Permutation Entropy Analysis to Intracranial Hypertension from a Porcine Model
Abstract
:1. Introduction
2. Brief Bibliographical Review
- In [10], a dataset of nine Sprague Dawley rats was exposed to cycles of hypotension (45–50 mmHg) for 15 min, followed by resuscitation and equilibration. Approximate entropy was calculated with parameters and r = 0.2 SD; its value resulted in the hypotension periods of 1.086 (), and after resuscitation, 1.242 (), which were statistically significantly different from those of the basal periods 0.691 (). However, there is no clear definition of the power of the statistical test used or why it uses these parameter values.
- In [42], data collected from 93 patients from 1998 to 2003 who were admitted to the Pediatric Intensive Care Unit of the Doernbecher Children’s Hospital (Oregon Health and Science University) were used. The data contribute to the casuistry of the decrease in complexity, measured using ApEnt, during periods of intracranial hypertension. The value of the parameters used in the estimation of ApEnt is not explicit.
- A retrospective analysis of 11 clinical intracranial hypertension episodes—a case series over a 30-month period from April 2000 to January 2003 is presented in [13]. They found that ApEn is lower during the intracranial hypertension period than during the stable and recovering periods.
- In [13], 11 episodes of intracranial hypertension from 7 subjects requiring ventriculostomy catheters for intracranial pressure monitoring and/or cerebral spinal fluid drainage were analyzed, with parameters
- In [11], 12 patients submitted to a preoperative study for the diagnosis of normotensive hydrocephalus. These patients showed oscillatory episodes of ICP in their nocturnal recordings. The ApEnt calculated on the ICP oscillatory state had a value of 0.23 () was statistically different from the baseline period 0.41 (). In this case, there was also no consideration of the statistical power of the test and no clarification of the value of the parameters used to calculate ApEnt.
- In [13], a retrospective study of 11 ICP segments belonging to 7 traumatic brain-injured (TBI) pediatric patients is presented. ApEnt was calculated in three different periods: stable: 0.442 (), critical 0.26 (), and recovery 0.40 () using the parameter values (, r = 0.2 SD).
- The ICP time series was from four patients with hypertensive episodes using wavelet entropy. The results found are consistent with the general casuistry; the change in complexity is negative in the periods of the hypertension plateau. The wavelet entropy gives information that the wavelet energy is distributed differently in the basal condition than in the plateau, in which it is concentrated in low-frequency bands. This allows us to infer that the episodes of intracranial hypertension could be caused by a rearrangement of the oscillatory energy in the brain.
- A total of 120 ICP signals recorded during the infusion test were analyzed by wavelet entropy to characterize the different stages of the infusion test as a function of this entropy. The lowest value of the WE was found in the basal phase, a growth of this entropy was in the infusion phase, and the highest value was in the plateau phase, to finally decrease slightly in the recovery phase.
- In [16], a database of 325 patients with traumatic brain injury who were admitted to the Neurosciences Critical Care Unit, Addenbrooke’s Hospital, Cambridge, United Kingdom, between 2002 and 2010 was used. They reported that reduced complexity of ICP calculated in 3 h moving windows, might predict death in traumatic brain injured patients. The parameters used to calculate a multiscale of the sample entropy were not explicitly explained in [16], but they said that was used in [16].
- In [17], 30 patients were admitted to the neurocritical care unit at Addenbrooke’s Hospital between February 2005 and June 2006. The parameters used were , r = 0.2 SD for SampEnt estimation. It also contributes to the casuistry of the decrease in complexity during periods of intracranial hypertension.
- In [19], 69 signals were selected from 33 patients with normal pressure hydrocephalus and 36 patients with a secondary form of normal pressure hydrocephalus. The possibility of discriminating patients with different types of hydrocephalus by infusion tests using permutation entropy was discussed. PE-ICP was calculated using , . It was found that permutation entropy is able to distinguish between periods of “norm” and hydrocephalus, although it is not able to classify patients with different types of hydrocephalus.
- In [18], an analysis of the MIMIC-III waveform database matched subset containing all the complete data (vital signs, signals, clinical analysis, and annotations) of a subset of patients from the MIMIC-III database [36] is presented. The authors show that there is a PE-ICPd decrease in the period of hypertension; using the missing values technique, they showed that the increase in the period of hypertension is related to a decrease in the degree of freedom of the system to adapt, which would be an indication that the complexity decreased by the failure of the mechanisms of cerebral autoregulation, according to the theory proposed in [8].
