New Horizons in Probiotics: Unraveling the Potential of Edible Microbial Polysaccharides through In Vitro Digestion Models
Abstract
:1. Introduction
2. In Vitro Digestion Models
2.1. The Earliest—SHIME
2.2. Widely Used—TIM
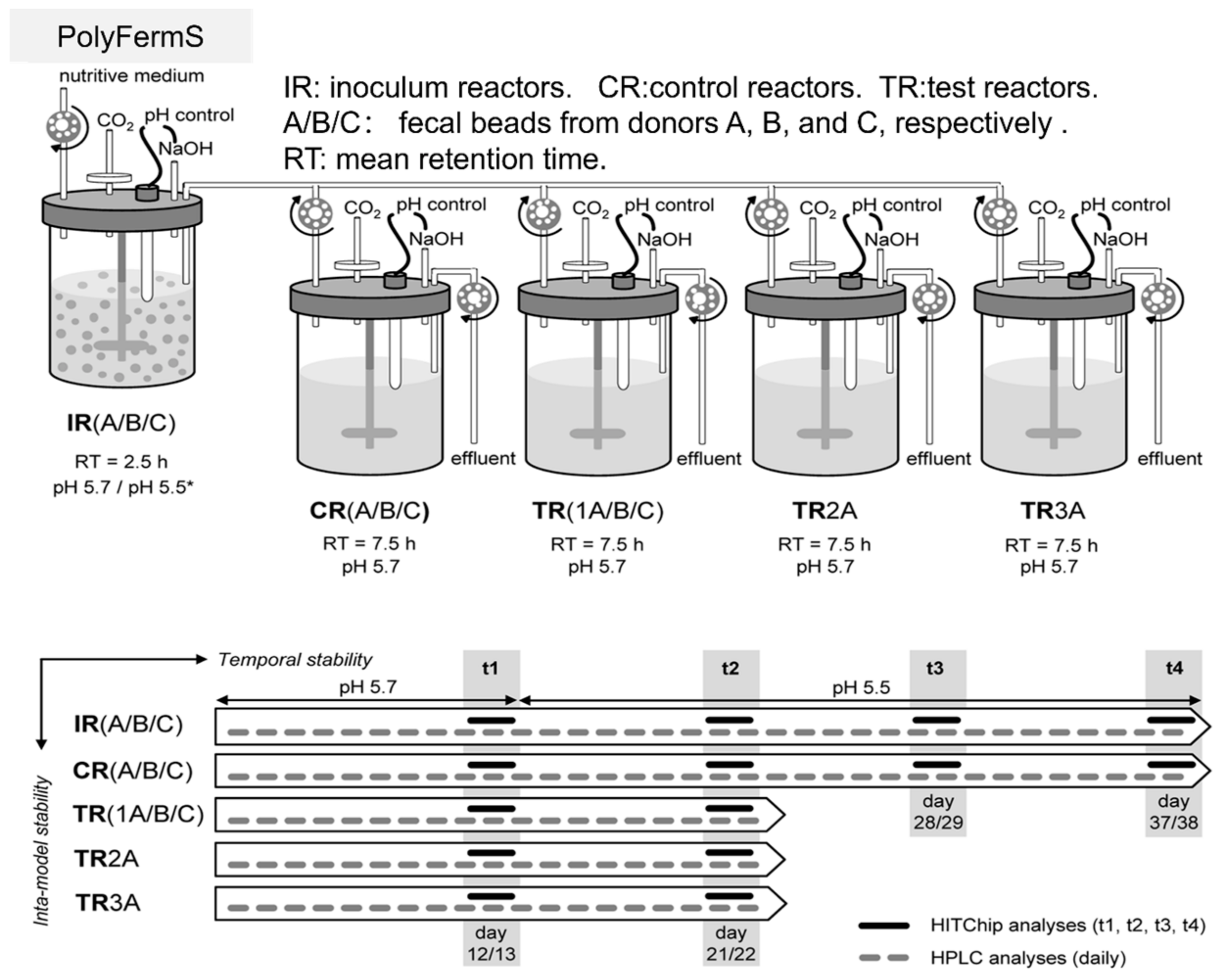
2.3. Unique—PolyFermS
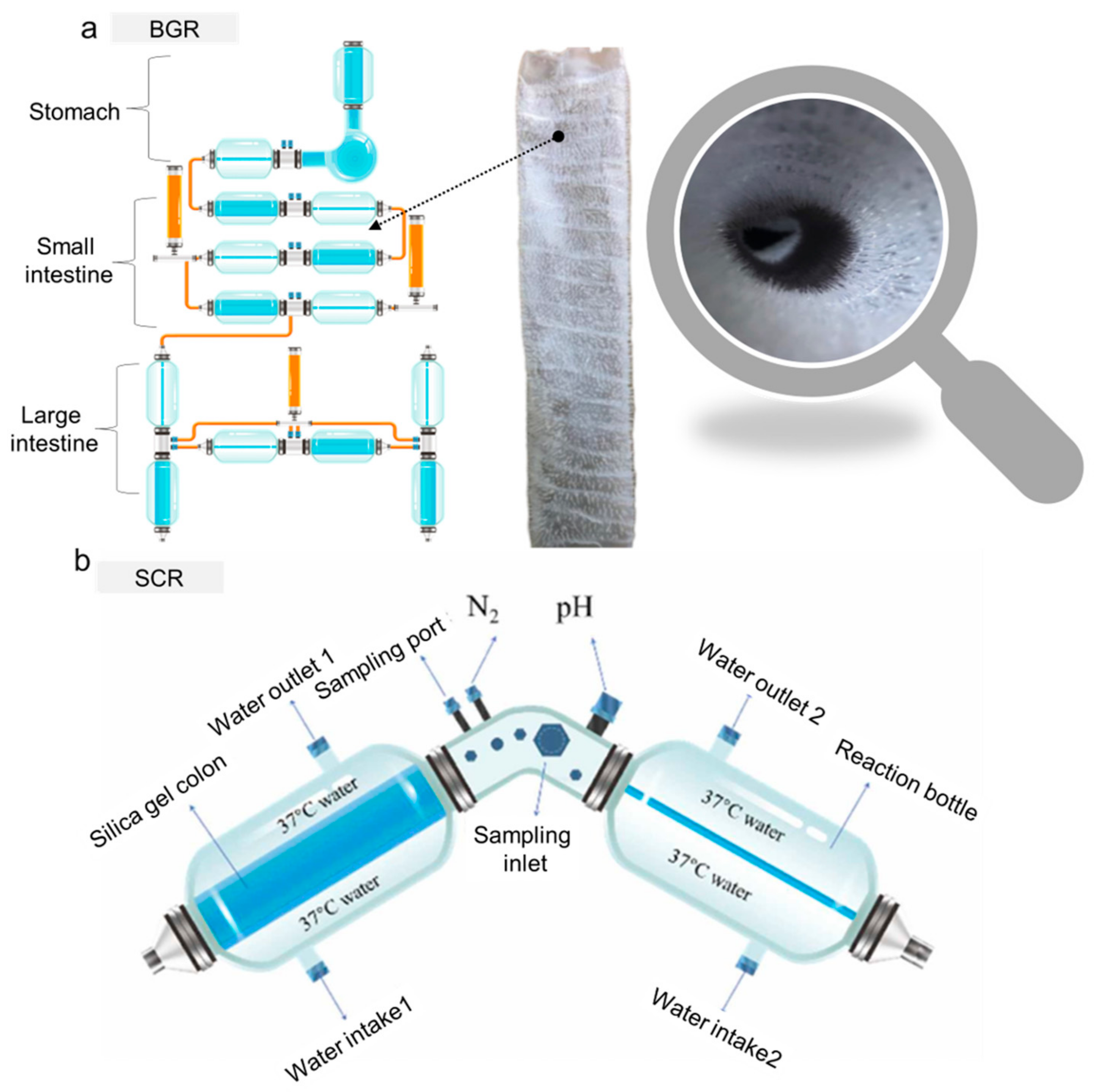
2.4. Emerging—BGR
3. Factors Influencing the Impacts of Microbial Polysaccharides and Oligosaccharides on the Intestine
3.1. Glycosidic Bonds and Monosaccharide Compositions
3.2. Polymerization
3.3. Linear and Branched Structures
4. Application of XG in Intestinal Reactors
4.1. XG
4.2. Application of XG in In Vitro Biomimetic Models
4.2.1. Fluid Dynamics Evaluation
4.2.2. Synbiotics for Auxiliary Colonization
4.2.3. Probiotic Potential
5. Application of GG in Intestinal Reactors
5.1. GG
5.2. Application of GG in In Vitro Digestion
6. Conclusions and Future Trends
- Multi-faceted exploration of interactions: a diverse range of microbial gums and various digestion methods (such as static digestion and in vitro dynamic digestion) should be employed to comprehensively explore the interactions among diet, host health, and metabolic regulation.
- Innovations in preparation methods and expanded applications: new preparation methods for microbial gums as prebiotics need to be developed, and novel applications as synbiotics also warrant exploration.
- Detailed mechanistic insights: extensive research is required to elucidate the detailed mechanisms underlying the relationship between microbial gum structure and host health, mediated by the gut microbiota.
- Personalized nutrition with microbial polysaccharides: the development and application of personalized nutritional microbial gums, based on the functional characteristics of the gut microbiota.
- Structural, functional, and environmental associations: given that microbial gums vary with bacterial sources and processing history, attention should be paid to the associations of these variations with structure and activity. These factors often co-determine the metabolic pathways and cross-feeding interactions within the gut microbial community, ultimately influencing host health.
