Evaluating the Impact of Modic Changes on Operative Treatment in the Cervical and Lumbar Spine: A Systematic Review and Meta-Analysis
Abstract
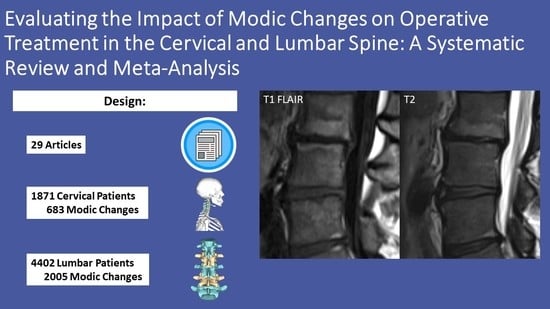
:1. Introduction
2. Materials and Methods
2.1. Study Eligibility
2.2. Data Collection Quality Assessment
2.3. Meta-Analysis
3. Results

| Authors | Patient Population | Modic Subtypes | Age | Gender | Patients | Any MC (%) | MC-I (%) | MC-II (%) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Yang et al. [32] (2019) | Patients with cervical radiculopathy due to single-level disc herniation | I, II | 45.2 (7.3) | 131 | 223 | 41 (18.4%) | 10 (4.5%) | 29 (13%) | 7 |
| Baker et al. [33] (2020) | Patients with symptomatic degenerative pathology refractory to conservative management | I, II, III | NR | NR | 861 | 356 (41.3%) | 70 (8.1%) | 218 (25.3%) | 9 |
| Huang et al. [34] (2020) | Patients who underwent single-level ACDF with MC-II | II | 50.4 (1.6) | 58 | 116 | 24 (20.7%) | 0 | 24 (20.7%) | 8 |
| Li et al. [35] (2015) | Patients who underwent single-level ACDF with MC-II | II | 47.0 (7.2) | 134 | 248 | 35 (14.1%) | 0 | 35 (14.1%) | 7 |
| Li et al. [36] (2017) | Patients with chronic axial symptoms resulting from single-level radiculopathy or myelopathy | II | 56.1 (6.1) | 36 | 76 | 76 (100%) | 0 | 76 (100%) | 7 |
| Zhou et al. [37] (2018) | Patients with cervical spondylotic myelopathy | NR | 56.1 (7.3) | 56 | 117 | 28 (23.9%) | NR | NR | 8 |
| Li et al. [38] (2022) | Patients with MCs cervical spondylotic myelopathy with hernia behind the vertebrae or OPLL | I, II | 55.0 (22.2) | 67 | 124 | 61 (49.2%) | 20 (16.1%) | 41 (33.1%) | 6 |
| Li et al. [39] (2015) | Patients with chronic axial symptoms resulting from single-level cervical disk degeneration nonresponsive to appropriate nonsurgical treatment for at least 6 months | I, II | 55.8 (6.5) | 49 | 106 | 62 (58.5%) | 23 (21.7%) | 39 (36.8%) | 7 |
| Authors | Patient Population | MC Subtypes | Age | Gender | Patients | Any MC (%) | MC-I (%) | MC-II (%) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Kumarasamy et al. [21] (2021) | Patients with LBP and single level lumbar disc herniation | I, II | 42.5 (12.6) | 107 | 309 | 86 (27.8%) | 6 (1.9%) | 68 (22%) | 7 |
| Jiao et al. [40] (2021) | Patients with LBP and either LDH, spinal stenosis, or spondylolisthesis who underwent single-segment TLIF with a PEEK cage | I, II | 56.7 (8.9) | 49 | 89 | 51 (57.3%) | 20 (22.5%) | 31 (60.8%) | 6 |
| el Barzouhi et al. [41] (2014) | Patients with sciatica | I, II | 43.2 (10.1) | 140 | 263 | 112 (42.6%) | 4 (1.5%) | 106 (40.3%) | 8 |
| Ulrich et al. [42] (2020) | Patients with claudication and lumbar stenosis | I, II | 66.8 (6.3) | 96 | 205 | 143 (69.8%) | 22 (15.4%) | 93 (65.0%) | 8 |
| Chung et al. [43] (2021) | Patients with lumbar DDD | I, II, III | 64.7 (9.1) | 54 | 86 | NR | NR | NR | 7 |
| MacLean et al. [23] (2021) | Patients with single level LDH | I, II, III | 53 (13) | 101 | 179 | 110 (61.5%) | 28 (15.6%) | 63 (35.2%) | 7 |
| Udby et al. [44] (2020) | Patients with bilateral or unilateral radiculopathy | I, II | 50.5 | 310 | 620 | 290 (46.8%) | 73 (11.8%) | 217 (35%) | 7 |
| Sørlie et al. [45] (2012) | Patients with one-level lumbar disc herniation | I, II | 41.2 (12.1) | 66 | 178 | 104 (58.4%) | 36 (20.2%) | 68 (38.2%) | 8 |
| Gornet et al. [46] (2014) | Patients with back pain due to DDD with pre-op ODI ≥ 30 | I, II | NR | NR | 89 | NR | NR | NR | 8 |
| Ohtori et al. [47] (2010) | Patients with LBP and leg pain due to lumbar spinal canal stenosis | I, II | 65.4 | 16 | 33 | 33 (100%) | 21 (63.6%) | 12 (36.4%) | 6 |
| Cao at al [48] (2014) | Patients with one-level LDH and MCs | I, II | NR | NR | 91 | 91 (100%) | 42 (46.2%) | 60 (65.9%) | 7 |
