The Caucasian Whortleberry Extract/Myrtle Essential Oil Loaded Active Films: Physicochemical Properties and Effects on Quality Parameters of Wrapped Turkey Breast Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
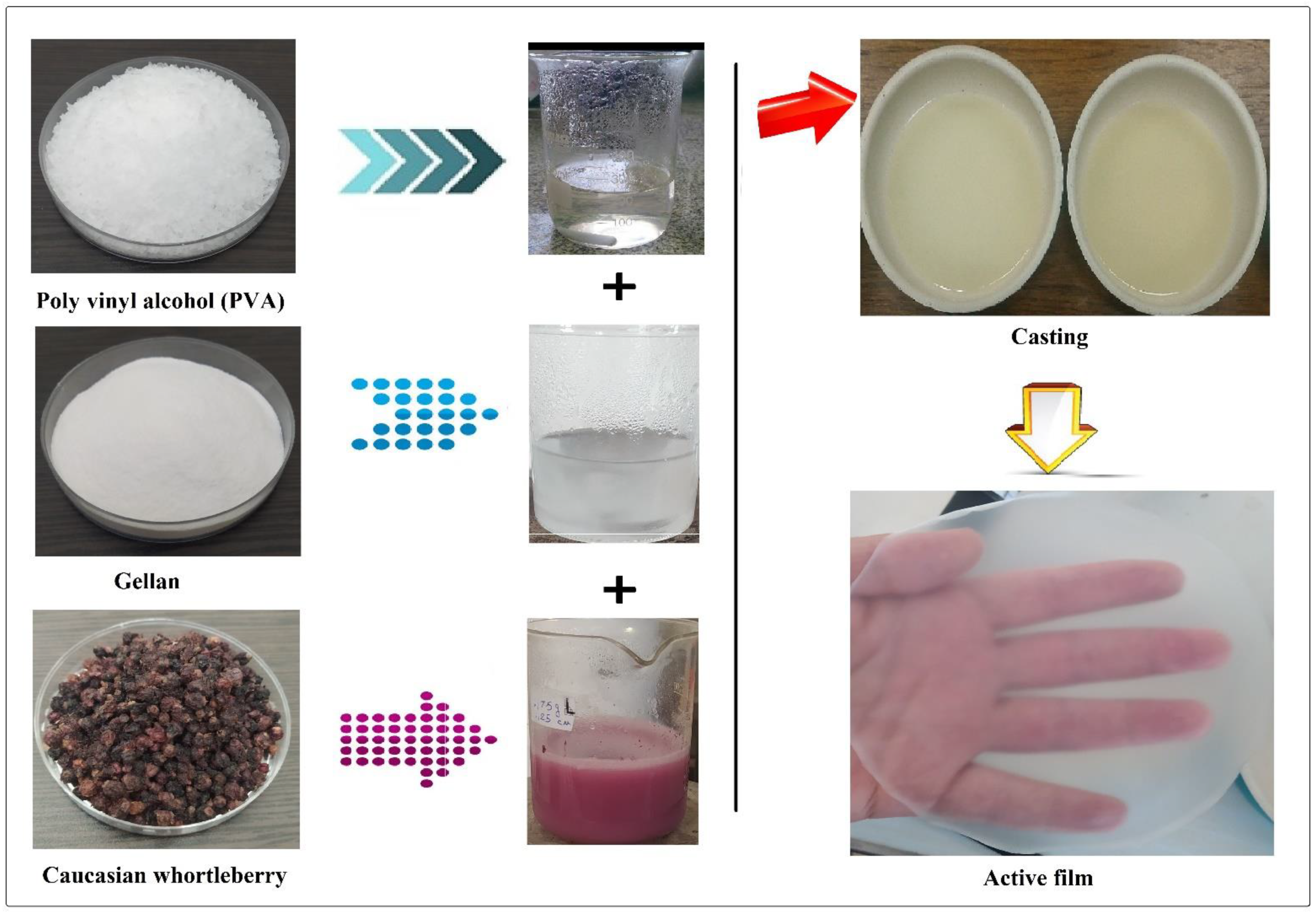
2.2. Preparation of CWE
2.3. Preparation of G/PVA/MEO/CWE Films
2.4. Characterization of Films
2.4.1. Mechanical Properties
2.4.2. Film Thickness Properties
2.4.3. Determination of Water Vapor Permeability (WVP)
2.4.4. Moisture Content (MC)
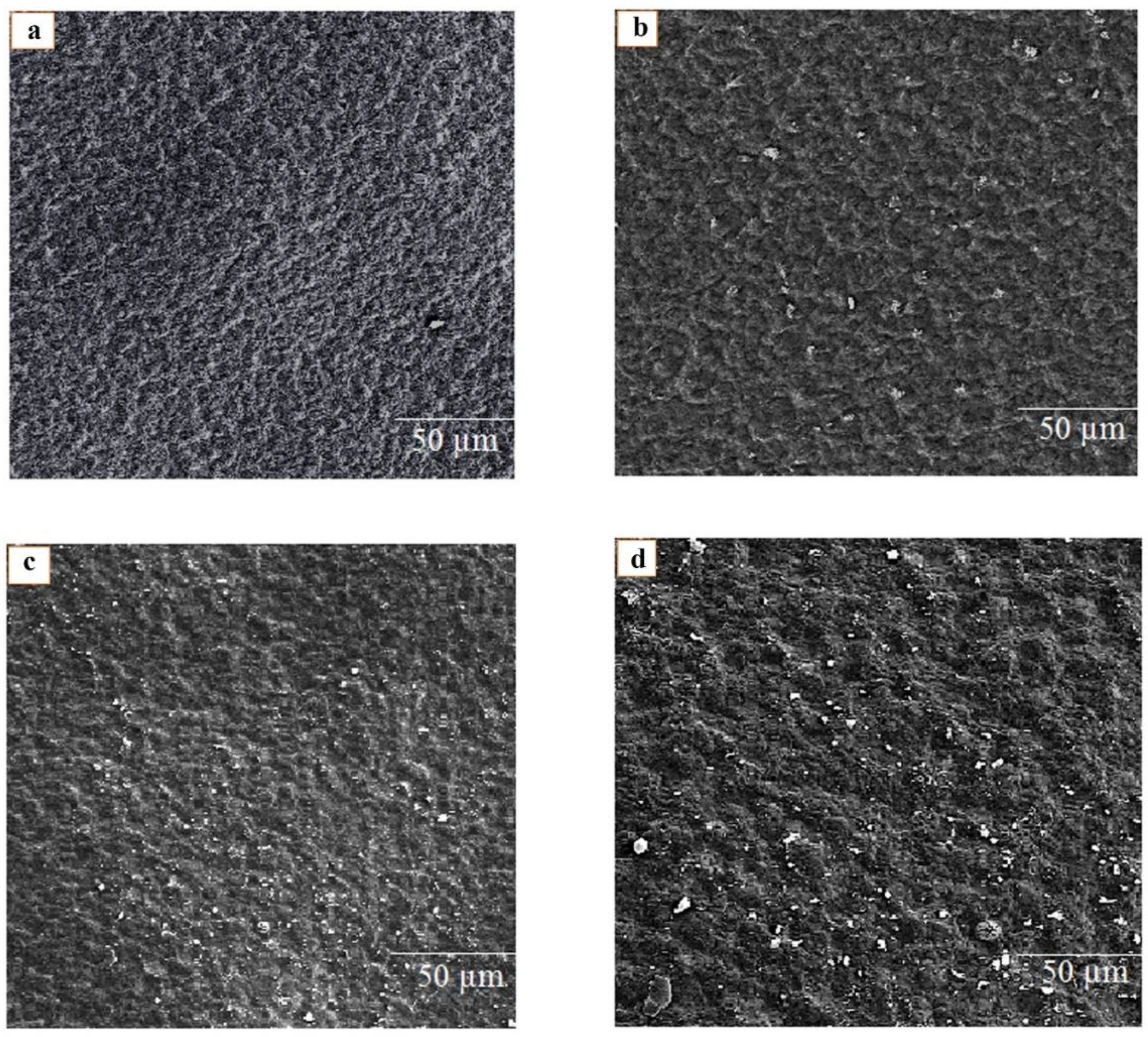
2.4.5. Field Emission Scanning Electron Microscopy (FE-SEM)
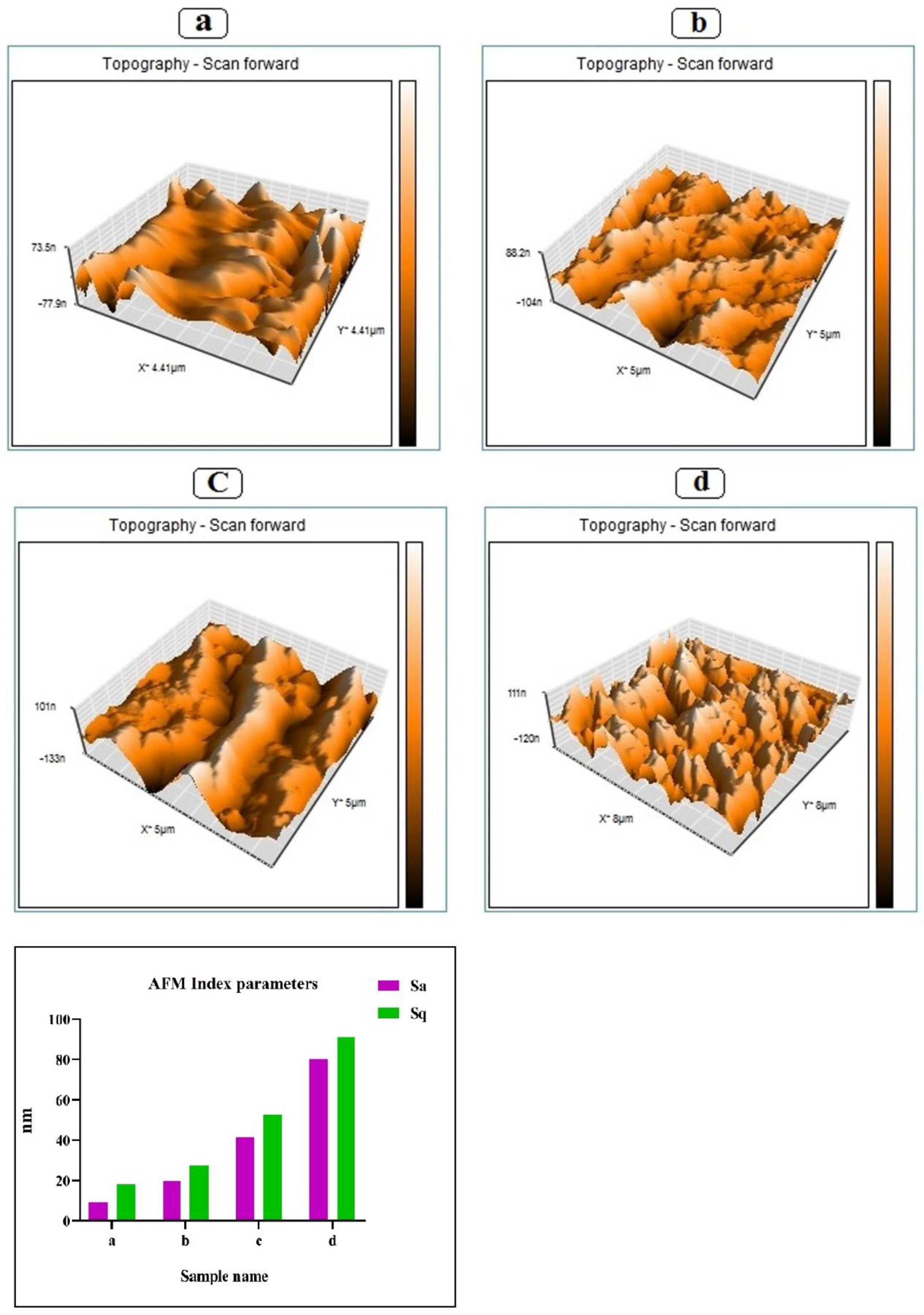
2.4.6. Atomic Force Microscopy (AFM)
2.4.7. Antibacterial Properties
MIC and MBC Studies
The Antibacterial Activity Determination of G/PVA/MEO/CWE Films
Turkey Breast Meat Preparation and Treatments
Microbiological Analysis of Turkey Breast Meat Samples
Inoculation of Turkey Breast Meat Samples by General Food-Borne Pathogenic Microorganisms
2.4.8. Chemical Analyses of Turkey Breast Meat
2.4.9. Sensory Assessment
2.4.10. Statistical Analysis
3. Results and Discussion
3.1. Mechanical Properties
3.2. Water Vapor Permeability (WVP) and Moisture Content (MC) Properties
3.3. Surface Morphology of Biodegradable Films Using FE-SEM
3.4. Atomic Force Microscope (AFM)
3.5. The MIC and MBC Evaluation for MEO and CWE
3.6. Antibacterial Activity of G/PVA /MEO/CWE Films
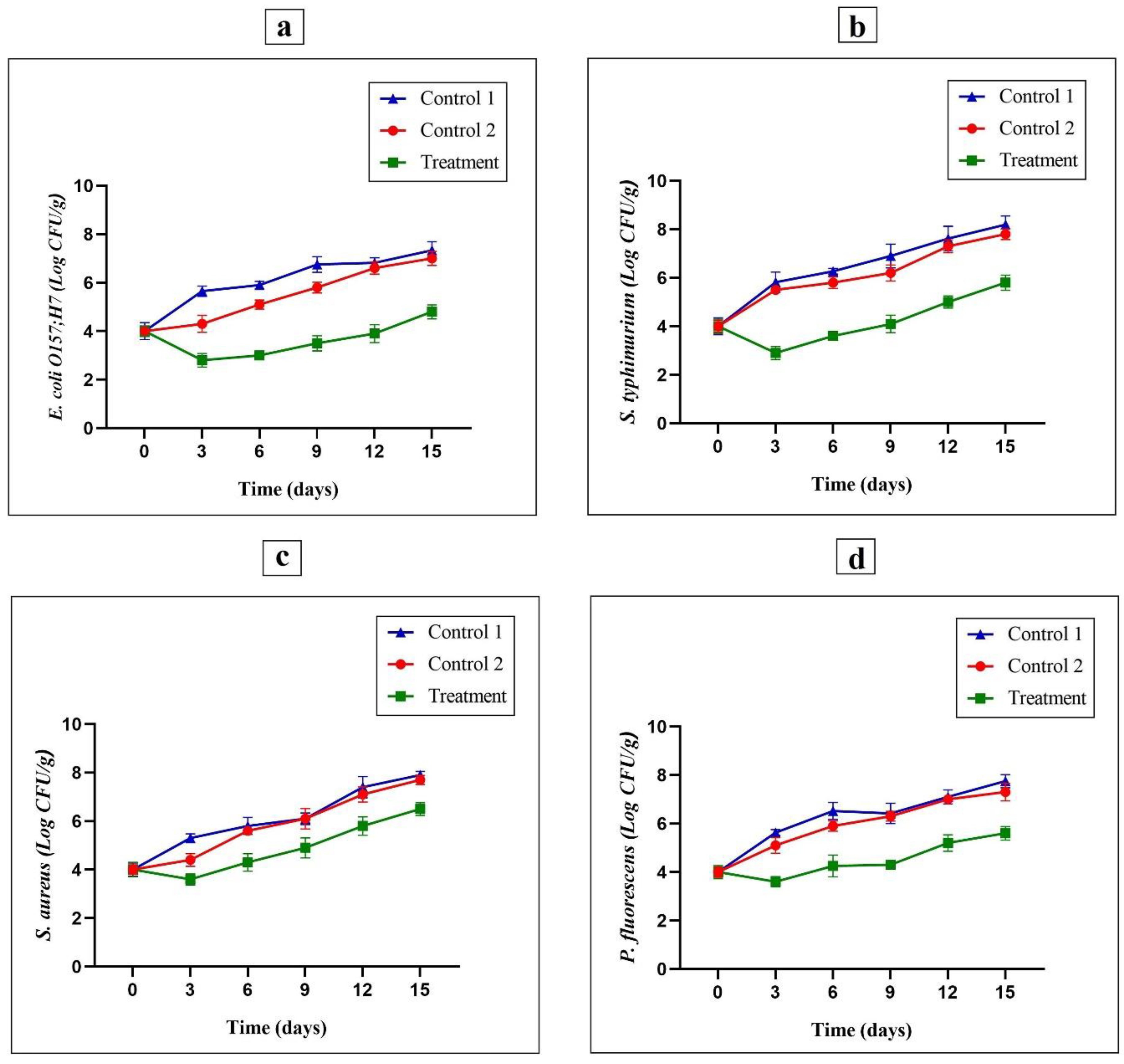
3.7. Microbial Analysis of Samples
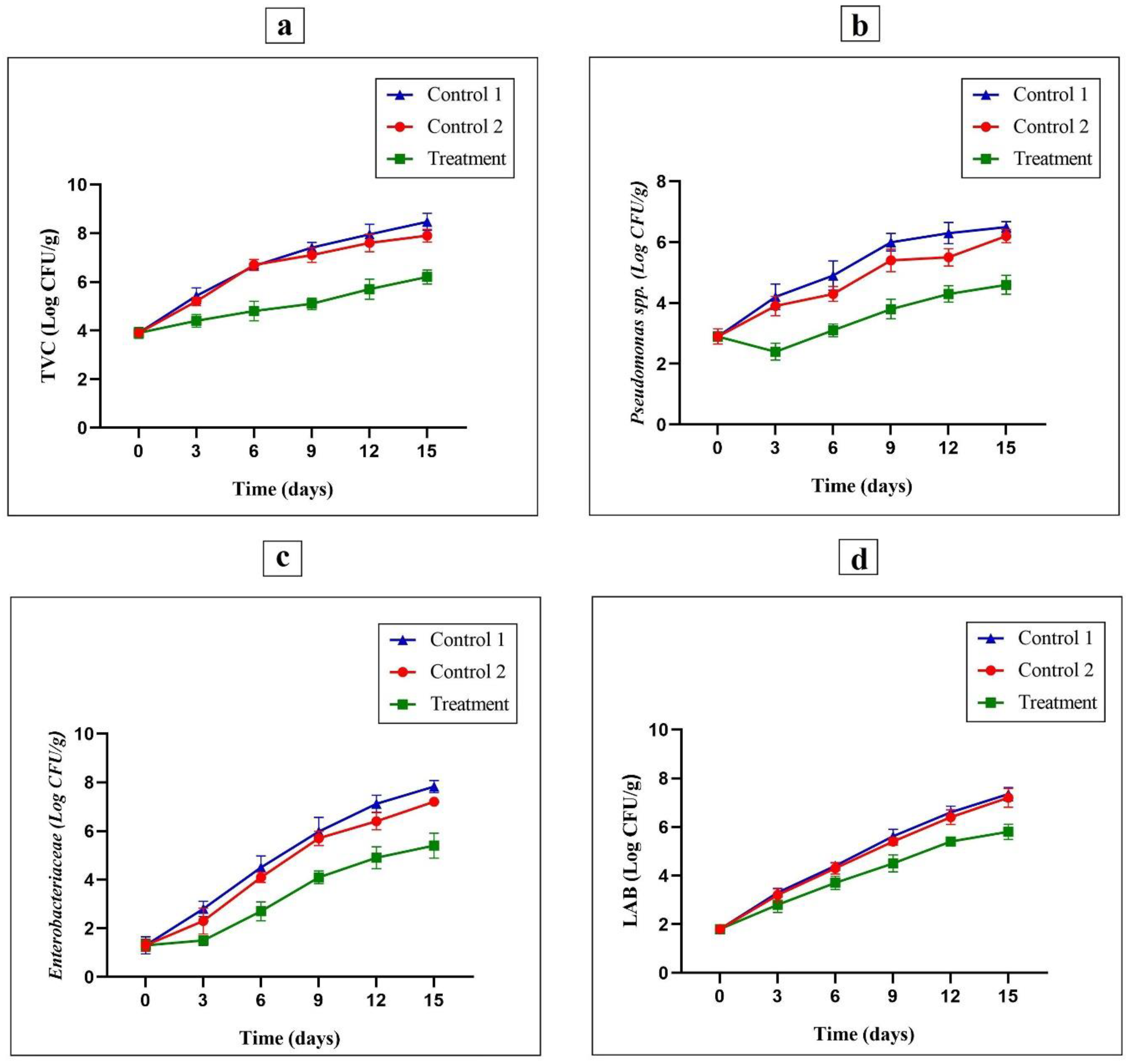
3.7.1. Determination of TVC Values
3.7.2. Pseudomonas spp. Count
3.7.3. Enterobacteriaceae Count
3.7.4. LAB
3.8. Evaluating the Inoculated Foodborne Pathogenic Microbes
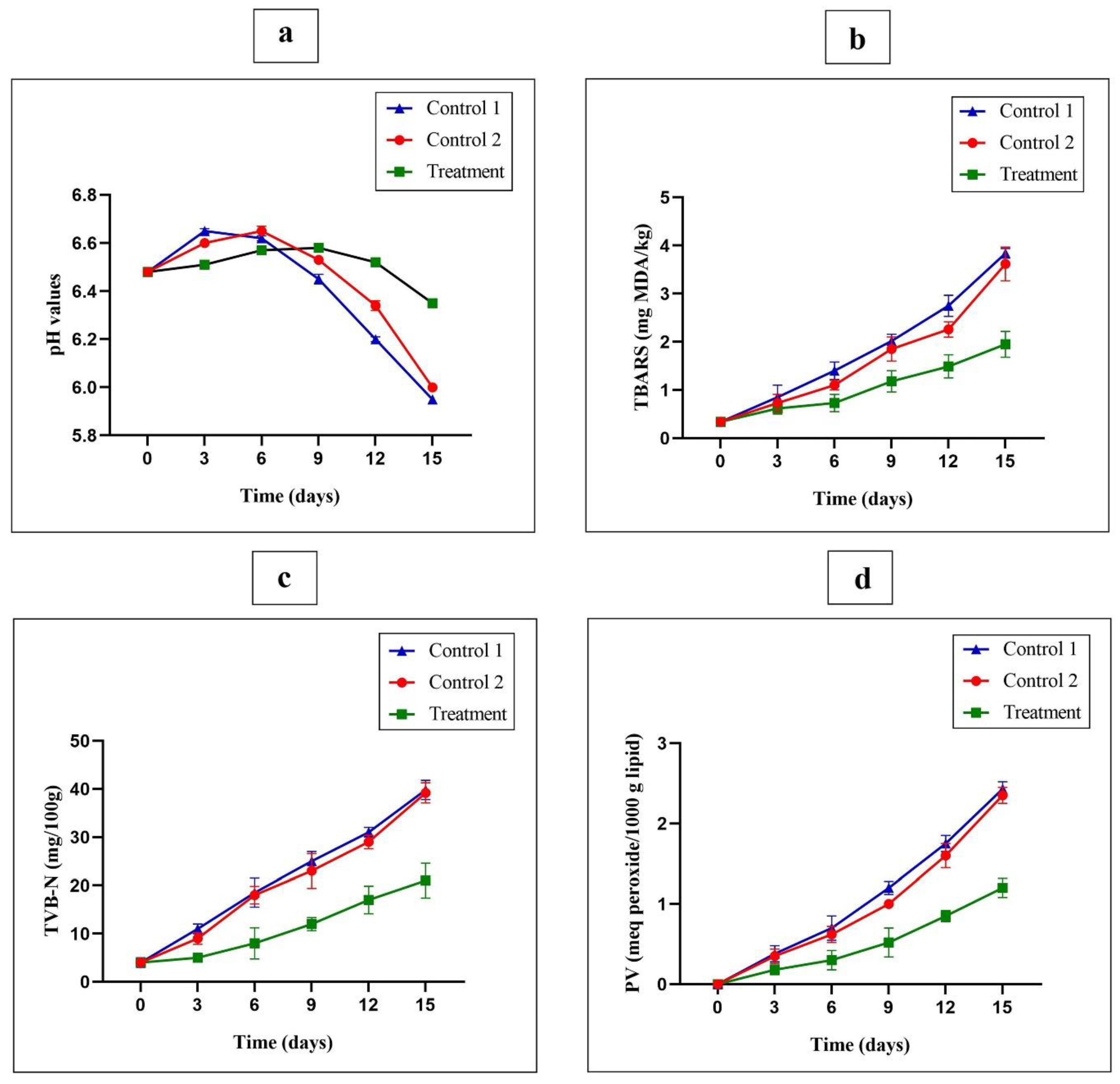
3.9. Chemical Analysis on Wrapped Turkey Breast Meat
3.9.1. Changes in the pH Values
3.9.2. Changes in the TBARS
3.9.3. Changes in the TVB-N
3.9.4. Peroxide Value Measurement
3.10. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | Polyvinyl alcohol |
| G | Gellan |
| MEO | Myrtle essential oil |
| CWE | Caucasian whortleberry extract |
| CW | Caucasian whortleberry |
| WVP | Water vapor permeation |