- More realistic models for digestion detection and evaluation: in vitro experiments are constrained by model simplifications and experimental conditions, and they are unable to fully capture the diversity and dynamics of food in the actual intestines. Continuous updates and iterations are essential to overcome the limitations of in vitro digestion. Researchers must exercise caution in interpreting in vitro experiment results at this stage, combining them with other research methods for a comprehensive understanding.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Baral, K.C.; Bajracharya, R.; Lee, S.H.; Han, H.K. Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology. Int. J. Nanomed. 2021, 16, 7535–7556. [Google Scholar] [CrossRef] [PubMed]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Tech. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Zhang, H.; Wu, J.; Zhu, L.; Zhan, X. In vitro assessment of prebiotic properties of oligosaccharides derived from four microbial polysaccharides. LWT 2021, 147, 111544. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladanyi, M.; Juhasz, R.; Szecsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, Z.G.; Xiao, Z.G.; Saleh, A.S.M. Impact of calcium ions and degree of oxidation on the structural, physicochemical, and in-vitro release properties of resveratrol-loaded oxidized gellan gum hydrogel beads. Int. J. Biol. Macromol. 2022, 196, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Khoobbakht, F.; Mirmoghtadaie, L.; Hosseini, S.M. Characterization of gellan gum-chitosan based hydrogel to evaluate as a potential gelatin substitute. Food Hydrocoll. 2023, 145, 109038. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Chen, C.; Li, P.; Lu, B. The gut microbiota-artery axis: A bridge between dietary lipids and atherosclerosis? Prog. Lipid Res. 2023, 89, 101209. [Google Scholar] [CrossRef]
- Gu, Z.; Meng, S.; Wang, Y.; Lyu, B.; Li, P.; Shang, N. A novel bioactive postbiotics: From microbiota-derived extracellular nanoparticles to health promoting. Crit. Rev. Food Sci. Nutr. 2023, 63, 6885–6899. [Google Scholar] [CrossRef] [PubMed]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Meng, T.; He, W.; Huang, H.; Liu, C.; Fu, X.; He, J.; Yin, Y.; Xiao, D. Dietary Chito-oligosaccharides Improve Intestinal Immunity via Regulating Microbiota and Th17/Treg Balance-Related Immune Signaling in Piglets Challenged by Enterotoxigenic E. coli. J. Agric. Food Chem. 2021, 69, 15195–15207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, D.; Wang, X.; Asare, P.T.; Zhang, Q.; Na, L.; Shao, L. Gut Microbiota Targeted Approach in the Management of Chronic Liver Diseases. Front. Cell. Infect. Microbiol. 2022, 12, 774335. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, F.C.; de Barros Ranke, F.F.; de Oliva-Neto, P. Evaluation of xylooligosaccharides and fructooligosaccharides on digestive enzymes hydrolysis and as a nutrient for different probiotics and Salmonella typhimurium. LWT 2020, 118, 108761. [Google Scholar] [CrossRef]
- Huang, C.X.; Yu, Y.X.; Li, Z.; Yan, B.W.; Pei, W.H.; Wu, H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: A review. Front. Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fang, J.; Huang, W.; Liu, S.; Zhang, X.; Gong, G.; Huang, L.; Lin, X.; Wang, Z. The intervention effects of konjac glucomannan with different molecular weights on high-fat and high-fructose diet-fed obese mice based on the regulation of gut microbiota. Food Res. Int. 2023, 165, 112498. [Google Scholar] [CrossRef]
- Ferreira, V.C.; Barroso, T.L.C.T.; Castro, L.E.N.; da Rosa, R.G.; Oliveira, L.D. An overview of prebiotics and their applications in the food industry. Eur. Food Res. Technol. 2023, 249, 2957–2976. [Google Scholar] [CrossRef]
- Yang, H.; Hou, Y.; Pan, Y.; Zhang, T.; Meng, Q.; Han, J.; Liu, W.; Qu, D. Effect of chewing ability on in vivo oral digestive characteristics and in vitro gastrointestinal starch hydrolysis of three different types of cooked rice. Food Funct. 2023, 14, 9324–9336. [Google Scholar] [CrossRef]
- Xavier, A.A.O.; Mariutti, L.R.B. Static and semi-dynamicdigestion methods: State of the art and recent achievements towards standardization. Curr. Opin. Food Sci. 2021, 41, 260–273. [Google Scholar] [CrossRef]
- Nadia, J.B.J.; Singh, R.P.; Singh, H.; Bornhorst, G.M. Structural breakdown of starch-based foods during gastric digestion and its link to glycemic response: In vivo and in vitro considerations. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2660–2698. [Google Scholar] [CrossRef] [PubMed]
- Pautong, P.A.; Añonuevo, J.J.; de Guzman, M.K.; Sumayao, R.; Henry, C.J.; Sreenivasulu, N. Evaluation of digestion methods and starch structure components as determinants for predicting the glycemic index of rice. LWT-Food Sci. Technol. 2022, 168, 113929. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Madalena, D.A.; Pinheiro, A.C.; Vicente, A.A. Rice in vitro digestion: Application of INFOGEST harmonized protocol for glycemic index determination and starch morphological study. J. Food Sci. Technol. 2020, 57, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hu, J.; Zuo, S.; Zhang, S.; Li, M.; Nie, S. In vitro gastrointestinal digestion and fermentation models and their applications in food carbohydrates. Crit. Rev. Food Sci. Nutr. 2022, 62, 5349–5371. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Son, H.; Lee, G.; Lee, S.; Unno, T.; Shin, J.H. Role, relevance, and possibilities of in vitro fermentation models in human dietary, and gut-microbial studies. Biotechnol. Bioeng. 2022, 119, 3044–3061. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F. Simulating human gastrointestinal motility in dynamic in vitro models. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3804–3833. [Google Scholar] [CrossRef]
