Exploring the Potential of Plant-Derived Exosome-like Nanovesicle as Functional Food Components for Human Health: A Review
Abstract
:1. Introduction
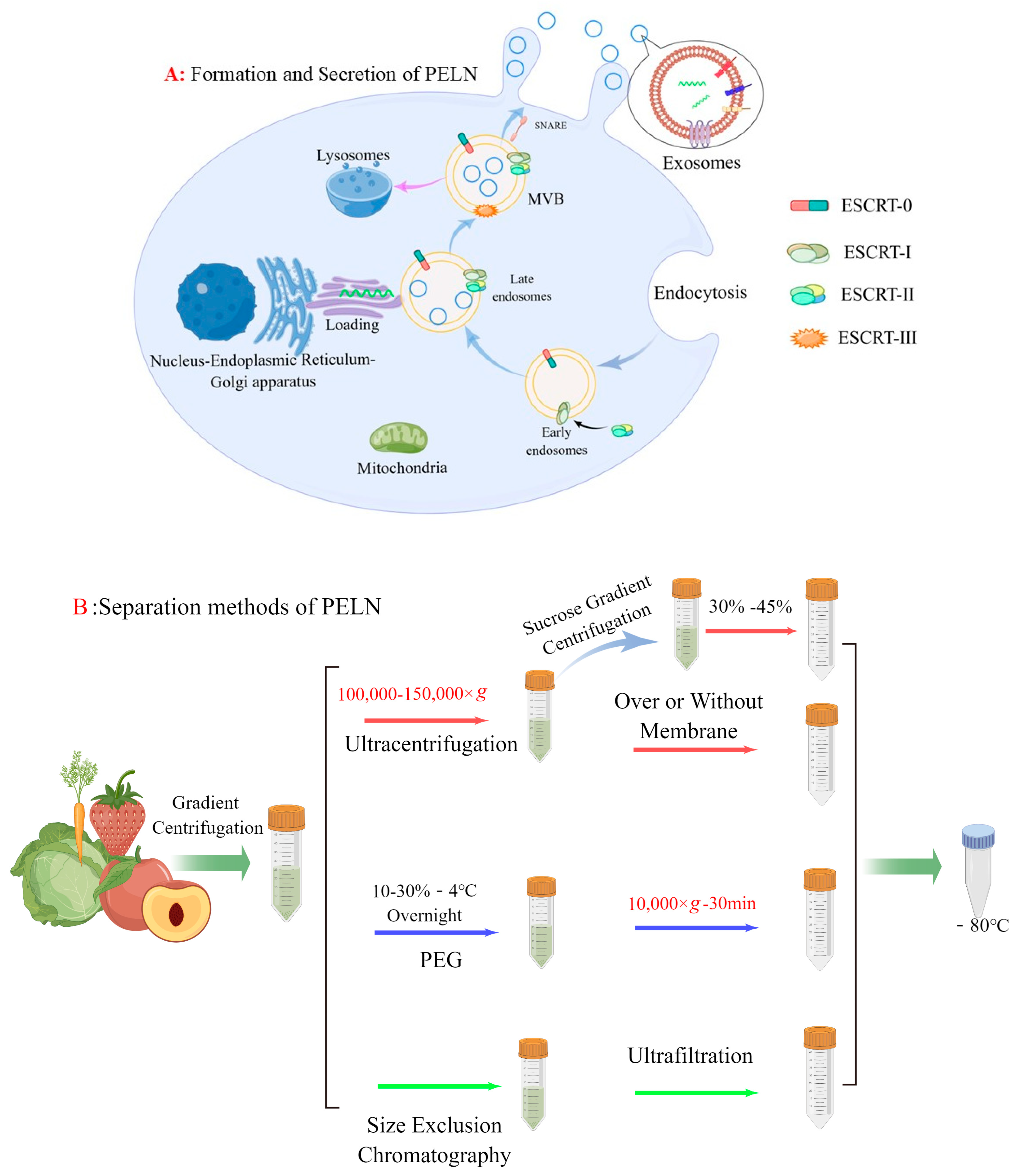
2. Isolation and Purification of PELN
2.1. Formation of PELN
2.2. Extraction Methods
2.3. Strategies for Enhancing the Production of PELN
2.4. Characterization of PELN
3. Composition of PELN
3.1. Protein and Lipids
3.2. Nucleic Acids
3.3. Other Phytochemical Components
4. Biological Function of PELN
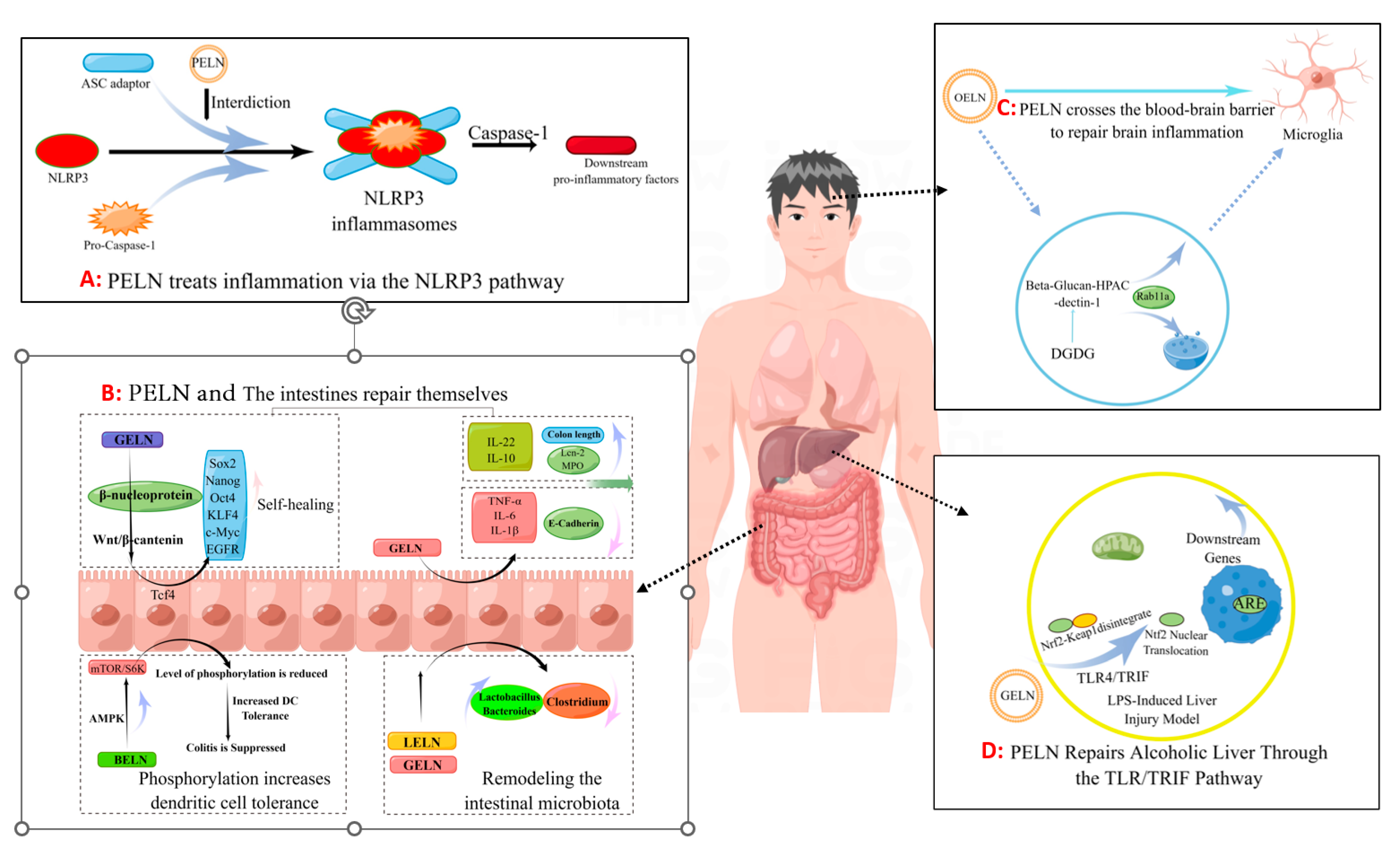
4.1. Absorption and Distribution of PELN in the Body
4.2. Reconstructing Gut Microbiota
4.3. Ameliorating Inflammation
4.4. Tumor Inhibition
5. Potential Application of PELN
5.1. Stability of PELN
5.2. Security of PELN
5.3. Endocytic Uptake and Intracellular Internalization of PELN
6. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A Comprehensive Review on the Applications of Exosomes and Liposomes in Regenerative Medicine and Tissue Engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Mu, J.; Teng, Y.; Zhang, X.; Sundaram, K.; Sriwastva, M.K.; Kumar, A.; Lei, C.; Zhang, L.; Liu, Q.M. Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex Into Microglial Exosomes. Small 2022, 18, 2105385. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Li, X.; An, T.T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M.J.; Eudy, J. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J. Extracell. Vesicles 2021, 10, e12069. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Dico, A.L.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-W.; Ye, C.; Wang, K.-X.; Yang, X.; Zhu, P.-Y.; Hu, K.; Lan, T.; Huang, L.-Y.; Wang, W.; Gu, B. Momordica. charantia-derived extracellular vesicles-like nanovesicles protect cardiomyocytes against radiation injury via attenuating DNA damage and mitochondria dysfunction. Front. Cardiovasc. Med. 2022, 9, 864188. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 2018, 24, 637–652. e638. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef] [PubMed]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, W.; Yang, D. ESCRT System: A Versatile Protein Transport and Membrane Shearing Machine. Chin. J. Biochem. Mol. Biol. 2013, 29, 11. [Google Scholar]
- Futter, C.E.; Pearse, A.; Hewlett, L.J.; Hopkins, C.R. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 1996, 132, 1011–1023. [Google Scholar] [CrossRef]
- Li, W.; Xing, Y.; Wang, Y.; Xu, T.; Song, E.; Feng, W. A non-canonical target-binding site in Munc18-1 domain 3b for assembling the Mint1-Munc18-1-syntaxin-1 complex. Structure 2023, 31, 68–77. e65. [Google Scholar] [CrossRef]