3. Materials and Methods
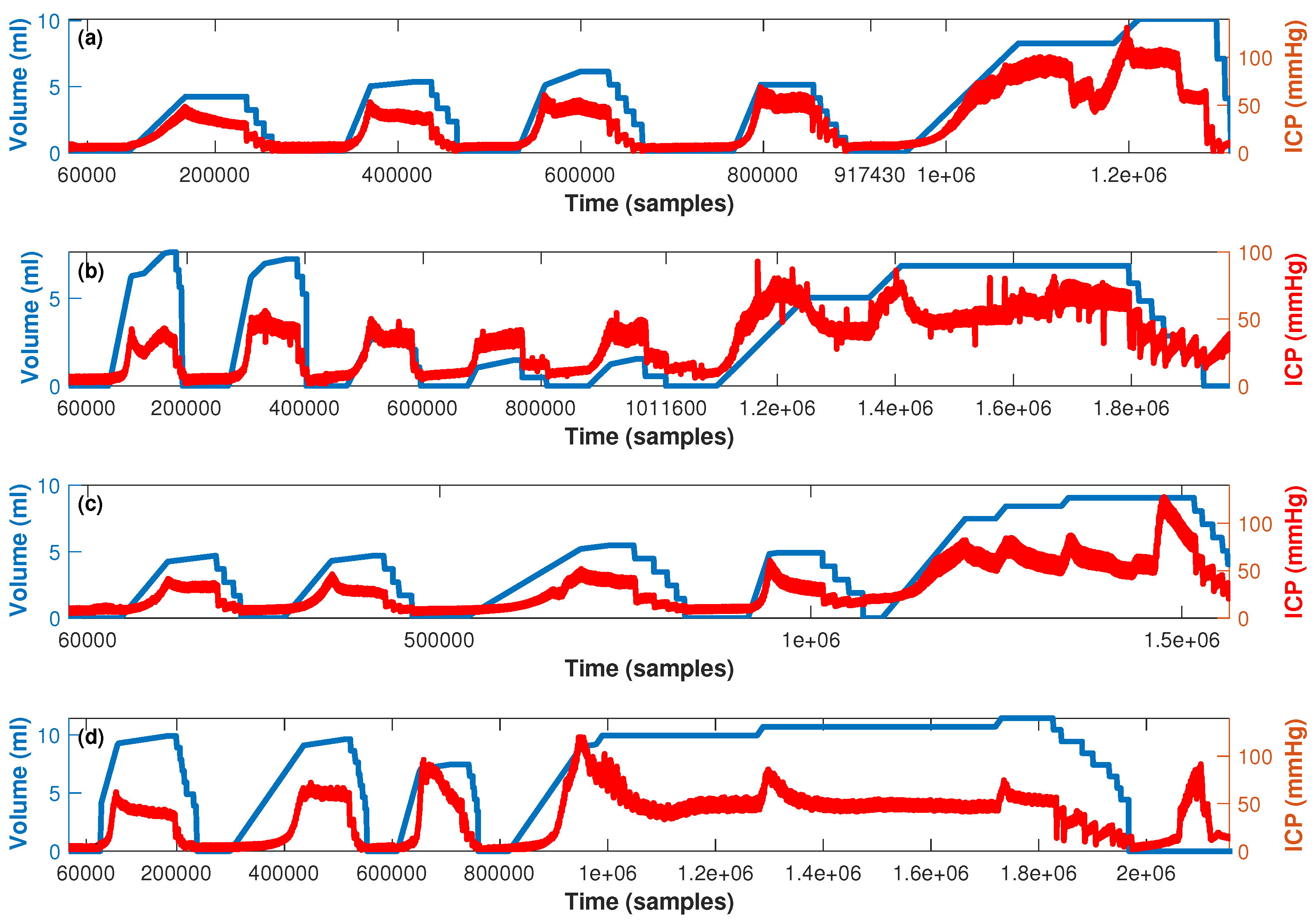
3.1. Experimental Data
- Anesthesia stage: General anesthesia was induced through an atrial vein with 6 mg/kg fentanyl, 4 mg/kg propofol, and 1.2–1.5 mg/kg rocuronium. Intubation was performed with a 7.0 mm cuffed tube. Anesthesia was maintained with a continuous intravenous infusion of propofol 0.25–0.30 mcg/kg/min, remifentanil 0.5 mcg/kg/min, and pancuronium 0.04 mgmg/kg/h. Mechanical ventilation was performed in a pressure-controlled mode with a positive pressure at the end of the expiration of 5 cm H2O and a FiO2 of 0.40. The respiratory rate and tidal volume were adjusted to maintain normocarbia throughout the experiment, which was controlled with a spirometer. Normovolemia was maintained by infusing a complete electrolyte solution at a rate of 10–15 mL/kg/h.