- Venema, K. The TNO In Vitro Model of the Colon (TIM-2). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 293–304. [Google Scholar] [CrossRef]
- Minekus, M. The TNO Gastro-Intestinal Model (TIM). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 37–46. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Cotter, P.D. Relevance of organ(s)-on-a-chip systems to the investigation of food-gut microbiota-host interactions. Crit. Rev. Microbiol. 2022, 48, 463–488. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, S.; Yu, Y.; Wu, R.; Wang, J.; Chen, X.D.; Szeto, I.M.; Wu, P.; Jin, Y. Impact of casein-to-whey protein ratio on gastric emptying, proteolysis, and peptidome profile of fermented milk during in vitro dynamic gastrointestinal digestion in preschool children. Food Chem. 2023, 405, 134840. [Google Scholar] [CrossRef]
- Li, Z.T.; Zhu, L.; Zhang, W.L.; Zhan, X.B.; Gao, M.J. New dynamic digestion model reactor that mimics gastrointestinal function. Biochem. Eng. J. 2020, 154, 107431. [Google Scholar] [CrossRef]
- Molly, K.; Woestyne, M.V.; Smet, I.D.; Verstraete, W. Validation of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) Reactor Using Microorganism-associated Activities. Microb. Ecol. Health Dis. 2009, 7, 191–200. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Van den Abbeele, P.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIMER®). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., Lopez-Exposito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 305–317. [Google Scholar] [CrossRef]
- Alander, M.; De Smet, I.; Nollet, L.; Verstraete, W.; von Wright, A.; Mattila-Sandholm, T. The effect of probiotic strains on the microbiota of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). Int. J. Food Microbiol. 1999, 46, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Rovalino-Córdova, A.M.; Fogliano, V.; Capuano, E. Effect of bean structure on microbiota utilization of plant nutrients: An in-vitro study using the simulator of the human intestinal microbial ecosystem (SHIME®). J. Funct. Foods 2020, 73, 104087. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Hao, L.; Du, P.; Li, C.; Han, H.; Xu, H.; Liu, L. The bioavailability of soybean polysaccharides and their metabolites on gut microbiota in the simulator of the human intestinal microbial ecosystem (SHIME). Food Chem. 2021, 362, 130233. [Google Scholar] [CrossRef]
- Yang, L.; Kang, X.C.; Dong, W.J.; Wang, L.; Liu, S.F.; Zhong, X.H.; Liu, D.B. Prebiotic properties of Ganoderma lucidum polysaccharides with special enrichment of Bacteroides ovatus and B. uniformis in vitro. J. Funct. Foods 2022, 92, 105069. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef]
- Verwei, M.; Minekus, M.; Zeijdner, E.; Schilderink, R.; Havenaar, R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms. Int. J. Pharm. 2016, 498, 178–186. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Y.; Dong, Q.; Tang, X.; Xin, Y.; Yin, B.; Zhu, J.; Kou, X.; Ho, C.T.; Huang, Q. Development of organogel-based emulsions to enhance the loading and bioaccessibility of 5-demethylnobiletin. Food Res. Int. 2021, 148, 110592. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Chassard, C.; Haug, M.C.; Fourmestraux, C.; Derrien, M.; Lacroix, C. Design and Investigation of PolyFermS In Vitro Continuous Fermentation Models Inoculated with Immobilized Fecal Microbiota Mimicking the Elderly Colon. PLoS ONE 2015, 10, e0142793. [Google Scholar] [CrossRef]
- Pham, V.T.; Chassard, C.; Rifa, E.; Braegger, C.; Geirnaert, A.; Rocha Martin, V.N.; Lacroix, C. Lactate Metabolism Is Strongly Modulated by Fecal Inoculum, pH, and Retention Time in PolyFermS Continuous Colonic Fermentation Models Mimicking Young Infant Proximal Colon. mSystems 2019, 4, e00264-18. [Google Scholar] [CrossRef]
- Zihler Berner, A.; Fuentes, S.; Dostal, A.; Payne, A.N.; Vazquez Gutierrez, P.; Chassard, C.; Grattepanche, F.; de Vos, W.M.; Lacroix, C. Novel Polyfermentor intestinal model (PolyFermS) for controlled ecological studies: Validation and effect of pH. PLoS ONE 2013, 8, e77772. [Google Scholar] [CrossRef] [PubMed]
- Isenring, J. Adaptive Evolutionary Engineering and Characterization of the Invasion Process of Lactiplantibacillus Plantarum in Human PolyFermS Continuous Colonic Fermentation Model; ETH Zurich: Zürich, Switzerland, 2021. [Google Scholar]
- Tanner, S.A.; Zihler Berner, A.; Rigozzi, E.; Grattepanche, F.; Chassard, C.; Lacroix, C. In vitro continuous fermentation model (PolyFermS) of the swine proximal colon for simultaneous testing on the same gut microbiota. PLoS ONE 2014, 9, e94123. [Google Scholar] [CrossRef]
- Pennacchia, A. Modelling the Impact of Antibiotics and Resistant Strains in Chicken and Human Gut in the In Vitro PolyFermS Fermentation Model; ETH Zurich: Zürich, Switzerland, 2023. [Google Scholar]