- Bao, H.; Das, D.; Courtney, N.A.; Jiang, Y.; Briguglio, J.S.; Lou, X.; Roston, D.; Cui, Q.; Chanda, B.; Chapman, E.R. Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature 2018, 554, 260–263. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.; Hällbrink, M.; Seow, Y.; Bultema, J.J. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 879–883. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Proteomic Profiling Methods Protoc. 2015, 1295, 179–209. [Google Scholar]
- Leng, Y.; Yang, L.; Pan, S.; Zhan, L.; Yuan, F. Characterization of blueberry exosome-like nanoparticles and miRNAs with potential cross-kingdom human gene targets. Food Sci. Hum. Wellness 2024, 13, 869–878. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, N.; Madurga, R.; Rackov, G.; Palacín-Aliana, I.; Núñez-Torres, R.; Asensi-Puig, A.; Carrión-Navarro, J.; Esteban-Rubio, S.; Peinado, H.; González-Neira, A. Polyethylene glycol improves current methods for circulating extracellular vesicle-derived DNA isolation. J. Transl. Med. 2019, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Kim, J.; Shin, H.; Kim, J.; Kim, J.; Park, J. Isolation of high-purity extracellular vesicles by extracting proteins using aqueous two-phase system. PLoS ONE 2015, 10, e0129760. [Google Scholar] [CrossRef]
- Kırbaş, O.K.; Bozkurt, B.T.; Asutay, A.B.; Mat, B.; Ozdemir, B.; Öztürkoğlu, D.; Ölmez, H.; İşlek, Z.; Şahin, F.; Taşlı, P.N. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci. Rep. 2019, 9, 19159. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef]
- Ding, L.; Yang, X.; Gao, Z.; Effah, C.Y.; Zhang, X.; Wu, Y.; Qu, L. A holistic review of the state-of-the-art microfluidics for exosome separation: An overview of the current status, existing obstacles, and future outlook. Small 2021, 17, 2007174. [Google Scholar] [CrossRef]
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega 2021, 6, 17635–17641. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Pan, Q.; Cao, H.X.; Xin, F.Z.; Zhao, Z.H.; Yang, R.X.; Zeng, J.; Zhou, H.; Fan, J.G. Lipotoxic hepatocyte-derived exosomal microRNA 192-5p activates macrophages through rictor/Akt/forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology 2020, 72, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Zimmermann, M.; Mitterbauer, A.; Ellinger, A.; Gruber, F.; Narzt, M.-S.; Zellner, M.; Gyöngyösi, M.; Madlener, S.; Simader, E. Analysis of the secretome of apoptotic peripheral blood mononuclear cells: Impact of released proteins and exosomes for tissue regeneration. Sci. Rep. 2015, 5, 16662. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, S.; Li, S.; Wu, J.; Ma, R.; Cao, H.; Zhu, Y.; Feng, J. Exosomes: Decreased sensitivity of lung cancer A549 cells to cisplatin. PLoS ONE 2014, 9, e89534. [Google Scholar] [CrossRef] [PubMed]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 1–10. [Google Scholar] [CrossRef]