| Lurie et al. [49] (2013) | Patients with radicular pain due to intervertebral disc herniation | I, II | 41.7 (11.4) | 522 | 307 | 80 (26.1%) | 27 (8.8%) | 53 (17.3%) | 7 |
| Xu et al. [50] (2019) | Patients with unilateral radicular pain due to one-level intracanal disc herniation | I, II | 40.0 (12.5) | 104 | 276 | 94 (34.1%) | 44 (15.9%) | 50 (18.1%) | 6 |
| Djurasovic et al. [51] (2012) | Patients with “disc pathology” listed as primary surgical indication | I, II, III | 47 | 23 | 51 | NR | NR | NR | 7 |
| Masala et al. [52] (2014) | Patients with LBP without radicular symptoms unresponsive to conservative therapy for 6 months with type I MC | I | 40.3 (8.2) | 133 | 218 | 218 (100%) | 218 (100%) | 0 | 6 |
| Ohtori et al. [53] (2010) | Patients with one-level LDH | I | 35.5 | 19 | 45 | 23 (51.1%) | 23 (51.1%) | 0 | 6 |
| Rahme et al. [54] (2010) | Patients with one-level LDH | I, II, III | 54 | 14 | 41 | 32 (78%) | 6 (14.6%) | 26 (63.4%) | 7 |
| Blondel et al. [55] (2011) | Patients with chronic LBP due to single-level DDD | I, II | 42.1 | 101 | 221 | 114 (51.6%) | 65 (29.4%) | 49 (22.2%) | 8 |
| Gautschi et al. [56] (2016) | Patients with LBP due to disc herniation, spinal stenosis or DDD requiring lumbar fusion | I, II, III | 58.6 (15.5) | 144 | 338 | 202 (59.8%) | NR | NR | 7 |
| Hellum et al. [57] (2012) | Patients with LBP due to LDD with an ODI ≥ 30% | I, II | 41.2 (7.0) | 81 | 152 | 131 (85%) | 48 (31.6%) | 55 (36.2%) | 9 |
| Kwon et al. [58] (2009) | Patients who underwent PLIF | I, II, III | 47.4 | 232 | 351 | 92 (26.2%) | 26 (7.4%) | 55 (15.7%) | 7 |
3.1. Cervical Spine
3.2. Lumbar—Discectomy/Microdiscectomy
3.3. Lumbar Fusion
3.4. Other Lumbar Surgeries
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Section/Topic | Number | Checklist Item | Reported on Page Number |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 4 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2,3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 2 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 2,3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 3 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 3 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 3 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 3 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | NA |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 3 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 3–16 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 3, Table 1 and Table 2 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 3–16, tables |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 9, 15 (Figure 2 and Figure 3) |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 3, Table 1 and Table 2 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., health care providers, users, and policy makers). | 16, 17 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 16, 17 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 16, 17 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 2 |
References
- De Roos, A.; Kressel, H.; Spritzer, C.; Dalinka, M. MR imaging of marrow changes adjacent to end plates in degenerative lumbar disk disease. Am. J. Roentgenol. 1987, 149, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199. [Google Scholar] [CrossRef]
- Modic, M.T.; Masaryk, T.J.; Ross, J.; Carter, J.R. Imaging of degenerative disk disease. Radiology 1988, 168, 177–186. [Google Scholar] [CrossRef]