- Poeker, S.A.; Geirnaert, A.; Berchtold, L.; Greppi, A.; Krych, L.; Steinert, R.E.; de Wouters, T.; Lacroix, C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS). Sci. Rep. 2018, 8, 4318. [Google Scholar] [CrossRef] [PubMed]
- Poeker, S.A.; Lacroix, C.; de Wouters, T.; Spalinger, M.R.; Scharl, M.; Geirnaert, A. Stepwise Development of an in vitro Continuous Fermentation Model for the Murine Caecal Microbiota. Front. Microbiol. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Fehlbaum, S.; Chassard, C.; Schwab, C.; Voolaid, M.; Fourmestraux, C.; Derrien, M.; Lacroix, C. In vitro Study of Lactobacillus paracasei CNCM I-1518 in Healthy and Clostridioides difficile Colonized Elderly Gut Microbiota. Front. Nutr. 2019, 6, 184. [Google Scholar] [CrossRef] [PubMed]
- Bircher, L.; Schwab, C.; Geirnaert, A.; Greppi, A.; Lacroix, C. Planktonic and Sessile Artificial Colonic Microbiota Harbor Distinct Composition and Reestablish Differently upon Frozen and Freeze-Dried Long-Term Storage. Msystems 2020, 5, e00521-19. [Google Scholar] [CrossRef] [PubMed]
- Naimi, S.; Zirah, S.; Taher, M.B.; Theolier, J.; Fernandez, B.; Rebuffat, S.F.; Fliss, I. Microcin J25 Exhibits Inhibitory Activity Against Salmonella Newport in Continuous Fermentation Model Mimicking Swine Colonic Conditions. Front. Microbiol. 2020, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Isenring, J.; Geirnaert, A.; Hall, A.R.; Jans, C.; Lacroix, C.; Stevens, M.J. In vitro gut modeling as a tool for adaptive evolutionary engineering of Lactiplantibacillus plantarum. Msystems 2021, 6, e01085-20. [Google Scholar] [CrossRef]
- Gosciniak, A.; Eder, P.; Walkowiak, J.; Cielecka-Piontek, J. Artificial Gastrointestinal Models for Nutraceuticals Research-Achievements and Challenges: A Practical Review. Nutrients 2022, 14, 2560. [Google Scholar] [CrossRef]
- Naimi, S.; Zirah, S.; Greppi, A.; Lacroix, C.; Rebuffat, S.; Fliss, I. Impact of microcin J25 on the porcine microbiome in a continuous culture model. Front. Microbiol. 2022, 13, 930392. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ji, H.; Jiang, Y.; Gao, M.; Zhan, X.; Jin, Z. In-vitro dynamic fermentation simulation colon reactor for gut microbiota incubation. Biochem. Eng. J. 2023, 193, 108877. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Hu, G.; Sun, Z.; Zhan, X.; Gao, M. Akkermansia muciniphila fermentation culture based on a novel bionic large intestine dynamic digestion model. Food Biosci. 2021, 43, 101260. [Google Scholar] [CrossRef]
- Li, Z.T.; Hu, G.A.; Zhu, L.; Zhao, Z.C.; Yun, J.; Gao, M.J.; Zhan, X.B. In vitro digestion and fecal fermentation of highly resistant starch rice and its effect on the gut microbiota. Food Chem. 2021, 361, 130095. [Google Scholar] [CrossRef]
- Li, Z.T.; Han, S.X.; Pu, J.Y.; Wang, Y.Y.; Jiang, Y.; Gao, M.J.; Zhan, X.B.; Xu, S. In Vitro Digestion and Fecal Fermentation of Low-Gluten Rice and Its Effect on the Gut Microbiota. Foods 2023, 12, 855. [Google Scholar] [CrossRef]
- Yu, D.; Zhu, L.; Gao, M.; Yin, Z.; Zhang, Z.; Zhu, L.; Zhan, X. A Comparative Study of the Effects of Whole Cereals and Refined Cereals on Intestinal Microbiota. Foods 2023, 12, 2847. [Google Scholar] [CrossRef]
- Milani, C.; Lugli, G.A.; Duranti, S.; Turroni, F.; Mancabelli, L.; Ferrario, C.; Mangifesta, M.; Hevia, A.; Viappiani, A.; Scholz, M.; et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Sci. Rep. 2015, 5, 15782. [Google Scholar] [CrossRef]
- Goh, Y.J.; Klaenhammer, T.R. Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu. Rev. Food Sci. Technol. 2015, 6, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Zunga, M.; Yebra, M.J.; Monedero, V. Complex Oligosaccharide Utilization Pathways in Lactobacillus. Curr. Issues Mol. Biol. 2021, 40, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Barrangou, R.; Hachem, M.A.; Lahtinen, S.J.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional analysis of prebiotic uptake and catabolism by Lactobacillus acidophilus NCFM. PLoS ONE 2012, 7, e44409. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Homer, K.A.; Hosie, A.H. Two closely related ABC transporters in Streptococcus mutans are involved in disaccharide and/or oligosaccharide uptake. J. Bacteriol. 2008, 190, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.A.A.; Molenaar, D.; de Vos, W.A.; Gibson, G.R.; Kolida, S. Identification of Prebiotic Fructooligosaccharide Metabolism in Lactobacillus plantarum WCFS1 through Microarrays. Appl. Environ. Microb. 2007, 73, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.Q.; Sun, Y.; Ye, H.; Hu, B.; Zeng, X.X. Influences of structures of galactooligosaccharides and fructooligosaccharides on the fermentation by human intestinal microbiota. J. Funct. Foods 2015, 13, 158–168. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Y.; Zhang, S.; Gao, X.; Liu, F.; Zhang, H.; Dai, Y.; Wang, Y.; Lu, F. The Vitro Fermentation of Six Functional Oligosaccharides by Clostridium butyricum TK2 and Clostridium butyricum CB8. Food Sci. Technol. Res. 2014, 20, 1005–1011. [Google Scholar] [CrossRef]
- Snelders, J.; Olaerts, H.; Dornez, E.; Van de Wiele, T.; Aura, A.M.; Vanhaecke, L.; Delcour, J.A.; Courtin, C.M. Structural features and feruloylation modulate the fermentability and evolution of antioxidant properties of arabinoxylanoligosaccharides during in vitro fermentation by human gut derived microbiota. J. Funct. Foods 2014, 10, 1–12. [Google Scholar] [CrossRef]