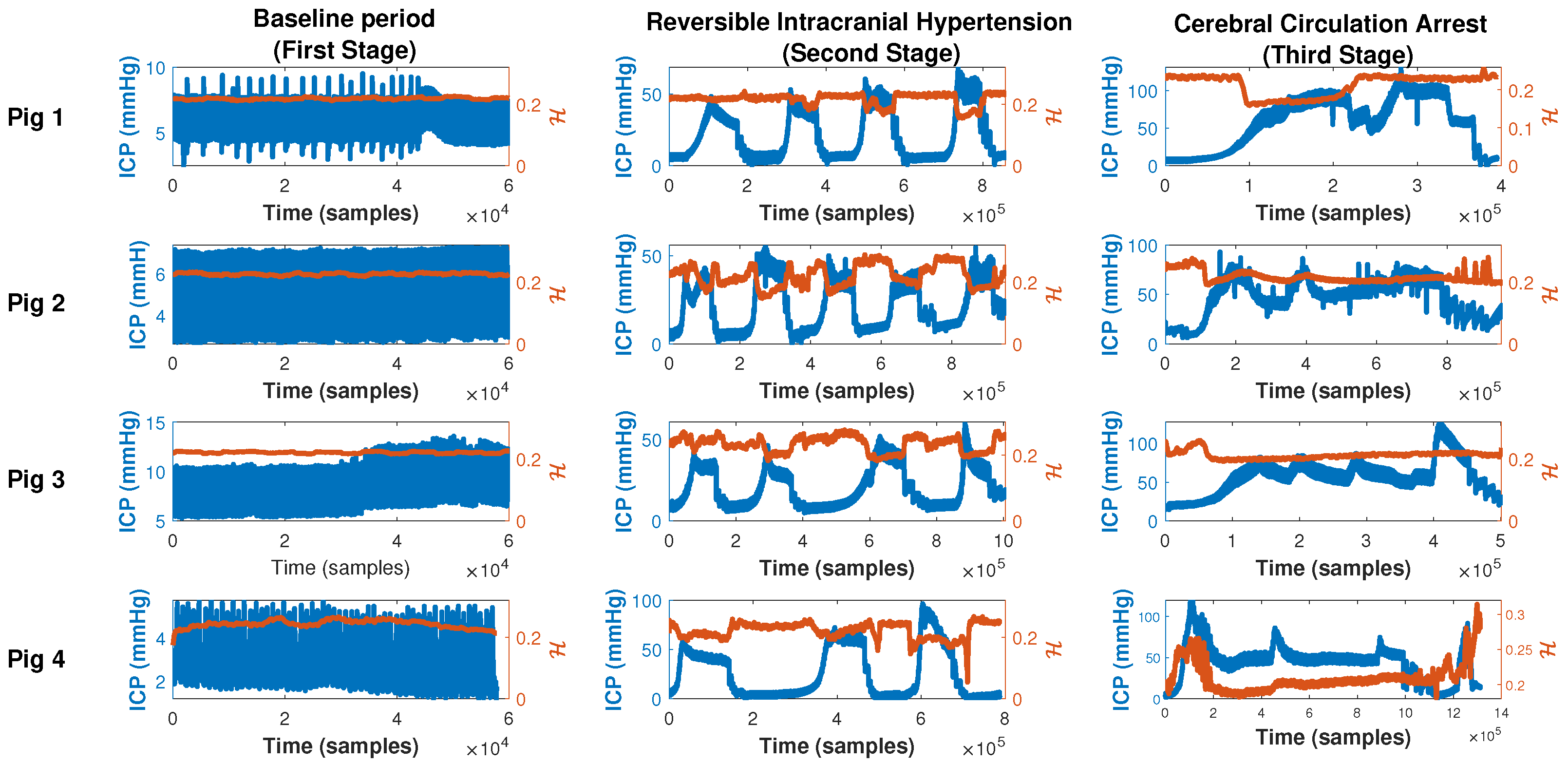
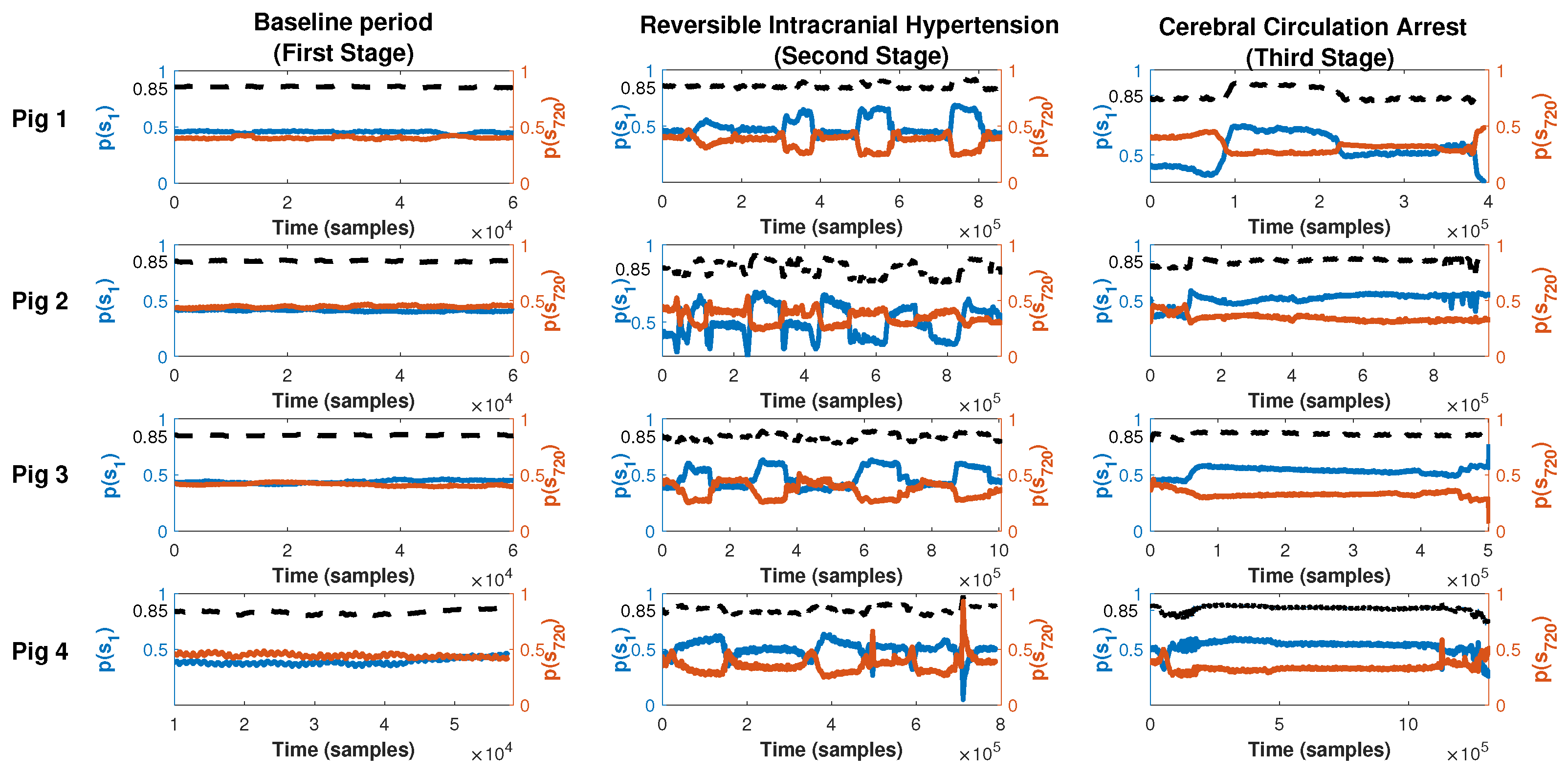
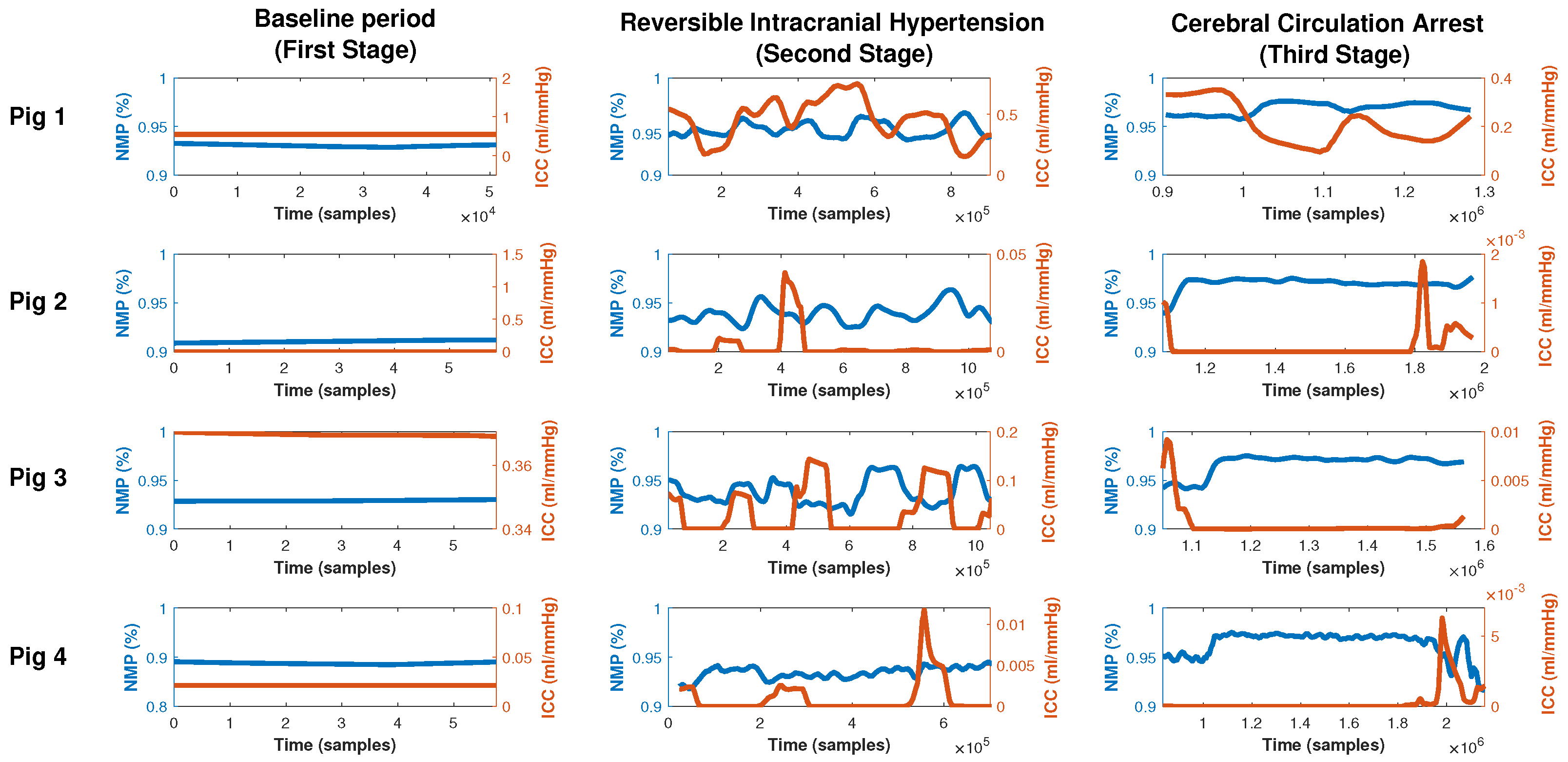
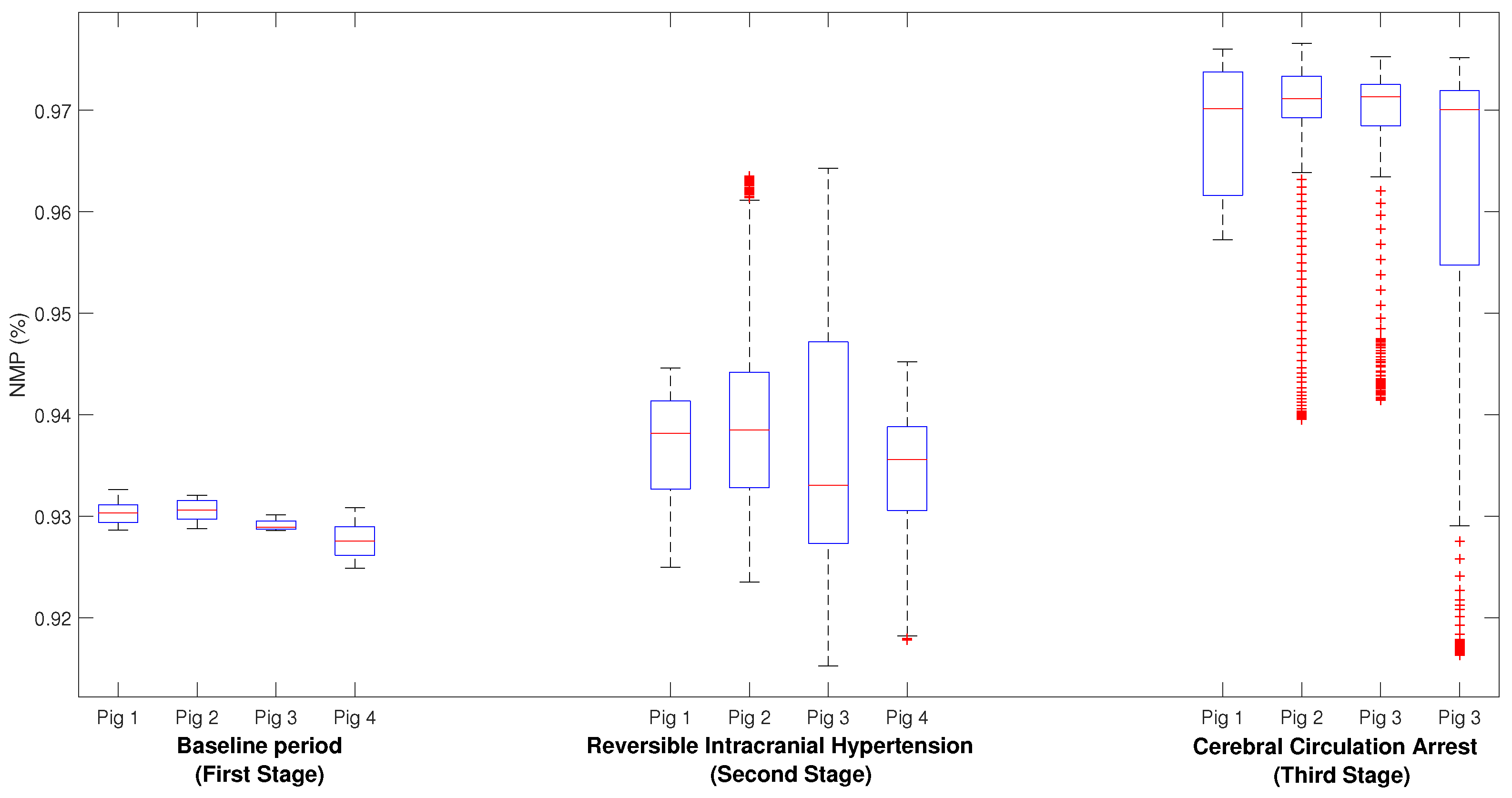
- First stage (baseline period): In this stage, after proper anesthesia, the ICP catheter was placed through a burr hole 20 mm anterior to the coronal suture, and 15 mm from the sagittal suture (midline) and, once the correct measurement of ICP was obtained, a duration of 5 min was recorded.