| MC | Moisture content |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| TBARS | Thiobarbituric acid reactive substances |
| PV | Peroxide value |
| TVB-N | Total volatile basic nitrogen |
| UTS | Ultimate tensile strength |
| SB | Strain at break |
References
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Iyyappan, J.; Gnanasekaran, R.; Ishwarya, G.; Harshini, R.P.; Dhithya, V.; Chandran, M.; Kanishka, V.; Gomathi, K. Advances in bio food packaging—An overview. Heliyon 2021, 7, e07998. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Ghanbarzadeh, B.; Ayaseh, A.; Ostadrahimi, A.; Ehsani, A.; Alizadeh-Sani, M.; Adun, P.A. The optimization of physico-mechanical properties of bionanocomposite films based on gluten/carboxymethyl cellulose/cellulose nanofiber using response surface methodology. Polym. Test. 2019, 78, 105989. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-T.; Tsai, I.L.; Ho, Y.-C.; Hang, Y.-H.; Lin, C.; Tsai, M.-L.; Mi, F.-L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef] [PubMed]
- Kochkina, N.E.; Lukin, N.D. Structure and properties of biodegradable maize starch/chitosan composite films as affected by PVA additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xu, F.; Yuan, L.; Hu, H.; Yao, X.; Liu, J. Comparison of the physical and functional properties of starch/polyvinyl alcohol films containing anthocyanins and/or betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef]
- Bajalan, I.; Pirbalouti, A.G. Variation in antibacterial activity and chemical compositions of essential oil from different populations of myrtle. Ind. Crops Prod. 2014, 61, 303–307. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Mirzakhani, M.; Pirbalouti, A.G. Essential oil variation among 21 wild myrtle (Myrtus communis L.) populations collected from different geographical regions in Iran. Ind. Crops Prod. 2013, 51, 328–333. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Ballington, J.R. Vaccinium species of section Hemimyrtillus: Their value to cultivated blueberry and approaches to utilization1This article is part of a Special Issue entitled “A tribute to Sam Vander Kloet FLS: Pure and applied research from blueberries to heathland ecology”. Botany 2012, 90, 347–353. [Google Scholar] [CrossRef]