- Iwaya, H.; Lee, J.S.; Yamagishi, S.; Shinoki, A.; Lang, W.; Thawornkuno, C.; Kang, H.K.; Kumagai, Y.; Suzuki, S.; Kitamura, S.; et al. The delay in the development of experimental colitis from isomaltosyloligosaccharides in rats is dependent on the degree of polymerization. PLoS ONE 2012, 7, e50658. [Google Scholar] [CrossRef]
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307. [Google Scholar] [CrossRef]
- Nobre, C.; do Nascimento, A.K.C.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Cavalcanti, M.T.H.; Teixeira, J.A.; Porto, A.L.F. Process development for the production of prebiotic fructo-oligosaccharides by penicillium citreonigrum. Bioresour. Technol. 2019, 282, 464–474. [Google Scholar] [CrossRef]
- Hu, Y.; Heyer, C.M.E.; Wang, W.; Zijlstra, R.T.; Ganzle, M.G. Digestibility of branched and linear alpha-gluco-oligosaccharides in vitro and in ileal-cannulated pigs. Food Res. Int. 2020, 127, 108726. [Google Scholar] [CrossRef]
- Jonathan, M.C.; van Brussel, M.; Scheffers, M.S.; Kabel, M.A. Characterisation of branched gluco-oligosaccharides to study the mode-of-action of a glucoamylase from Hypocrea jecorina. Carbohydr. Polym. 2015, 132, 59–66. [Google Scholar] [CrossRef]
- Sworn, G. Xanthan Gum; Elsevier: Amsterdam, The Netherlands, 2021; pp. 833–853. [Google Scholar]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohyd. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Lopes, B.D.; Lessa, V.L.; Silva, B.M.; Carvalho, M.A.D.; Schnitzler, E.; Lacerda, L.G. Xanthan gum: Properties, production conditions, quality and economic perspective. J. Food Nutr. Res 2015, 54, 185–194. [Google Scholar]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Fang, Y. Introduction to food hydrocolloids. In Food Hydrocolloids: Functionalities and Applications; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 1–28. [Google Scholar]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Preichardt, L.D.; Vendruscolo, C.T.; Gularte, M.A.; Moreira, A.D. The role of xanthan gum in the quality of gluten free cakes: Improved bakery products for coeliac patients. Int. J. Food Sci. Tech. 2011, 46, 2591–2597. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic gum, xanthan gum and orange oil on flavor release from diluted orange beverage emulsion. Food Chem. 2008, 107, 1161–1172. [Google Scholar] [CrossRef]
- Hemar, Y.; Tamehana, M.; Munro, P.A.; Singh, H. Viscosity, microstructure and phase behavior of aqueous mixtures of commercial milk protein products and xanthan gum. Food Hydrocoll. 2001, 15, 565–574. [Google Scholar] [CrossRef]
- Le, Y.; Yang, H.S. Xanthan gum modified fish gelatin and binary culture modulates the metabolism of probiotics in fermented milk mainly via amino acid metabolism pathways. Food Res. Int. 2022, 161, 111844. [Google Scholar] [CrossRef] [PubMed]
- Keppler, S.; O’Meara, S.; Bakalis, S.; Fryer, P.J.; Bornhorst, G.M. Characterization of individual particle movement during in vitro gastric digestion in the Human Gastric Simulator (HGS). J. Food Eng. 2020, 264, 109674. [Google Scholar] [CrossRef]
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carbohydr. Polym. 2019, 208, 431–440. [Google Scholar] [CrossRef]
- Cinquin, C.; Le Blay, G.; Fliss, I.; Lacroix, C. Immobilization of infant fecal microbiota and utilization in an in vitro colonic fermentation model. Microb. Ecol. 2004, 48, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-T.; Nie, X.-R.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Xu, J.; Sun, W.; Li, H.; Gao, Z.; Hu, G.; Wu, J.; Zhang, H.; Li, Z.; Gao, M.; Zhu, L.; et al. Xanthan gum oligosaccharides ameliorate glucose metabolism and related gut microbiota dysbiosis in type 2 diabetic mice. Food Biosci. 2022, 50, 102002. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Dong, D.; Zhang, Y.; Zhang, S.; Pan, Y.; Ji, H.; Zhang, Z.; Huang, X.; Zhang, L.; et al. Effects of xanthan gum, konjac glucomannan, and arabinogalactan on the in vitro digestion and fermentation characteristics of biscuits. Food Funct. 2023, 14, 6036–6048. [Google Scholar] [CrossRef] [PubMed]
- Espert, M.; Salvador, A.; Sanz, T. Rheological and microstructural behaviour of xanthan gum and xanthan gum-Tween 80 emulsions during in vitro digestion. Food Hydrocoll. 2019, 95, 454–461. [Google Scholar] [CrossRef]
- Espert, M.; Constantinescu, L.; Sanz, T.; Salvador, A. Effect of xanthan gum on palm oil in vitro digestion. Application in starch-based filling creams. Food Hydrocoll. 2019, 86, 87–94. [Google Scholar] [CrossRef]
- Vera, C.N.; Laguna, L.; Zura, L.; Puente, L.; Muñoz, L.A. Evaluation of the physical changes of different soluble fibres produced during an in vitro digestion. J. Funct. Foods 2019, 62, 103518. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Sun, W.X.; Yang, W.H.; Chen, S.G.; Liu, D.H.; Tian, J.H.; Ye, X.Q. The Influence of Xanthan Gum on Rheological Properties and In Vitro Digestibility of Kudzu (Pueraria lobata) Starch. Starch-Stärke 2020, 72, 1900139. [Google Scholar] [CrossRef]
- Chen, M.S.; Guo, L.P.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.X.; Mao, J.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses Ⅰ: In vitro digestion process. Food Hydrocoll. 2020, 107, 105971. [Google Scholar] [CrossRef]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 109859. [Google Scholar] [CrossRef]