- Second stage (reversible intracranial hypertension): An 8 French Foley catheter (FC) was placed through another burr hole 20 mm anterior to the coronal suture and a 15 mm sagittal suture contralateral to the ICP catheter. After this, several reversible intracranial hypertension episodes were generated using 0.9% saline in the FC with a continuous infusion pump to control the volume and rate of infusion. After reaching the target ICP value—after 5 min—deflation was carried out progressively at a rate of 1 mL/min.
- Third stage (cerebral circulatory arrest): Intracranial hypertension was induced using the same Foley catheter and the infusion pump to a target cerebral perfusion pressure of less than 10 mmHg for no less than one hour and an EEG compatible with electrocerebral silence.
3.2. Intracranial Compliance
3.3. Permutation Entropy
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, M.H. Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J. Cereb. Blood Flow Metab. 2016, 36, 1338–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnut, R.M.; Temkin, N.; Carney, N.; Dikmen, S.; Rondina, C.; Videtta, W.; Petroni, G.; Lujan, S.; Pridgeon, J.; Barber, J.; et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N. Engl. J. Med. 2012, 367, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observational cohort study. Lancet Neurol. 2021, 20, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C.; Goldenberg, F.D. Intracranial pressure in traumatic brain injury: From thresholds to heuristics. Crit. Care Med. 2020, 48, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C.; Desai, M.; Damoulakis, G.; Zeiler, F.A. Intracranial Pressure Threshold Heuristics in Traumatic Brain Injury: One, None, Many! Neurocritical Care 2020, 32, 672–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolis, G.; Nicolis, G. Introduction to Nonlinear Science; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Kumar, A. Chaos theory: Impact on and applications in medicine. J. Health Allied Sci. NU 2012, 2, 93–99. [Google Scholar] [CrossRef]
- Vaillancourt, D.E.; Newell, K.M. Changing complexity in human behavior and physiology through aging and disease. Neurobiol. Aging 2002, 23, 1–11. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Goldberger, A.L. Loss of’complexity’and aging: Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Beaumont, A.; Marmarou, A. Approximate entropy: A regularity statistic for assessment of intracranial pressure. In Intracranial Pressure and Brain Biochemical Monitoring; Springer: Berlin/Heidelberg, Germany, 2002; pp. 193–195. [Google Scholar]
- Hu, X.; Miller, C.; Vespa, P.; Bergsneider, M. Adaptive computation of approximate entropy and its application in integrative analysis of irregularity of heart rate variability and intracranial pressure signals. Med. Eng. Phys. 2008, 30, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Hornero, R.; Aboy, M.; Abásolo, D. Analysis of intracranial pressure during acute intracranial hypertension using Lempel–Ziv complexity: Further evidence. Med. Biol. Eng. Comput. 2007, 45, 617–620. [Google Scholar] [CrossRef]
- Hornero, R.; Aboy, M.; Abasolo, D.; McNames, J.; Wakeland, W.; Goldstein, B. Complex analysis of intracranial hypertension using approximate entropy. Crit. Care Med. 2006, 34, 87–95. [Google Scholar] [CrossRef]
- Gao, L.; Smielewski, P.; Czosnyka, M.; Ercole, A. Cerebrovascular signal complexity six hours after intensive care unit admission correlates with outcome after severe traumatic brain injury. J. Neurotrauma 2016, 33, 2011–2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.W.; Czosnyka, M.; Shieh, J.S.; Smielewska, A.; Pickard, J.D.; Smielewski, P. Complexity of intracranial pressure correlates with outcome after traumatic brain injury. Brain 2012, 135, 2399–2408. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.W.; Czosnyka, M.; Shieh, J.S.; Pickard, J.D.; Smielewski, P. Continuous monitoring of the complexity of intracranial pressure after head injury. In Intracranial Pressure and Brain Monitoring XV; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–35. [Google Scholar]
- Soehle, M.; Gies, B.; Smielewski, P.; Czosnyka, M. Reduced complexity of intracranial pressure observed in short time series of intracranial hypertension following traumatic brain injury in adults. J. Clin. Monit. Comput. 2013, 27, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ciarrocchi, N.; Quiróz, N.; Traversaro, F.; San Roman, E.; Risk, M.; Goldemberg, F.; Redelico, F.O. The complexity of intracranial pressure as an indicator of cerebral autoregulation. Commun. Nonlinear Sci. Numer. Simul. 2019, 75, 192–199. [Google Scholar] [CrossRef]
- Adjei, T.; Abásolo, D.; Santamarta, D. Intracranial pressure for the characterization of different types of hydrocephalus: A Permutation Entropy study. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 4198–4201. [Google Scholar]
- Khan, J.; Mariappan, R.; Venkatraghavan, L. Entropy as an indicator of cerebral perfusion in patients with increased intracranial pressure. J. Anaesthesiol. Clin. Pharmacol. 2014, 30, 409. [Google Scholar]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.; Müller, A.; Wessel, N. Practical considerations of permutation entropy. Eur. Phys. J. Spec. Top. 2013, 222, 249–262. [Google Scholar] [CrossRef]
- Traversaro, F.; Legnani, W.; Redelico, F.O. Influence of the signal to noise ratio for the estimation of Permutation Entropy. Phys. A Stat. Mech. Its Appl. 2020, 553, 124134. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Pigolotti, S.; Torrent, M.; Masoller, C. Numerical and experimental study of the effects of noise on the permutation entropy. New J. Phys. 2015, 17, 093002. [Google Scholar] [CrossRef]