- Hasanloo, T.; Sepehrifar, R.; Hajimehdipoor, H. Levels of phenolic compounds and their effects on antioxidant capacity of wild Vaccinium arctostaphylos L. (Qare-Qat) collected from different regions of Iran. Turk. J. Biol. 2011, 35, 371–377. [Google Scholar] [CrossRef]
- Soltani, R.; Hakimi, M.; Asgary, S.; Ghanadian, S.M.; Keshvari, M.; Sarrafzadegan, N. Evaluation of the effects of Vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: A randomized, double-blind, placebo-controlled clinical trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 217451. [Google Scholar] [CrossRef] [Green Version]
- Musavi, A.K.; Mazandarani, M.; Rahimian, S.; Ghafourian, M. Ecological Requirement, Ethnopharmacology, Phytochemical and Antioxidant Capacity of Vaccinium arctostaphylos L. in Gilan Province (North of Iran). J. Med. Plants By-Prod. 2016, 5, 89–95. [Google Scholar]
- Contini, C.; Álvarez, R.; O’sullivan, M.; Dowling, D.P.; Gargan, S.Ó.; Monahan, F.J. Effect of an active packaging with citrus extract on lipid oxidation and sensory quality of cooked turkey meat. Meat Sci. 2014, 96, 1171–1176. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sarriés, M.V.; Tateo, A.; Polidori, P.; Franco, D.; Lanza, M. Carcass characteristics, meat quality and nutritional value of horsemeat: A review. Meat Sci. 2014, 96, 1478–1488. [Google Scholar] [CrossRef]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Khah, M.D.; Ghanbarzadeh, B.; Roufegarinejad Nezhad, L.; Ostadrahimi, A. Effects of virgin olive oil and grape seed oil on physicochemical and antimicrobial properties of pectin-gelatin blend emulsified films. Int. J. Biol. Macromol. 2021, 171, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Ghanbarzadeh, B.; Divband, B. Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. Int. J. Food Microbiol. 2020, 312, 108375. [Google Scholar] [CrossRef] [PubMed]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti khiabani, M.; Akhondzadeh basti, A.; Abdulkhani, A. The effects of gelatin-CMC films incorporated with chitin nanofiber and Trachyspermum ammi essential oil on the shelf life characteristics of refrigerated raw beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava starch− chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef]
