes in lung epithelial ion transport and liquid movement. Annu. Rev. Physiol. 1992, 54, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Olver, R.E.; Walters, D.V. Developmental regulation of lumenal lung fluid and electrolyte transport. Respir. Physiol. Neurobiol. 2007, 159, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Liebowitz, M.; Katheria, A.; Sauberan, J.; Singh, J.; Nelson, K.; Hassinger, D.C.; Aucott, S.W.; Kaempf, J.; Kimball, A.; Fernandez, E.; et al. Lack of Equipoise in the PDA-TOLERATE Trial: A Comparison of Eligible Infants Enrolled in the Trial and Those Treated Outside the Trial. J. Pediatr. 2019, 213, 222–226.e2. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, A.S.; Gardin, J.M.; Martin, R.P.; Parisi, A.F.; Popp, R.L.; Quinones, M.A.; Stevenson, J.G. Guidelines for optimal physician training in echocardiography Recommendations of the American Society of Echocardiography Committee for Physician training in Echocardiography. Am. J. Cardiol. 1987, 60, 158–163. [Google Scholar] [CrossRef]
- Popat, H.; Robledo, K.P.; Sebastian, L.; Evans, N.; Gill, A.; Kluckow, M.; Sinhal, S.; De Waal, K.; Tarnow-Mordi, W.; Osborn, D. Interobserver agreement and image quality of functional cardiac ultrasound measures used in a randomised trial of delayed cord clamping in preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 103, F257–F263. [Google Scholar] [CrossRef]
- Bedetti, G.; Gargani, L.; Corbisiero, A.; Frassi, F.; Poggianti, E.; Mottola, G. Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc. Ultrasound 2006, 4, 34. [Google Scholar] [CrossRef] [Green Version]

| Score | Sonographic Appearance |
|---|---|
| 0 | presence of <3 well-spaced B-lines |
| 1 | presence of ≥3 well-spaced B-lines |
| 2 | B-lines are difficult to count, or partially coalescent |
| 3 | fully coalescent B-Lines, with or without minor consolidations limited to the subpleural space |
| 4 | extended consolidations |
| Non-hsPDA Group (n = 35) | hsPDA Group (n = 46) | p Value | |
|---|---|---|---|
| GA, weeks | 25.0 (24.4, 25.4) | 24.8 (24.2, 25.2) | 0.058 |
| Birth weight, g | 718 ± 109 | 688 ± 110 | 0.069 |
| Male | 24 (68.6) | 24 (52.2) | 0.173 |
| Apgar score 1 min | 6 (5, 8) | 6 (5, 8) | 0.427 |
| Apgar score 5 min | 9 (8, 10) | 9 (8, 10) | 0.972 |
| Spontaneous vaginal delivery | 26 (74.3) | 36 (78.3) | 0.676 |
| Antenatal steroids, full course | 23 (65.7) | 27 (58.7) | 0.520 |
| MSAF | 1 (2.9) | 1 (2.2) | 1.000 |
| PROM > 18 h | 8 (22.9) | 14 (30.4) | 0.448 |
| EOS | 10 (28.6) | 12 (26.1) | 0.803 |
| EPS | 32 (91.4) | 43 (93.5) | 0.998 |
| MV | 29 (82.8) | 38 (82.6) | 0.977 |
| LUS | 30.3 ± 4.3 | 38.2 ± 2.8 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, H.; Huang, Z.; Lin, B.; Zhao, J.; Fu, Y.; Yu, Y.; Sun, H.; Yang, C. The Predictive Value of Lung Ultrasound Score on Hemodynamically Significant Patent Ductus Arteriosus among Neonates ≤25 Weeks. Diagnostics 2023, 13, 2263. https://doi.org/10.3390/diagnostics13132263
Zong H, Huang Z, Lin B, Zhao J, Fu Y, Yu Y, Sun H, Yang C. The Predictive Value of Lung Ultrasound Score on Hemodynamically Significant Patent Ductus Arteriosus among Neonates ≤25 Weeks. Diagnostics. 2023; 13(13):2263. https://doi.org/10.3390/diagnostics13132263
Chicago/Turabian StyleZong, Haifeng, Zhifeng Huang, Bingchun Lin, Jie Zhao, Yongping Fu, Yanliang Yu, Hongyan Sun, and Chuanzhong Yang. 2023. "The Predictive Value of Lung Ultrasound Score on Hemodynamically Significant Patent Ductus Arteriosus among Neonates ≤25 Weeks" Diagnostics 13, no. 13: 2263. https://doi.org/10.3390/diagnostics13132263