- Traversaro, F.; Redelico, F.O. Characterization of autoregressive processes using entropic quantifiers. Phys. A Stat. Mech. Its Appl. 2018, 490, 13–23. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E. A mathematical theory of communication. ACM SIGMOBILE Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef] [Green Version]
- Jordan, D.; Stockmanns, G.; Kochs, E.F.; Pilge, S.; Schneider, G. Electroencephalographic order pattern analysis for the separation of consciousness and unconsciousness: An analysis of approximate entropy, permutation entropy, recurrence rate, and phase coupling of order recurrence plots. J. Am. Soc. Anesthesiol. 2008, 109, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Cui, S.; Voss, L.J. Using permutation entropy to measure the electroencephalographic effects of sevoflurane. J. Am. Soc. Anesthesiol. 2008, 109, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Liang, Z.; Voss, L.J.; Sleigh, J.W. Multiscale permutation entropy analysis of EEG recordings during sevoflurane anesthesia. J. Neural Eng. 2010, 7, 046010. [Google Scholar] [CrossRef]
- Olofsen, E.; Sleigh, J.; Dahan, A. Permutation entropy of the electroencephalogram: A measure of anaesthetic drug effect. Br. J. Anaesth. 2008, 101, 810–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Cardoso-Cruz, H.; Silva, F.; Galhardo, V.; Antunes, L. Comparison of anesthetic depth indexes based on thalamocortical local field potentials in rats. J. Am. Soc. Anesthesiol. 2010, 112, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Campos, S.; Monteiro, J.; Venâncio, C.; Costa, B.; Guedes de Pinho, P.; Antunes, L. Performance of anesthetic depth indexes in rabbits under propofol anesthesia: Prediction probabilities and concentration-effect relations. J. Am. Soc. Anesthesiol. 2011, 115, 303–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinkel, S.; Marwan, N.; Kurths, J. Order patterns recurrence plots in the analysis of ERP data. Cogn. Neurodyn. 2007, 1, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Schinkel, S.; Marwan, N.; Kurths, J. Brain signal analysis based on recurrences. J.-Physiol.-Paris 2009, 103, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Pollard, T.J.; Shen, L.; Lehman, L.w.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Anthony Celi, L.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Scott, D.J.; Villarroel, M.; Clifford, G.D.; Saeed, M.; Mark, R.G. Open-access MIMIC-II database for intensive care research. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 8315–8318. [Google Scholar]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [Green Version]
- Rosso, O.A.; Blanco, S.; Yordanova, J.; Kolev, V.; Figliola, A.; Schürmann, M.; Başar, E. Wavelet entropy: A new tool for analysis of short duration brain electrical signals. J. Neurosci. Methods 2001, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bonal, A.; Marshak, A. Approximate entropy and sample entropy: A comprehensive tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornero, R.; Aboy, M.; Abásolo, D.; McNames, J.; Goldstein, B. Interpretation of approximate entropy: Analysis of intracranial pressure approximate entropy during acute intracranial hypertension. IEEE Trans. Biomed. Eng. 2005, 52, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Hu, X.; Yao, D. Improved wavelet entropy calculation with window functions and its preliminary application to study intracranial pressure. Comput. Biol. Med. 2013, 43, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Scalzo, F.; Bergsneider, M.; Vespa, P.; Chad, M.; Hu, X. Wavelet entropy characterization of elevated intracranial pressure. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 2924–2927. [Google Scholar]
- Wostyn, P. Intracranial pressure and Alzheimer’s disease: A hypothesis. Med. Hypotheses 1994, 43, 219–222. [Google Scholar] [CrossRef]
- Wostyn, P. Can chronic increased intracranial pressure or exposure to repetitive intermittent intracranial pressure elevations raise your risk for Alzheimer’s disease? Med. Hypotheses 2004, 62, 925–930. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, N. Alzheimer Disease: Intracranial Pressure and Glaucoma. In Intraocular and Intracranial Pressure Gradient in Glaucoma; Springer: Berlin/Heidelberg, Germany, 2019; pp. 307–309. [Google Scholar]
- Ciarrocchi, N.M.; Pose, F.; Saez, P.; del Carman Garcia, M.; Padilla, F.; Plou, P.; Hem, S.; Karippacheril, J.G.; Gutiérrez, A.F.; Redelico, F.O. Reversible focal intracranial hypertension swine model with continuous multimodal neuromonitoring. J. Neurosci. Methods 2022, 373, 109561. [Google Scholar] [CrossRef] [PubMed]
- Karippacheril, J.; Ho, T. Data acquisition from S/5 GE Datex anesthesia monitor using VSCapture: An open source.NET/Mono tool. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 423. [Google Scholar] [CrossRef]
- Petersen, K.; Bøgevig, S.; Riis, T.; Andersson, N.; Dalhoff, K.; Holst, J.; Knop, F.; Faber, J.; Petersen, T.; Christensen, M. High-Dose Glucagon Has Hemodynamic Effects Regardless of Cardiac Beta-Adrenoceptor Blockade: A Randomized Clinical Trial. J. Am. Heart Assoc. 2020, 9, e016828. [Google Scholar] [CrossRef]
- Pybus, D. Expanding the role of mobile devices in the operating room: Direct wireless connection to the anesthesia monitor. J. Cardiothorac. Vasc. Anesth. 2015, 29, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadesse, G.; Zhu, T.; Le, N.T.N.; Hung, N.; Duong, H.; Khanh, T.; Quang, P.; Tran, D.; Yen, L.; Doorn, R.; et al. Severity detection tool for patients with infectious disease. Healthc. Technol. Lett. 2020, 7, 45–50. [Google Scholar] [CrossRef]
- Dubost, C.; Humbert, P.; Oudre, L.; Labourdette, C.; Vayatis, N.; Vidal, P.P. Quantitative assessment of consciousness during anesthesia without EEG data. J. Clin. Monit. Comput. 2021, 35, 993–1005. [Google Scholar] [CrossRef]
- Design and Development of a Tracheostomy Tube Test Device. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A952164&dswid=1009 (accessed on 14 April 2021).