- Fan, H.Y.; Duquette, D.; Dumont, M.-J.; Simpson, B.K. Salmon skin gelatin-corn zein composite films produced via crosslinking with glutaraldehyde: Optimization using response surface methodology and characterization. Int. J. Biol. Macromol. 2018, 120, 263–273. [Google Scholar] [CrossRef]
- Chen, Z.; Zong, L.; Chen, C.; Xie, J. Development and characterization of PVA-Starch active films incorporated with β-cyclodextrin inclusion complex embedding lemongrass (Cymbopogon citratus) oil. Food Packag. Shelf Life 2020, 26, 100565. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H. Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. Int. J. Biol. Macromol. 2011, 48, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Pirnia, M.; Shirani, K.; Tabatabaee Yazdi, F.; Moratazavi, S.A.; Mohebbi, M. Characterization of antioxidant active biopolymer bilayer film based on gelatin-frankincense incorporated with ascorbic acid and Hyssopus officinalis essential oil. Food Chem. X 2022, 14, 100300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, J.; Hou, X.; Wu, H.; Li, Q.; Li, S.; Luo, Q.; Li, M.; Liu, X.; Shen, G.; et al. Physicochemical, antibacterial, and biodegradability properties of green Sichuan pepper (Zanthoxylum armatum DC.) essential oil incorporated starch films. LWT 2022, 161, 113392. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT—Food Sci. Technol. 2016, 72, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Al-Mariri, A.; Safi, M. In Vitro Antibacterial Activity of Several Plant Extracts and Oils against Some Gram-Negative Bacteria. Iran. J. Med. Sci. 2014, 39, 36–43. [Google Scholar] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Caillet, S.; Côté, J.; Sylvain, J.-F.; Lacroix, M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control 2012, 23, 419–428. [Google Scholar] [CrossRef]