- Mitra, D.; Cassar-Pullicino, V.N.; Mccall, I.W. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur. Radiol. 2004, 14, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Teraguchi, M.; Hashizume, H.; Oka, H.; Cheung, J.P.; Samartzis, D.; Muraki, S.; Akune, T.; Kawaguchi, H.; Nakamura, K.; et al. A Prospective, 3-year Longitudinal Study of Modic Changes of the Lumbar Spine in a Population-based Cohort. Spine 2022, 47, 490–497. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Finnin, M.A.; Wang, Y.; Wluka, A.E.; Urquhart, D.M.; O’Sullivan, R.; Jones, G.; Cicuttini, F.M. The natural history of Modic changes in a community-based cohort. Jt. Bone Spine 2017, 84, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, J.; Karppinen, J.; Niinimäki, J.; Haapea, M.; Grönblad, M.; Luoma, K.; Rinne, E. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet. Disord. 2015, 16, 98. [Google Scholar] [CrossRef]
- Kuisma, M.; Karppinen, J.; Niinimäki, J.; Kurunlahti, M.; Haapea, M.; Vanharanta, H.; Tervonen, O. A Three-Year Follow-up of Lumbar Spine Endplate (Modic) Changes. Spine 2006, 31, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Albert, H.B.; Manniche, C. Modic changes following lumbar disc herniation. Eur. Spine J. 2007, 16, 977–982. [Google Scholar] [CrossRef]
- Karchevsky, M.; Schweitzer, M.E.; Carrino, J.A.; Zoga, A.; Montgomery, D.; Parker, L. Reactive endplate marrow changes: A systematic morphologic and epidemiologic evaluation. Skelet. Radiol. 2005, 34, 125–129. [Google Scholar] [CrossRef]
- Brandt, J.; Haibel, H.; Cornely, D.; Golder, W.; Gonzalez, J.; Reddig, J.; Thriene, W.; Sieper, J.; Braun, J. Successful treatment of active ankylosing spondylitis with the anti–tumor necrosis factor α monoclonal antibody infliximab. Arthritis Care Res. 2000, 43, 1346–1352. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, X.; Ren, H.; Huang, B.; Chen, J.; Liu, J.; Shan, Z.; Zhu, Z.; Zhao, F. Low virulence bacterial infections in cervical intervertebral discs: A prospective case series. Eur. Spine J. 2018, 27, 2496–2505. [Google Scholar] [CrossRef]
- Crockett, M.T.; Kelly, B.S.; Van Baarsel, S.; Kavanagh, E.C. Modic Type 1 Vertebral Endplate Changes: Injury, Inflammation, or Infection? Am. J. Roentgenol. 2017, 209, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Fujiwara, H.; Nishiwaki, Y.; Daimon, K.; Okada, E.; Nojiri, K.; Watanabe, M.; Katoh, H.; Shimizu, K.; Ishihama, H.; et al. Modic changes in the cervical spine: Prospective 20-year follow-up study in asymptomatic subjects. J. Orthop. Sci. 2019, 24, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Maigne, J.-Y.; Genevay, S.; Marty, M.; Gandjbakhch, F.; Rozenberg, S.; Foltz, V. Inflammatory pain pattern and pain with lumbar extension associated with Modic 1 changes on MRI: A prospective case–control study of 120 patients. Eur. Spine J. 2013, 23, 493–497. [Google Scholar] [CrossRef]
- Luoma, K.; Vehmas, T.; Kerttula, L.; Grönblad, M.; Rinne, E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur. Spine J. 2016, 25, 2873–2881. [Google Scholar] [CrossRef]
- Kerttula, L.; Luoma, K.; Vehmas, T.; Grönblad, M.; Kääpä, E. Modic type I change may predict rapid progressive, deforming disc degeneration: A prospective 1-year follow-up study. Eur. Spine J. 2012, 21, 1135–1142. [Google Scholar] [CrossRef]
- Herlin, C.; Kjaer, P.; Espeland, A.; Skouen, J.S.; Leboeuf-Yde, C.; Karppinen, J.; Niinimäki, J.; Sørensen, J.S.; Storheim, K.; Jensen, T.S. Modic changes—Their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS ONE 2018, 13, e0200677. [Google Scholar] [CrossRef]
- Laustsen, A.F.; Bech-Azeddine, R. Do Modic changes have an impact on clinical outcome in lumbar spine surgery? A systematic literature review. Eur. Spine J. 2016, 25, 3735–3745. [Google Scholar] [CrossRef]
- Djuric, N.; Yang, X.; Ostelo, R.W.J.G.; van Duinen, S.G.; Nijeholt, G.J.L.; van der Kallen, B.F.W.; Peul, W.C.; Vleggeert-Lankamp, C.L.A. Disc inflammation and Modic changes show an interaction effect on recovery after surgery for lumbar disc herniation. Eur. Spine J. 2019, 28, 2579–2587. [Google Scholar] [CrossRef]