- Beninson, L.A.; Brown, P.N.; Loughridge, A.B.; Saludes, J.P.; Maslanik, T.; Hills, A.K.; Woodworth, T.; Craig, W.; Yin, H.; Fleshner, M. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142-5p and miR-203). PLoS ONE 2014, 9, e108748. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, J.; Wang, M.; Wang, Y.; Dong, M.; Ma, X.; Sun, B.; Liu, S.; Zhao, Z.; Chen, L. Lipid-induced DRAM recruits STOM to lysosomes and induces LMP to promote exosome release from hepatocytes in NAFLD. Sci. Adv. 2021, 7, eabh1541. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.d.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liao, X.; Tian, Y.; Li, G. Exosome separation using microfluidic systems: Size-based, immunoaffinity-based and dynamic methodologies. Biotechnol. J. 2017, 12, 1600699. [Google Scholar] [CrossRef] [PubMed]
- Parisse, P.; Rago, I.; Ulloa Severino, L.; Perissinotto, F.; Ambrosetti, E.; Paoletti, P.; Ricci, M.; Beltrami, A.; Cesselli, D.; Casalis, L. Atomic force microscopy analysis of extracellular vesicles. Eur. Biophys. J. 2017, 46, 813–820. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022, 9, 2103222. [Google Scholar] [CrossRef] [PubMed]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef] [PubMed]
- Ly, N.P.; Han, H.S.; Kim, M.; Park, J.H.; Choi, K.Y. Plant-derived nanovesicles: Current understanding and applications for cancer therapy. Bioact. Mater. 2023, 22, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 18. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061. e1018. [Google Scholar] [CrossRef]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Gd, B. Immune recognition. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar]
- Record, M. Exosome-like nanoparticles from food: Protective nanoshuttles for bioactive cargo. Mol. Ther. 2013, 21, 1294–1296. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, M.-L.; Zhu, W.-J.; Cao, Y.-N.; Peng, L.-X.; Yan, Z.-Y.; Zhao, G. In vitro effects of Tartary buckwheat-derived nanovesicles on gut microbiota. J. Agric. Food Chem. 2022, 70, 2616–2629. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Leidal, A.M.; Huang, H.H.; Marsh, T.; Solvik, T.; Zhang, D.; Ye, J.; Kai, F.; Goldsmith, J.; Liu, J.Y.; Huang, Y.-H. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 2020, 22, 187–199. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Chapado, L.A.; Martin-Hernandez, R.; Hernandez de la Red, S.; Tome-Carneiro, J.; Gil-Zamorano, J.; Ruiz-Roso, M.B.; Del Saz, A.; Crespo, M.C.; del Pozo-Acebo, L.; Arantes Ferreira Peres, W. Connection between miRNA mediation and the bioactive effects of broccoli (Brassica oleracea var. italica): Exogenous miRNA resistance to food processing and GI digestion. J. Agric. Food Chem. 2021, 69, 9326–9337. [Google Scholar] [CrossRef]