- Bardají, D.K.R.; Reis, E.B.; Medeiros, T.C.T.; Lucarini, R.; Crotti, A.E.M.; Martins, C.H.G. Antibacterial activity of commercially available plant-derived essential oils against oral pathogenic bacteria. Nat. Prod. Res. 2016, 30, 1178–1181. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Fei, L.; Ding, Y.-C.; Ye, X.-Q.; Ding, Y.-T. Antibacterial effect of cinnamon oil combined with thyme or clove oil. Agric. Sci. China 2011, 10, 1482–1487. [Google Scholar]
- Brink, I.; Šipailienė, A.; Leskauskaitė, D. Antimicrobial properties of chitosan and whey protein films applied on fresh cut turkey pieces. Int. J. Biol. Macromol. 2019, 130, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control 2018, 92, 37–46. [Google Scholar] [CrossRef]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Indicators of food microbial quality and safety. In Modern Food Microbiology; James, M.J., Martin, J.L., Eds.; Springer: Boston, MA, USA, 2005; pp. 473–495. [Google Scholar]
- Kakaei, S.; Shahbazi, Y. Effect of chitosan-gelatin film incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil on survival of Listeria monocytogenes and chemical, microbial and sensory properties of minced trout fillet. LWT—Food Sci. Technol. 2016, 72, 432–438. [Google Scholar] [CrossRef]
- Olsson, C.; Ahrné, S.; Pettersson, B.; Molin, G. DNA based classification of food associated Enterobacteriaceae previously identified by Biolog GN microplates. Syst. Appl. Microbiol. 2004, 27, 219–228. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Benjakul, S. Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. Int. J. Food Microbiol. 2011, 149, 247–253. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.T.Y.; Barbut, S.; Ross, K.; Diarra, M.S.; Balamurugan, S. The effect of cranberry pomace ethanol extract on the growth of meat starter cultures, Escherichia coli O157: H7, Salmonella enterica serovar Enteritidis and Listeria monocytogenes. LWT 2019, 115, 108452. [Google Scholar]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Bher, A.; Jacob, H.; Auras, R. Compression molded LLDPE films loaded with bimetallic (Ag-Cu) nanoparticles and cinnamon essential oil for chicken meat packaging applications. LWT 2018, 93, 329–338. [Google Scholar] [CrossRef]