- Kumarasamy, D.; Rajasekaran, S.; Anand, K.S.S.V.; Soundararajan, D.C.R.; Shetty, T.A.P.; Kanna, P.R.M.; Pushpa, B.T. Lumbar Disc Herniation and Preoperative Modic Changes: A Prospective Analysis of the Clinical Outcomes After Microdiscectomy. Glob. Spine J. 2021, 12, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Karis, D.S.; Vleggeert-Lankamp, C.L. Association between Modic changes, disc degeneration, and neck pain in the cervical spine: A systematic review of literature. Spine J. 2019, 20, 754–764. [Google Scholar] [CrossRef] [PubMed]
- MacLean, M.A.; Kureshi, N.; Shankar, J.; Stewart, S.A.; Christie, S.D. Modic Change and Clinical Assessment Scores in Patients Undergoing Lumbar Surgery for Disk Herniation. Clin. Spine Surgery A Spine Publ. 2020, 34, E205–E210. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 June 2022).
- Shahmohammadi, M.R.; Behrouzian, S. Effect of preoperative modic change in the outcome of patients with low back pain following posterior spinal fusion or laminectomy. Asian J. Neurosurg. 2019, 14, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Fujita, N.; Hosogane, N.; Hikata, T.; Watanabe, K.; Tsuji, O.; Nagoshi, N.; Yagi, M.; Kaneko, S.; Fukui, Y.; et al. Presence of Modic type 1 change increases risk of postoperative pyogenic discitis following decompression surgery for lumbar canal stenosis. J. Orthop. Sci. 2017, 22, 988–993. [Google Scholar] [CrossRef]
- Ba, J.D.B.; Sayari, A.J.; Harada, G.K.; Tao, Y.; Louie, P.K.; Basques, B.A.; Galbusera, F.; Niemeyer, F.; Wilke, H.; An, H.S.; et al. The Modic-endplate-complex phenotype in cervical spine patients: Association with symptoms and outcomes. J. Orthop. Res. 2021, 40, 449–459. [Google Scholar] [CrossRef]
- Baker, J.D.; Sayari, A.J.; Tao, Y.; Louie, P.K.; Basques, B.A.; Galbusera, F.; Niemeyer, F.; Wilke, H.; An, H.S.; Samartzis, D. Endplate abnormalities, Modic changes and their relationship to alignment parameters and surgical outcomes in the cervical spine. J. Orthop. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Erinç, S.; Talmaç, M.A.; Kemah, B.; Özdemir, M.H. The effect of modic changes on the fusion rates of posterior interbody fusion surgery modic changes and posterior interbody fusion. J. Neurosurg. Sci. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Esposito, P.; Pinheiro-Franco, J.; Froelich, S.; Maitrot, D. Predictive value of MRI vertebral end-plate signal changes (MODIC) on outcome of surgically treated degenerative disc disease: Results of a cohort study including 60 patients. Neurochirurgie 2006, 52, 315–322. [Google Scholar] [CrossRef]
- Ghodsi, S.M.; Rouhani, R.; Abdollahzade, S.; Khadivi, M.; Jouibari, M.F. Frequency of Vertebral Endplate Modic Changes in Patients with Unstable Lumbar Spine and Its Effect on Surgical Outcome. Asian Spine J. 2015, 9, 737–740. [Google Scholar] [CrossRef]
- Yang, X.; Donk, R.; Arts, M.P.; Vleggeert-Lankamp, C.L. Are Modic Vertebral End-Plate Signal Changes Associated with Degeneration or Clinical Outcomes in the Cervical Spine? World Neurosurg. 2019, 129, e881–e889. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.D.; Harada, G.K.; Tao, Y.; Louie, P.K.; Basques, B.A.; Galbusera, F.; Niemeyer, F.; Wilke, H.-J.; An, H.S.; Samartzis, D. The Impact of Modic Changes on Preoperative Symptoms and Clinical Outcomes in Anterior Cervical Discectomy and Fusion Patients. Neurospine 2020, 17, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Hong, Y.; Liu, H.; Duan, Y.; Wang, B.; Chen, H.; Ding, C.; Rong, X.; Wu, T. Is the bone fusion affected by Modic-2 changes in single-level anterior cervical discectomy and fusion? Medicine 2020, 99, e18597. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lei, T.; Shen, Y. The Impact of Modic-2 changes on the clinical outcomes of single-level anterior cervical discectomy and fusion. Eur. Spine J. 2015, 24, 2936–2940. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Ding, W.; Zhang, Y.; Shen, Y. Comparison of Clinical Outcomes After Anterior Cervical Discectomy and Fusion Versus Cervical Total Disk Replacement in Patients with Modic-2 Changes on MRI. Clin. Spine Surgery A Spine Publ. 2017, 30, E1088–E1092. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Li, T.; Xue, Y. Preoperative Modic changes are related to axial symptoms after anterior cervical discectomy and fusion. J. Pain Res. 2018, 11, 2617–2623. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Tong, T.; Shen, Y. Influence of Modic Changes on Cage Subsidence and Intervertebral Fusion after Single-Level Anterior Cervical Corpectomy and Fusion. J. Investig. Surg. 2020, 35, 301–307. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wei, J.; Shen, Y. A study on the cervical spondylotic myelopathy treated by anterior cervical diskectomy and fusion in accordance with Modic changes with a 2-year minimum follow-up. J. Orthop. Surg. Res. 2015, 10, 11. [Google Scholar] [CrossRef]
- Jiao, J.; Li, J.; Luo, Y.; Zhang, W. Clinical and radiographic outcomes of hybrid graft in patients with Modic changes undergoing transforaminal lumbar interbody fusion. J. Orthop. Surg. Res. 2021, 16, 486. [Google Scholar] [CrossRef]
- El Barzouhi, A.; Vleggeert-Lankamp, C.L.; van der Kallen, B.F.; Nijeholt, G.J.L.; Hout, W.B.V.D.; Koes, B.W.; Peul, W.C. Back pain’s association with vertebral end-plate signal changes in sciatica. Spine J. 2014, 14, 225–233. [Google Scholar] [CrossRef]
- Ulrich, N.H.; Burgstaller, J.M.; Gravestock, I.; Winklhofer, S.; Porchet, F.; Pichierri, G.; Wertli, M.M.; Steurer, J.; Farshad, M.; The LSOS Study Group. The influence of endplate (Modic) changes on clinical outcomes in lumbar spinal stenosis surgery: A Swiss prospective multicenter cohort study. Eur. Spine J. 2020, 29, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.-S.; Lee, H.-D.; Jeon, C.-H. The Impact of Vertebral End Plate Lesions on the Radiological Outcome in Oblique Lateral Interbody Fusion. Glob. Spine J. 2021, 11, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Udby, P.M.; Ohrt-Nissen, S.; Bendix, T.; Paulsen, R.; Støttrup, C.; Andresen, A.; Brorson, S.; Carreon, L.Y.; Andersen, M. Are Modic Changes Associated with Health-related Quality of Life After Discectomy. Spine 2020, 45, 1491–1497. [Google Scholar] [CrossRef]
- Sørlie, A.; Moholdt, V.; Kvistad, K.A.; Nygaard, P.; Ingebrigtsen, T.; Iversen, T.; Kloster, R.; Solberg, T.K. Modic type I changes and recovery of back pain after lumbar microdiscectomy. Eur. Spine J. 2012, 21, 2252–2258. [Google Scholar] [CrossRef]
- Gornet, M.F.; Schranck, F.; Wharton, N.D.; Beall, D.P.; Jones, E.; Myers, M.E.; Hipp, J.A. Optimizing success with lumbar disc arthroplasty. Eur. Spine J. 2014, 23, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Koshi, T.; Suzuki, M.; Orita, S.; Eguchi, Y.; Ochiai, N.; Kishida, S.; et al. Change in Modic Type 1 and 2 Signals After Posterolateral Fusion Surgery. Spine 2010, 35, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Chen, Z.; Zheng, Y.; Wang, Y.; Jiang, L.; Yang, Y.; Zhuang, C.; Liang, Y.; Zheng, T.; Gong, Y.; et al. Comparison of simple discectomy and instrumented posterior lumbar interbody fusion for treatment of lumbar disc herniation combined with Modic endplate changes. Chin. Med. J. 2014, 127, 2789–2794. [Google Scholar] [PubMed]
- Lurie, J.D.; Moses, R.A.; Tosteson, A.N.A.; Tosteson, T.D.; Carragee, E.J.; Carrino, J.A.; Kaiser, J.A.; Herzog, R.J. Magnetic Resonance Imaging Predictors of Surgical Outcome in Patients with Lumbar Intervertebral Disc Herniation. Spine 2013, 38, 1216–1225. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Wang, B.; Lv, G.-H.; Wu, P.; Dai, Y.; Jiang, B.; Zheng, Z.; Xiao, S. Percutaneous Endoscopic Lumbar Discectomy for Lumbar Disc Herniation with Modic Changes via a Transforaminal Approach: A Retrospective Study. Pain Physician 2019, 22, E601–E608. [Google Scholar]