- Zhu, W.-J.; Liu, Y.; Cao, Y.-N.; Peng, L.-X.; Yan, Z.-Y.; Zhao, G. Insights into health-promoting effects of plant microRNAs: A review. J. Agric. Food Chem. 2021, 69, 14372–14386. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, H.; Liu, G.; Chen, A.; Calvo, A.; Cai, Q.; Jin, H. Fungal small RNAs ride in extracellular vesicles to enter plant cells through clathrin-mediated endocytosis. bioRxiv 2023, 14, 4383. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J. Use of nanovesicles from orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Jinsong, N.; Xinrui, W.; Bo, S.; Yuehui, W.; Jiaxiang, W. Effect of 20(S)-ginsenoside Rg3 in anti-B16 melanoma metastasis. J. Jilin Univ. Med. Sci. 2004, 30, 540–542. [Google Scholar]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, H.; Shi, W.; Chen, L.; Chen, T.; Chen, G.; Wang, W.; Lan, J.; Huang, Z.; Zhang, J. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnol. 2021, 19, 439. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Guerriero, G.; Hausman, J.-F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: Focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Nemidkanam, V.; Chaichanawongsaroj, N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS ONE 2022, 17, e0262884. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-Induced change on gene expression in EA. hy926 cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Teng, Y.; He, L.; Sayed, M.; Mu, J.; Xu, F.; Zhang, X.; Kumar, A.; Sundaram, K.; Sriwastva, M.K. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. Iscience 2021, 24, 102511. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics 2022, 12, 1220. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Rhee, W.J. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Canup, B.S.; Ngo, V.L.; Denning, T.L.; Garg, P.; Laroui, H. Internalization of garlic-derived nanovesicles on liver cells is triggered by interaction with CD98. ACS Omega 2020, 5, 23118–23128. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Hossain, M.N.; De Leo, V.; Tamborra, R.; Laselva, O.; Ingrosso, C.; Daniello, V.; Catucci, L.; Losito, I.; Sollitto, F.; Loizzi, D. Characterization of anti-proliferative and anti-oxidant effects of nano-sized vesicles from Brassica oleracea L. (Broccoli). Sci. Rep. 2022, 12, 14362. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Cristaldi, M.; Monteleone, F.; Fontana, S.; Alessandro, R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J. Proteom. 2018, 173, 1–11. [Google Scholar] [CrossRef]
- Wang, B.; Guo, X.-J.; Cai, H.; Zhu, Y.-H.; Huang, L.-Y.; Wang, W.; Luo, L.; Qi, S.-H. Momordica charantia-derived extracellular vesicles-like nanovesicles inhibited glioma proliferation, migration, and invasion by regulating the PI3K/AKT signaling pathway. J. Funct. Foods 2022, 90, 104968. [Google Scholar] [CrossRef]