- Agrimonti, C.; White, J.C.; Tonetti, S.; Marmiroli, N. Antimicrobial activity of cellulosic pads amended with emulsions of essential oils of oregano, thyme and cinnamon against microorganisms in minced beef meat. Int. J. Food Microbiol. 2019, 305, 108246. [Google Scholar] [CrossRef] [PubMed]
- Sogut, E.; Seydim, A.C. The effects of chitosan-and polycaprolactone-based bilayer films incorporated with grape seed extract and nanocellulose on the quality of chicken breast fillets. LWT 2019, 101, 799–805. [Google Scholar] [CrossRef]
- Konuk Takma, D.; Korel, F. Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van Der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT—Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef]
| CWE (mg/mL) | MEO (mg/mL) | PVA (wt%) | G (wt%) | Sample |
|---|---|---|---|---|
| 0 | 0 | 1 | 2 | G/PVA |
| 3 | 0 | 1 | 2 | G/PVA/CWE 3 |
| 6 | 0 | 1 | 2 | G/PVA/CWE 6 |
| 9 | 0 | 1 | 2 | G/PVA/CWE 9 |
| 0 | 3 | 1 | 2 | G/PVA/MEO 3 |
| 0 | 6 | 1 | 2 | G/PVA/MEO 6 |
| 0 | 9 | 1 | 2 | G/PVA/MEO 9 |
| 3 | 3 | 1 | 2 | G/PVA/CWE 3/MEO 3 |
| 6 | 3 | 1 | 2 | G/PVA/ CWE 3/MEO 6 |
| 9 | 3 | 1 | 2 | G/PVA/ CWE 3/MEO 9 |
| 3 | 6 | 1 | 2 | G/PVA/ CWE 6/MEO 3 |
| 6 | 6 | 1 | 2 | G/PVA/ CWE 6/MEO 6 |
| 9 | 6 | 1 | 2 | G/PVA/ CWE 6/MEO 9 |
| 3 | 9 | 1 | 2 | G/PVA/CWE 9/MEO 3 |
| 6 | 9 | 1 | 2 | G/PVA/CWE 9/MEO 6 |
| 9 | 9 | 1 | 2 | G/PVA/CWE 9/MEO 9 |
| CWE and MEO (mg /mL Film Solution) | UTS (Mpa) | SB (%) | Thickness (mm) |
|---|---|---|---|
| G/PVA | 21.12 ± 0.31 a | 28.42 ± 0.42 k | 0.10 ± 0.00 b |
| G/PVA/CWE 3 | 20.86 ± 0.86 ab | 30.65 ± 1.03 jk | 0.10 ± 0.00 b |
| G/PVA/CWE 6 | 20.17 ± 0.52 abc | 33.10 ± 1.65 j | 0.10 ± 0.00 b |
| G/PVA/CWE 9 | 19.45 ± 0.45 bc | 36.38 ± 0.38 i | 0.10 ± 0.00 b |
| G/PVA/MEO 3 | 19.05 ± 0.30 cd | 40.94 ± 0.94 h | 0.10 ± 0.00 b |
| G/PVA/MEO 6 | 16.66 ± 0.66 ef | 54.73 ± 0.51 f | 0.11 ± 0.00 ab |
| G/PVA/MEO 9 | 12.52 ± 1.11 i | 67.11 ± 0.11 c | 0.11 ± 0.00 ab |
| G/PVA/CWE 3/MEO 3 | 18.67 ± 1.40 cd | 41.64 ± 1.64 h | 0.11± 0.00 ab |
| G/PVA/ CWE 3/MEO 6 | 15.25 ± 0.25 fg | 56.22 ± 0.44 ef | 0.11 ± 0.01 ab |
| G/PVA/ CWE 3/MEO 9 | 11.40 ± 1.20 ij | 70.37 ± 0.22 b | 0.12 ± 0.00 ab |
| G/PVA/ CWE 6/MEO 3 | 17.81 ± 0.83 de | 44.87 ± 0.57 g | 0.11 ± 0.00 ab |
| G/PVA/ CWE 6/MEO 6 | 14.14 ± 0.64 gh | 58.79 ± 0.83 de | 0.12 ± 0.00 ab |