- Djurasovic, M.; Carreon, L.Y.; Crawford III, C.H.C.; Zook, J.D.; Bratcher, K.R.; Glassman, S.D. The influence of preoperative MRI findings on lumbar fusion clinical outcomes. Eur. Spine J. 2012, 21, 1616–1623. [Google Scholar] [CrossRef]
- Masala, S.; Anselmetti, G.C.; Marcia, S.; Nano, G.; Taglieri, A.; Calabria, E.; Chiocchi, M.; Simonetti, G. Treatment of painful Modic type I changes by vertebral augmentation with bioactive resorbable bone cement. Neuroradiology 2014, 56, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Koshi, T.; Suzuki, M.; Orita, S.; Eguchi, Y.; Ochiai, N.; Kishida, S.; et al. Low Back Pain After Lumbar Discectomy in Patients Showing Endplate Modic Type 1 Change. Spine 2010, 35, E596–E600. [Google Scholar] [CrossRef] [PubMed]
- Rahme, R.; Moussa, R.; Bou-Nassif, R.; Maarrawi, J.; Rizk, T.; Nohra, G.; Samaha, E.; Okais, N. What happens to Modic changes following lumbar discectomy? Analysis of a cohort of 41 patients with a 3- to 5-year follow-up period. J. Neurosurg. Spine 2010, 13, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Blondel, B.; Tropiano, P.; Gaudart, J.; Huang, R.C.; Marnay, T. Clinical Results of Lumbar Total Disc Arthroplasty in Accordance with Modic Signs, With a 2-Year-Minimum Follow-up. Spine 2011, 36, 2309–2315. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Stienen, M.N.; Joswig, H.; Smoll, N.; Schaller, K.; Corniola, M.V. The usefulness of radiological grading scales to predict pain intensity, functional impairment, and health-related quality of life after surgery for lumbar degenerative disc disease. Acta Neurochir. 2016, 159, 271–279. [Google Scholar] [CrossRef]
- Hellum, C.; Johnsen, L.G.; Gjertsen, Ø.; Berg, L.; Neckelmann, G.; Grundnes, O.; Rossvoll, I.; Skouen, J.S.; Brox, J.I.; The Norwegian Spine Study Group; et al. Predictors of outcome after surgery with disc prosthesis and rehabilitation in patients with chronic low back pain and degenerative disc: 2-year follow-up. Eur. Spine J. 2012, 21, 681–690. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Chin, D.-K.; Jin, B.-H.; Kim, K.-S.; Cho, Y.-E.; Kuh, S.-U. Long Term Efficacy of Posterior Lumbar Interbody Fusion with Standard Cages alone in Lumbar Disc Diseases Combined with Modic Changes. J. Korean Neurosurg. Soc. 2009, 46, 322–327. [Google Scholar] [CrossRef]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Muraki, S.; Yamada, H.; Minamide, A.; Oka, H.; Ishimoto, Y.; Nagata, K.; Kagotani, R.; et al. Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2013, 22, 104–110. [Google Scholar] [CrossRef]
- Boden, S.D.; Davis, D.O.; Dina, T.S.; Patronas, N.J.; Wiesel, S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J. Bone Joint Surg. Am. 1990, 72, 403–408. [Google Scholar] [CrossRef]
- Lurie, J.D.; Tosteson, T.D.; Tosteson, A.N.A.; Zhao, W.; Morgan, T.S.; Abdu, W.A.; Herkowitz, H.; Weinstein, J.N. Surgical Versus Nonoperative Treatment for Lumbar Disc Herniation. Spine 2014, 39, 3–16. [Google Scholar] [CrossRef]
- Saifi, C.; Fein, A.W.; Cazzulino, A.; Lehman, R.A.; Phillips, F.M.; An, H.S.; Riew, K.D. Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. Spine J. 2018, 18, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Peterson, C.K.; Hodler, J. Degenerative Marrow (Modic) Changes on Cervical Spine Magnetic Resonance Imaging Scans. Spine 2011, 36, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Toyone, T.; Takahashi, K.; Kitahara, H.; Yamagata, M.; Murakami, M.; Moriya, H. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J. Bone Jt. Surg. Br. Vol. 1994, 76, 757–764. [Google Scholar] [CrossRef]
- Ha, K.-Y.; Molon, J.N.; Ahn, J.-H.; Kim, Y.-H. Fate of Osteophytes and Sclerosis in Fused Segments After Lumbar Fusion. Spine 2014, 39, E1110–E1115. [Google Scholar] [CrossRef]