- Özkan, İ.; Koçak, P.; Yıldırım, M.; Ünsal, N.; Yılmaz, H.; Telci, D.; Şahin, F. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci. Rep. 2021, 11, 14773. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jin, W.-Q.; Tang, X.-L.; Zhang, S.; Ma, R.; Zhao, D.-Q.; Sun, L.-W. Ginseng-derived nanoparticles inhibit lung cancer cell epithelial mesenchymal transition by repressing pentose phosphate pathway activity. Front. Oncol. 2022, 12, 942020. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef] [PubMed]
- Kantarcıoğlu, M.; Yıldırım, G.; Oktar, P.A.; Yanbakan, S.; Özer, Z.B.; Sarıca, D.Y.; Taşdelen, S.; Bayrak, E.; Balı, D.F.A.; Öztürk, S. Coffee-Derived Exosome-Like Nanoparticles: Are They the Secret Heroes? Turk. J. Gastroenterol. 2023, 34, 161. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.-M.; Yoon, E.J.; Cho, J.-S. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Han, J.; Wu, T.; Jin, J.; Li, Z.; Cheng, W.; Dai, X.; Yang, K.; Zhang, H.; Zhang, Z.; Zhang, H. Exosome-like nanovesicles derived from Phellinus linteus inhibit Mical2 expression through cross-kingdom regulation and inhibit ultraviolet-induced skin aging. J. Nanobiotechnol. 2022, 20, 455. [Google Scholar] [CrossRef] [PubMed]
- Timms, K.; Holder, B.; Day, A.; Mclaughlin, J.; Forbes, K.A.; Westwood, M. Watermelon-Derived Extracellular Vesicles Influence Human Ex Vivo Placental Cell Behavior by Altering Intestinal Secretions. Mol. Nutr. Food Res. 2022, 66, 2200013. [Google Scholar] [CrossRef]
- Aimaletdinov, A.M.; Gomzikova, M.O. Tracking of extracellular vesicles’ biodistribution: New methods and approaches. Int. J. Mol. Sci. 2022, 23, 11312. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Lehmann, C.J.; Cole, C.G.; Pamer, E.G. Translating Microbiome Research from and to the Clinic. Annu. Rev. Microbiol. 2022, 76, 435–460. [Google Scholar] [CrossRef]
- Kim, S.Q.; Kim, K.-H. Emergence of edible plant-derived nanovesicles as functional food components and nanocarriers for therapeutics delivery: Potentials in human health and disease. Cells 2022, 11, 2232. [Google Scholar] [CrossRef]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular vesicles in skin wound healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, J.; Sohn, Y.; Oh, C.-E.; Park, J.-H.; Yuk, J.-M.; Yeon, J.-H. Stability of plant leaf-derived extracellular vesicles according to preservative and storage temperature. Pharmaceutics 2022, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [PubMed]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of uptake. J. Circ. Biomark. 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zhao, W.; Wu, C.; Wang, X.; Chen, J.; Shi, X.; Sha, S.; Li, J.; Liang, X.; Yang, Y. Lemon-Derived Extracellular Vesicles Nanodrugs Enable to Efficiently Overcome Cancer Multidrug Resistance by Endocytosis-Triggered Energy Dissipation and Energy Production Reduction. Adv. Sci. 2022, 9, 2105274. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.-i.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef]