| G/PVA/ CWE 6/MEO 9 | 10.27 ± 0.37 jk | 73.29 ± 0. 35 ab | 0.12 ± 0.00 ab |
| G/PVA/CWE 9/MEO 3 | 16.95 ± 1.05 e | 46.16 ± 0.42 g | 0.12 ± 0.00 ab |
| G/PVA/CWE 9/MEO 6 | 12.93 ± 0.93 hi | 61.10 ± 0.29 d | 0.12 ± 0.00 ab |
| G/PVA/CWE 9/MEO 9 | 9.18 ± 1.06 k | 76.18 ± 0.65 a | 0.13 ± 0.00 a |
| CWE and MEO (mg /mL Film Solution) | WVP × 10−10 (g/mhPa) | MC (%) |
|---|---|---|
| G/PVA | 5.21 ± 0.21 j | 11.21 ± 0.18 j |
| G/PVA/CWE 3 | 4.76 ± 0.76 ij | 10.48 ± 0.48 i |
| G/PVA/CWE 6 | 4.32 ± 0.32 hi | 10.19 ± 0.34 i |
| G/PVA/CWE 9 | 3.91 ± 0.41 h | 9.86 ± 0.66 i |
| G/PVA/MEO 3 | 3.71 ± 0.21 gh | 9.11 ± 1.11 h |
| G/PVA/MEO 6 | 3.05 ± 1.73 efg | 7.58 ± 0.58 ef |
| G/PVA/MEO 9 | 1.93 ± 0.32 b cd | 5.12 ± 0.12 c |
| G/PVA/CWE 3/MEO 3 | 3.23 ± 0.13 fg | 8.53 ± 0.53 gh |
| G/PVA/ CWE 3/MEO 6 | 2.71 ± 1.30 ef | 7.21 ± 0.21 de |
| G/PVA/ CWE 3/MEO 9 | 1.53 ± 0.67 bc | 4.05 ± 1.10 1 b |
| G/PVA/ CWE 6/MEO 3 | 2.80 ± 0.43 ef | 8.02 ± 0.52 fg |
| G/PVA/ CWE 6/MEO 6 | 2.35 ± 0.35 de | 6.54 ± 0.46 d |
| G/PVA/ CWE 6/MEO 9 | 1.21 ± 0.21 ab | 3.19 ± 0.19 a |
| G/PVA/CWE 9/MEO 3 | 2.41 ± 1.60 de | 7.24 ± 0.34 de |
| G/PVA/CWE 9/MEO 6 | 1.84 ± 0.52 bcd | 5.72 ± 0.72 c |
| G/PVA/CWE 9/MEO 9 | 0.79 ± 0.29 a | 2.51 ± 1.51 a |
| MIC (mg/mL) | MBC(mg/mL) | |||||
|---|---|---|---|---|---|---|
| Bacteria | MEO | CWE | MO/CWE | MEO | CWE | MEO/CWE |
| S. typhimurium | 6 | 16 | 9 | 7 | 23 | 14 |
| S. aureus | 6 | 17 | 10 | 8 | 24 | 14 |
| E. coli | 5 | 15 | 9 | 6 | 18 | 11 |
| P. fluorescens | 5 | 16 | 9 | 6 | 22 | 12 |
| The Diameter of Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|
| Bacteria Strains | Control 1 | Control 2 | MEO | CWE | MEO/CWE |
| S. typhimurium | NI | NI | 14.65 ± 0.25 a | 13.97 ± 0.09 b | 16.72 ± 0.12 b |
| S. aureus | NI | NI | 12.55 ± 0.05 c | 10.78 ± 0.11 d | 13.10 ± 0.31 d |
| E. coli | NI | NI | 15.40± 0.10 a | 15.94 ± 0.06 a | 17.22 ± 0.07 a |
| P. fluorescens | NI | NI | 13.81± 0.21 b | 12.49 ± 0.19 c | 14.51 ± 0.14 c |
| Samples | Colour | Odor | Texture | Overall Acceptability | |
|---|---|---|---|---|---|
| Days | Groups | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| 0 | Control 1 Control 2 | 9.00 9.00 | 9.00 9.00 | 9.00 9.00 | 9.00 9.00 |