- Zhan, J.Q.; Yu, W.T.; Fu, J.J.; Li, G.S.; Hu, Y.Q.; Chen, Y.W. Peptides-carrageenan-xanthan gum: Printing mechanism and anti-oxidation under in vitro digestion. Food Biosci. 2023, 53, 102546. [Google Scholar] [CrossRef]
- Ozel, B.; Aydin, O.; Oztop, M.H. In vitro digestion of polysaccharide including whey protein isolate hydrogels. Carbohydr. Polym. 2020, 229, 115469. [Google Scholar] [CrossRef] [PubMed]
- Sworn, G.; Stouby, L. Gellan gum. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 855–885. [Google Scholar]
- Annaka, M. Anion specific conformational change in aqueous gellan gum solutions. Carbohydr. Polym. 2023, 305, 120437. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, Y.; Zhao, Y.; Fang, Y. Role of conformation transition of high acyl gellan in the design of double network hydrogels. Int. J. Biol. Macromol. 2023, 233, 123583. [Google Scholar] [CrossRef]
- Das, M.; Giri, T.K. Hydrogels based on gellan gum in cell delivery and drug delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101586. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Wang, X.; Zohar-Perez, C.; Zeng, Y.; Zou, Y.; Chen, Y.; Wu, S.; Wang, Y.; Arazi, S.; Nussinovitch, A.; Achmon, Y. Assessment of the environmental impact of agar, alginate, and gellan gum carbohydrate gum macro beads biodegradation in a simulated agricultural field system. Environ. Technol. Innov. 2023, 30, 103034. [Google Scholar] [CrossRef]
- Yan, B.; Zhao, Z.; Zhang, N.; Ruan, H.; Yu, X.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. 3D food printing curing technology based on gellan gum. J. Food Eng. 2022, 327, 111036. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, L.; Pan, C.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. 3D printed gellan gum/graphene oxide scaffold for tumor therapy and bone reconstruction. Compos. Sci. Technol. 2021, 208, 108763. [Google Scholar] [CrossRef]
- Dev, M.J.; Warke, R.G.; Warke, G.M.; Mahajan, G.B.; Patil, T.A.; Singhal, R.S. Advances in fermentative production, purification, characterization and applications of gellan gum. Bioresour. Technol. 2022, 359, 127498. [Google Scholar] [CrossRef] [PubMed]
- Sun, W. Immobilization of Bifidobacteria to Prolong Their Survival in Acidic Environments; University of Guelph: Guelph, ON, Canada, 1999. [Google Scholar]
- Nag, A.; Han, K.S.; Singh, H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int. Dairy J. 2011, 21, 247–253. [Google Scholar] [CrossRef]
- Dixit, R.; Verma, A.; Singh, U.P.; Soni, S.; Mishra, A.K.; Bansal, A.K.; Pandit, J.K. Preparation and Characterization of Gellan-Chitosan Polyelectrolyte Complex Beads. Lat. Am. J. Pharm. 2011, 30, 1186–1195. [Google Scholar]
- Rosas-Flores, W.; Ramos-Ramirez, E.G.; Salazar-Montoya, J.A. Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii using alginate and gellan gum. Carbohydr. Polym. 2013, 98, 1011–1017. [Google Scholar] [CrossRef]
- Gao, M.; Li, H.; Yang, T.; Li, Z.; Hu, X.; Wang, Z.; Jiang, Y.; Zhu, L.; Zhan, X. Production of prebiotic gellan oligosaccharides based on the irradiation treatment and acid hydrolysis of gellan gum. Carbohydr. Polym. 2022, 279, 119007. [Google Scholar] [CrossRef]
- Liu, L.Y.; Zhang, D.D.; Song, X.X.; Guo, M.; Wang, Z.W.; Geng, F.; Zhou, X.T.; Nie, S.P. Compound hydrogels derived from gelatin and gellan gum regulates the release of anthocyanins in simulated digestion. Food Hydrocoll. 2022, 127, 107487. [Google Scholar] [CrossRef]
- Vilela, J.A.P.; Perrechil, F.A.; Picone, C.S.F.; Sato, A.C.K.; Cunha, R.L.D. Preparation, characterization and in vitro digestibility of gellan and chitosan-gellan microgels. Carbohydr. Polym. 2015, 117, 54–62. [Google Scholar] [CrossRef]
- Nascimento da Costa, J.; Lima Nascimento, L.G.; Leal, A.R.; Danalache, F.; Moreira Leite, B.S.; Figueiredo, R.W.; Mata, P.; Alves, V.D.; Machado de Sousa, P.H. Effect of agar and gellan gum on structured guava (Psidium guajava L.): Rheological behavior and gastrointestinal digestion in vitro. Food Biosci. 2021, 42, 101165. [Google Scholar] [CrossRef]
- Meng, Y.; Hang, L.; Fang, S.; Li, Y.; Xu, X.; Zhang, F.; Chen, J. Fabrication of High-Acyl Gellan-Gum-Stabilized beta-Carotene Emulsion: Physicochemical Properties and In Vitro Digestion Simulation. Foods 2022, 11, 1742. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Z.G.; Xiao, Z.G. Preparation, physicochemical characterization and in vitro release behavior of resveratrol-loaded oxidized gellan gum/resistant starch hydrogel beads. Carbohydr. Polym. 2021, 260, 117794. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, J.; Han, L.; Han, K.; Wei, W.; Wu, T.; Li, J.; Zhang, M. Development and characterization of novel bigels based on monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel for lycopene delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef] [PubMed]




| Sample | In Vitro Digestion Model | Changes During Digestion | Key Findings | Reference |
|---|---|---|---|---|
| XG emulsion with/without Tween 80 | Three-step in vitro digestion model | Fat coagulation; increased viscoelasticity, due to weak points in XG structure; lower fat digestion rate in XG emulsion with Tween 80 | Tween 80-containing XG emulsion resists fat digestion and reduces FFA levels. XG solution shows decreased viscoelasticity and more flexible chains. Increased gastric viscoelasticity in XG emulsion, due to weak points in structure. | [94] |