| Authors | Objective | Study Design | Procedure | Follow-Up (Months) | Clinical Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|
| Yang et al. [32] (2019) | To report on the incidence of MC in patients with cervical radiculopathy due to disc herniation | Retrospective | ACDF vs. ACDA vs. ACD | 12 | NDI, MCS-12, PCS-12, Neck VAS, Arm VAS | MCs were not associated with a change in NDI, SF-12, VAS Surgical approach did not influence MRI-evidence of MCs |
| Baker et al. [33] (2020) | To study the association of MC with postoperative outcomes in ACDF patients | Retrospective | ACDF | 27.3 | VAS Neck, Vas arm, SF12, VR12 | Overall, MCs were not associated with post-operative PROs |
| Huang et al. [34] (2020) | To explore the impact of MC on bone fusion after single-level ACDF | Retrospective | ACDF | 33.2 | JOA score, VAS neck, fusion rates | MCs were not associated with post-operative PROs, but MC-II were associated with delayed fusion. |
| Li et al. [35] (2015) | To explore the impact of MC-II on the clinical outcomes of single-level ACDF | Retrospective | ACDF | 60 | JOA, NDI, neck VAS | MCs were not associated with post-operative PROs or fusion rates |
| Li et al. [36] (2017) | To compare the clinical and radiologic outcomes of patients with MC-II who underwent single level ACDF or ACDA | Retrospective | ACDF vs. ACDA | 60 | JOA, NDI, ROM, VAS neck, VAS arm | All patients improved from baseline, but the ACDA group showed greater improvement in VAS neck and axial ROM compared with the ACDF group at final follow-up. |
| Zhou et al. [37] (2018) | To compare the clinical and radiological outcomes between patients with or without axial symptoms in ACDF | Retrospective | ACDF | 12 | Axial symptoms | Patients with post-operative axial symptoms were more likely have had preoperative MCs on endplates adjacent to treated disc |
| Li et al. [38] (2022) | To determine the impact of MCs on cage subsidence and fusion after ACCF | Retrospective | ACCF | 24 | Cage subsidence, fusion rate | More patients with MCs experienced cage subsidence. MCs were not associated with post-operative PROs or fusion rates. |
| Li et al. [39] (2015) | To analyze the influence of MCs on the clinical results of cervical spondylotic myelopathy treated by ACDF | Retrospective | ACDF | 24 | JOA, percent recovered at final follow-up visit | All patients experienced significant improvement in all measures. MC-I patients reported significantly lower VAS at 3, 6, 12, and 24 months postop. MC-I patients had a higher JOA at 1-year |
| Authors | Objective | Study Design | Procedure | Follow-Up (Months) | Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|
| Kumarasamy et al. [21] (2021) | To evaluate the relationship between MC and clinical outcomes after a lumbar microdiscectomy | Prospective | Microdiscectomy | 12 | NRS pain, ODI, patient satisfaction by Mac Nab criteria | Patients with MC had less improvement in back pain and ODI scores at all follow-ups. However, MCID between the groups was not significant |
| Jiao et al. [40] (2021) | To analyze the influence of MCs the clinical and radiographic outcomes of transforaminal lumbar interbody fusion | Retrospective | TLIF | 23.4 | ODI, VAS back pain, VAS leg pain, cage subsidence, | MCs had no impact on fusion rates and clinical outcomes |
| el Barzouhi et al. [41] (2014) | To analyze the correlation between MCs and back pain in sciatica in patients with early surgery vs. conservative treatment | Prospective | Microdiscectomy | 12 | VAS back, 7-point Likert self-rating scale of global perceived recovery | Surgically treated patients showed an increase in extent of MCs (67% of pts) compared to conservatively treated patients (19%) No difference in post-operative back pain scores |