- Trukhan, V.; Horakova, L.; Rozanek, M. Program Extension for Data Analysis from Operating Rooms. In Proceedings of the 2020 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 29–30 October 2020. [Google Scholar] [CrossRef]
- McAlister, O.; Guldenring, D.; Finlay, D.; Bond, R.; Torney, H.; McCartney, B.; Davis, L.; Crawford, P.; Harvey, A. CPR Guideline Chest Compression Depths May Exceed Requirements for Optimal Physiological Response. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018. [Google Scholar] [CrossRef]
- Faul, M.; Wald, M.M.; Rutland-Brown, W.; Sullivent, E.E.; Sattin, R.W. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: Testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J. Trauma Acute Care Surg. 2007, 63, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monro, A. Observations on the Structure and Functions of the Nervous System; Edinburgh: Edinburgh, UK, 1783. [Google Scholar]
- Kellie, G. An Account of the Appearances Observed in the Dissection of Two of Three Individuals Presumed to Have Perished in the Storm of the 3d, and Whose Bodies Were Discovered in the Vicinity of Leith on the Morning of the 4th, November 1821; with Some Reflections on the Pathology of the Brain: Part I. Trans.-Med.-Chir. Soc. Edinb. 1824, 1, 84. [Google Scholar]
- Czosnyka, M.; Citerio, G. Brain Compliance: The Old Story with a New ‘et cetera’. Intensive Care Med. 2012, 38, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Ocamoto, G.N.; Russo, T.L.; Mendes Zambetta, R.; Frigieri, G.; Hayashi, C.Y.; Brasil, S.; Rabelo, N.N.; Spavieri Junior, D.L. Intracranial compliance concepts and assessment: A scoping review. Front. Neurol. 2021, 12, 756112. [Google Scholar] [CrossRef]
- Traversaro, F.; Risk, M.; Rosso, O.; Redelico, F. An empirical evaluation of alternative methods of estimation for Permutation Entropy in time series with tied values. arXiv 2017, arXiv:1707.01517. [Google Scholar]
- Amigó, J.M.; Kocarev, L.; Szczepanski, J. Order patterns and chaos. Phys. Lett. A 2006, 355, 27–31. [Google Scholar] [CrossRef]
- Traversaro, F.; Redelico, F.O. Confidence intervals and hypothesis testing for the Permutation Entropy with an application to epilepsy. Commun. Nonlinear Sci. Numer. Simul. 2018, 57, 388–401. [Google Scholar] [CrossRef] [Green Version]
- Vasan, A.; Friend, J. Medical devices for low-and middle-income countries: A review and directions for development. J. Med. Devices 2020, 14, 010803. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pose, F.; Ciarrocchi, N.; Videla, C.; Redelico, F.O. Permutation Entropy Analysis to Intracranial Hypertension from a Porcine Model. Entropy 2023, 25, 267. https://doi.org/10.3390/e25020267
Pose F, Ciarrocchi N, Videla C, Redelico FO. Permutation Entropy Analysis to Intracranial Hypertension from a Porcine Model. Entropy. 2023; 25(2):267. https://doi.org/10.3390/e25020267
Chicago/Turabian StylePose, Fernando, Nicolas Ciarrocchi, Carlos Videla, and Francisco O. Redelico. 2023. "Permutation Entropy Analysis to Intracranial Hypertension from a Porcine Model" Entropy 25, no. 2: 267. https://doi.org/10.3390/e25020267
APA StylePose, F., Ciarrocchi, N., Videla, C., & Redelico, F. O. (2023). Permutation Entropy Analysis to Intracranial Hypertension from a Porcine Model. Entropy, 25(2), 267. https://doi.org/10.3390/e25020267