| Sources | Isolation | Model | Omics | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Orange juice | UC | HFHSD | Lipids, Metabolism | TLR4—gut repair | [64] |
| Ginger | SDC | DSS | Lipids, Metabolism, Proteins | Gut epithelial cell proliferation, mitigating damage factors—gut repair | [6] |
| Broccoli | UC | DSS | AMPK-DCIncreased cell tolerance—colitis | [10] | |
| Tea | SDC | DSS | Lipids, Metabolism, Proteins | Homeostasis of the gut microbiota, prevention and treatment of gut inflammation | [71] |
| Grape | SDC | DSS | Lipids, | Wnt/β-cantenin-TCF4 Transcriptional mechanism—colitis | [9] |
| Ginger | SDC | Lipids, Proteins RNA | Wnt/TCF4 signaling pathway—gut homeostasis | [72] | |
| Grapefruit | |||||
| Carrot | |||||
| Ginger | SDC | Lipids, Proteins, miRNA | Lactobacillus rhamnosus—gut microbiota, gut inflammation | [14] | |
| Ginger | SDC | Inhibits the activation of NLRP3 inflammasomes | [73] | ||
| Blueberry | UC | Metabolism, Proteins, miRNA | Inhibits oxidative stress of TNF-α | [74] | |
| Lemon | SDC | Molecular pectin-enzyme R-NaseP-msp1, msp3-bile resistance | [75] | ||
| Garlic | SDC | HFD | c-Myc-regulates the cGAS/STING pathway—inflammation of the brain IDO1-AHR signaling pathway—insulin resistance | [76] | |
| Honey | SEC | miRNA | Inhibit the activation of the NLRP inflammatory plateau | [8] | |
| Carrot | SEC | Proteins | Nrf-2 antioxidant mechanism | [77] | |
| Oats | ODC | Alcohol Brain | HPCA (hippocampal calcin)/Rab11a/dectin-1-brain inflammation | [7] | |
| Lemon | UC | LPS | Metabolism, Proteins | Nuclear translocation of NF-κB—inhibits ERK1-2/NF-κB-anti-inflammatory | [11] |
| Shitake | UC | GalN/LPS | Proteins | Inhibits NLRP3 inflammasome—liver damage | [78] |
| Garlic | UC | LPS | Proteins | LPS-induced inflammation | [79] |
| Ginger | SDC | Alcohol Liver | Lipids, Metabolism | TLR4/TRIF-activates Nrf2 nuclear translocation—alcoholic liver | [80] |
| Ginger | SDC | Alcohol Brain | miRNA | miRNAs regulate viral protein expression—lung inflammation | [81] |
| Broccoli | SDC | Metabolism, Proteins | Antiproliferative and antioxidant | [82] | |
| Grapefruit | UC | A375 | Metabolism | P13K/AKT and MAPK/ERK, inhibit tumor growth | [12] |
| Ginseng | SDC | Macrophages | Proteins, Lipids, RNA | TLR-4 and MyD88-M2 to M1 polarization-apoptosis | [18] |
| Lemon | SDC | CML-Tumor | Proteins | Induction of TRAIL-mediated tumor growth | [11] |
| Lemon | SDC | Proteins | Phosphorylation-antitumor of ACACA-ERK1/2 and P38-MAPK | [83] | |
| Lemon | ELD | Gastric Cancer | Stage S-cell cycle arrest and apoptosis in gastric cancer cells | [26] | |
| Momordica charantia | SDC | p-AKT/AKT and p-PI3K/PI3K-glioma | [84] | ||