| Treatment | 9.00 | 9.00 | 9.00 | 9.00 | |
| 3 | Control 1 Control 2 | 7.5 ± 0.52 b 7.83 ± 0.71 b | 7.33 ± 0.81 b 7.50 ± 0.48 b | 7.16 ± 0.75 b 7.33 ± 0.61 b | 7.16 ± 0.51 b 7.50 ± 0.86 b |
| Treatment | 8.83 ± 0.32 a | 8.50 ± 0.81 a | 8.33 ± 0.54 a | 8.50 ± 0.48 a | |
| 6 | Control 1 Control 2 | 4.83 ± 0.51de 5.33 ± 0.28 d | 5.33 ± 0.40 c 5.83 ± 0.69 c | 6.16 ± 0.40 c 6.33 ± 0.78 c | 5.33 ± 0.38 c 5.83 ± 0.45 c |
| Treatment | 7.83 ± 0.54 b | 7.16 ± 0.40 b | 7.50 ± 0.40 b | 7.50 ± 0.51 b | |
| 9 | Control 1 Control 2 | 3.83 ± 1.63 fg 4.16 ± 0.82 ef | 3.33 ± 0.33 ef 3.83 ± 0.28 de | 3.50 ± 0.54 efg 3.83 ± 0.46 ef | 3.83 ± 0.40 d 4.16 ± 1.05 d |
| Treatment | 6.33 ± 0.55 c | 5.50 ± 0.51 c | 6.16 ± 0.54 c | 5.83 ± 0.44 c | |
| 12 | Control 1 Control 2 | 3.33 ± 2.63 gh 3.83 ± 0.71 fg | 2.83 ± 0.38 fg 3.16 ± 0.52 efg | 3.16 ± 0.40 f 3.50 ± 1.20 efg | 3.50 ± 0.63 de 3.83 ± 0.86 d |
| Treatment | 5.33 ± 0.55 d | 5.16 ± 0.51 c | 4.66 ± 0.40 d | 5.33 ± 0.54 c | |
| 15 | Control 1 Control 2 | 2.66 ± 1.40 h 2.83 ± 0.31 h | 2.33 ± 0.44 f 2.50 ± 1.61 fg | 2.83 ± 0.73 f 2.83 ± 0.49 g | 2.83 ± 0.54 e 2.83 ± 0.92 e |
| Treatment | 4.66 ± 0.67 de | 4.33 ± 0.51 d | 4.16 ± 0.40 de | 5.16 ± 0.51 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri, V.; Ghanbarzadeh, B.; Parastouei, K.; Baghersad, M.H. The Caucasian Whortleberry Extract/Myrtle Essential Oil Loaded Active Films: Physicochemical Properties and Effects on Quality Parameters of Wrapped Turkey Breast Meat. Foods 2022, 11, 3553. https://doi.org/10.3390/foods11223553
Bagheri V, Ghanbarzadeh B, Parastouei K, Baghersad MH. The Caucasian Whortleberry Extract/Myrtle Essential Oil Loaded Active Films: Physicochemical Properties and Effects on Quality Parameters of Wrapped Turkey Breast Meat. Foods. 2022; 11(22):3553. https://doi.org/10.3390/foods11223553
Chicago/Turabian StyleBagheri, Vahid, Babak Ghanbarzadeh, Karim Parastouei, and Mohammad Hadi Baghersad. 2022. "The Caucasian Whortleberry Extract/Myrtle Essential Oil Loaded Active Films: Physicochemical Properties and Effects on Quality Parameters of Wrapped Turkey Breast Meat" Foods 11, no. 22: 3553. https://doi.org/10.3390/foods11223553