| XG–palm oil system | Three-step in vitro digestion model | Decomposition of XG–palm oil system; structural changes; formation of metabolites | After gastric cultivation: increased extrusion force, modulus of elasticity, and viscosity in XG system; associated with higher water dilution and increased fat droplet size. | [95] |
| XG | Infogest COST Action | Aggregation, maintaining structure and viscosity during gastric stage | XG exhibits aggregation during digestion, maintaining its structure and viscosity during the gastric stage. | [96] |
| Kudzu starch and XG | Static digestion | Changes in molecular structure; formation of degradation products | Increasing XG concentration enhances interaction with kudzu starch, slowing down its hydrolysis during in vitro digestion. XG oligosaccharides may influence molecular structure. | [97] |
| XG | Infogest simulated digestion model | Highest viscosity of XG at the end of digestion, followed by GG and LBG, at low and high viscosities | XG significantly reduces glucose and free amino acid release at high viscosity; linear relationship with protein and starch digestion. | [98] |
| Whey Protein Isolate (WPI) and XG | Static digestion | Changes in emulsion particle size; degradation of XG; interaction between WPI and XG | WPI-XG stable emulsion affects digestion process of emulsion particles, resulting in lower digestion rates of fat and carotenoids. | [99] |
| Peptides–carrageenan–XG | Static digestion | Changes in molecular structure; formation of degradation products | ABTS ion, hydroxyl radical, and iron ion clearance rates were 62.83%, 74.81%, and 0.035 mg/mL, respectively. | [100] |
| XG | Static digestion | Changes in molecular structure (FTIR and NMR measurements) | After 2 h of exposure in SGF, changes in the molecular structure of XG were observed. | [101] |
| Sample | Changes During Digestion | Functional Evaluation | Key Findings | Reference |
|---|---|---|---|---|
| Anthocyanins encapsulated in GG | Anthocyanins retained in GG system in the stomach, released in the intestine. | Biocompatibility test with IEC-6 cells; GG system does not inhibit cell proliferation | GG-encapsulated anthocyanins effectively retained in the stomach and safely released in the intestine, showing potential as a safe and effective approach for treating and preventing intestinal diseases. | [116] |
| GG Microcapsules by physical cross-linking | Assessment of microcapsule size distribution, morphology, and Zeta potential before and after in vitro digestion | Evaluation of long-term stability | Microcapsules maintain shape after gastric digestion, disintegrate during intestinal digestion, suitable for intestinal delivery systems. Chitosan coating slows capsule disintegration in intestinal digestion, indicating coating aids in control of release characteristics. | [117] |
| Mixture of GG and Guava | Guava with added GG exhibits higher elasticity during time scan. | Evaluation of long-term stability, ascorbic acid, total antioxidant activity, and polyphenol content | Guava with added GG shows total antioxidant activity and average total extractable polyphenols close to values of guava pulp. | [118] |
| β-carotene Emulsion with High-acyl GG as emulsifier | Average particle size (MPS); emulsion yield (EY); dynamic stability | Bioavailability of β-carotene | Stable during oral and gastric digestion phases, MPS and ZP changes between 2.5 μm and 3.0 mV. In simulated intestinal digestion, β-carotene releases forms micelles; HA-β-carotene emulsion enhances release rate of FFA, improving β-carotene bioavailability. | [119] |
| Hydrogel beads comprising GG and Resistant Starch | Changes in molecular structure; introduction of carboxyl groups | pH sensitivity; drug loading efficiency | Gel beads exhibit good stability in simulated gastric fluid and continuous release of resveratrol in simulated intestinal fluid. | [120] |
| Mixture of Glycerol Monostearate–Beeswax Oleogel and High-acyl GG Hydrogel | Changes in molecular structure; rheological studies | Mechanical strength (storage modulus, hardness) increases with increasing oleogel content | Colloidal structure exhibits oil gel–water gel configuration; increasing oleogel content results in larger droplets. Rheological results indicate all colloids exhibit solid characteristics as storage modulus exceeds loss modulus. | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, S.; Zhang, T.; Gao, M.; Zhan, X. New Horizons in Probiotics: Unraveling the Potential of Edible Microbial Polysaccharides through In Vitro Digestion Models. Foods 2024, 13, 713. https://doi.org/10.3390/foods13050713
Wang Y, Zhu S, Zhang T, Gao M, Zhan X. New Horizons in Probiotics: Unraveling the Potential of Edible Microbial Polysaccharides through In Vitro Digestion Models. Foods. 2024; 13(5):713. https://doi.org/10.3390/foods13050713
Chicago/Turabian StyleWang, Yuying, Shengyong Zhu, Tiantian Zhang, Minjie Gao, and Xiaobei Zhan. 2024. "New Horizons in Probiotics: Unraveling the Potential of Edible Microbial Polysaccharides through In Vitro Digestion Models" Foods 13, no. 5: 713. https://doi.org/10.3390/foods13050713