| Garlic | ATP | Tumor Cells | S-phase cell cycle arrest-caspase-mediated apoptosis-anticancer | [85] | |
| Ginseng | UC | Tumor Cells | Proteins | Pentose phosphate pathway activity inhibits epithelial mesenchymal transformation in lung cancer cells | [86] |
| Wheat | Kits | Proteins | Wound healing potential | [15] | |
| Grapefruit | ATP | Proteins, miRNA | Upregulation of wound healing gene expression | [87] | |
| Momordica charantia | SDC | Proteins | AKT and ERK—mitochondrial dysfunction—cardioprotection | [13] | |
| Coffee | SEC | Liver Tumors | Liver fibrosis in chronic liver disease | [88] | |
| Portulaca oleracea | UC ATP | Cornecocytes | Upregulation of gene expression that builds a skin barrier and prevents water loss | [89] | |
| Green tea | |||||
| Ginseng | |||||
| Medlar | UC | HaCaT | miRNA | miR-CM1 inhibits Mical2 and inhibits ultraviolet ray-induced skin aging | [90] |
| Watermelon | UC | Caco-2 | Proteins | Alters gut communication with distant tissues—improves placental function and reduces FGR | [91] |
| Source | Grooming Process | Target | Loads | Function | Ref. |
|---|---|---|---|---|---|
| Grapefruit | Gut macrophages | Anti-inflammatory methotrexate (MTX) | Reduces the toxicity of MTX and improves its therapeutic efficacy for DSS-induced colitis in mice | [69] | |
| Grapefruit | Folic acid (FA) | Gut | Biotin siRNA, JSI-124 | [53] | |
| Grapefruit | FA-PEI-GNVs | Brain-GL-6 tumors | miRNA17 | Delayed brain tumor growth | [9] |
| Cherry | Nucleic acid | [104] | |||
| Cabbage | Therapeutic drugs | [16] | |||
| Lemon | Heparin-cRGD-EVs-doxorubicin | Liver, Spleen, kidney, and tumors | Doxorubicin | Multidrug resistance in cancer | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ren, C.; Zhan, R.; Cao, Y.; Ren, Y.; Zou, L.; Zhou, C.; Peng, L. Exploring the Potential of Plant-Derived Exosome-like Nanovesicle as Functional Food Components for Human Health: A Review. Foods 2024, 13, 712. https://doi.org/10.3390/foods13050712
Liu Y, Ren C, Zhan R, Cao Y, Ren Y, Zou L, Zhou C, Peng L. Exploring the Potential of Plant-Derived Exosome-like Nanovesicle as Functional Food Components for Human Health: A Review. Foods. 2024; 13(5):712. https://doi.org/10.3390/foods13050712
Chicago/Turabian StyleLiu, Yizhi, Chaoqin Ren, Ruiling Zhan, Yanan Cao, Yuanhang Ren, Liang Zou, Chuang Zhou, and Lianxin Peng. 2024. "Exploring the Potential of Plant-Derived Exosome-like Nanovesicle as Functional Food Components for Human Health: A Review" Foods 13, no. 5: 712. https://doi.org/10.3390/foods13050712
APA StyleLiu, Y., Ren, C., Zhan, R., Cao, Y., Ren, Y., Zou, L., Zhou, C., & Peng, L. (2024). Exploring the Potential of Plant-Derived Exosome-like Nanovesicle as Functional Food Components for Human Health: A Review. Foods, 13(5), 712. https://doi.org/10.3390/foods13050712