| Ulrich et al. [42] (2020) | To investigate if the MCs are predictive for outcomes in degenerative lumbar spinal stenosis patients undergoing decompression-alone or decompression with instrumented fusion surgery | Retrospective | Decompression vs. PLIF | 36 | SSM symptoms, SSM function, MCID in SSM symptoms, NRS pain, and EQ-5D sum score over time | MCs were not associated with clinical outcomes, independent of the chosen surgical operation. |
| Chung et al. [43] (2021) | To evaluate the influence of MC on the radiological outcomes in lumbar interbody fusion | Retrospective | OLIF | 28.6 | Cage subsidence, fusion rate | MCs were not associated with cage subsidence or impaired fusion |
| MacLean et al. [23] (2021) | To examine the relationship between preoperative MCs and postoperative clinical assessment scores for patients receiving lumbar discectomy or TLIF for lumbar disk herniation | Retrospective | TLIF vs. discectomy | 12 | VAS leg, SF12 physical, ODI | All patients experienced improved from baseline, but those with MC experienced the greatest improvement in disability. Outcomes were similar in discectomy vs. TLIF |
| Udby et al. [44] (2020) | To assess whether MCs are associated with health-related quality of life, long-term physical disability, back- or leg pain after discectomy | Retrospective | Discectomy | 24 | ODI, VAS back, VAS leg, Patient satisfaction scores, EQ-5D | MCs were not associated with differences in improvement in PROs, except for VAS back wherein patients with MC-I had worse scores than those with MC-II |
| Sørlie et al. [45] (2012) | To investigate whether the presence of preoperative MC-I represents a risk factor for persistent back pain 12 months after surgery amongst patients operated for lumbar disc herniation | Retrospective | Microdiscectomy | 12 | VAS back, VAS leg, ODI, EQ-5D, self-reported benefit of the operation and employment status | All patients improved in all outcomes at 1-year. In aggregate, MC were not associated with PROs. Patients with MC-I had less improvement of VAS Back and EQ-5D |
| Gornet et al. [46] (2014) | To determine which variables predict clinical outcomes following disc replacement | Prospective | Disc replacement | 60 | ODI, SF-36 | Patients with MC-II had better ODI scores at 5-year follow-up than those with no-MC or MC 1 |
| Ohtori et al. [47] (2010) | To investigate the changes in MCs after posterolateral fusion | Prospective | Posterolateral fusion | 24 | JOA, VAS back, ODI, fusion rate | MCs were not associated with post-operative PROs or fusion rates |
| Cao at al [48] (2014) | To compare the outcomes of simple discectomy and instrumented PLIF in patients with lumbar disc herniation and MCs | Retrospective | Instrumented PLIF vs. discectomy | 18 | JOA, VAS back, VAS leg | iPLIF resulted in superior outcomes for relief of LBP compared to simple discectomy. Both treatments similarly relieved radicular leg pain |
| Lurie et al. [49] (2013) | To determine whether baseline MRI and MCs are associated with differential outcomes with surgery or non-operative treatment | Retrospective | Open discectomy and decompression | 48 | ODI, bodily pain, sciatica and back pain symptoms, physical function | MC-I patients had poorer outcomes on all measures after surgery compared to MC-II or no MC |
| Xu et al. [50] (2019) | To assess the clinical outcomes of TF-PELD in the treatment of LDH and MCs | Retrospective | TF-PELD | 29.6 | ODI, VAS back, VAS leg, Patient satisfaction scores (Modified MacNab) | Patients with MC-I had poorer improvement in VAS back and ODI at 1 year and final follow-up compared to MC-II or no MC. Improvements in leg pain were comparable among groups |
| Djurasovic et al. [51] (2012) | To investigate relationship between MRI findings in patients with DDD and clinical improvement after lumbar fusion | Retrospective | PLF, TLIF, ALIF, circumferential fusion | 24 | NRS back and leg, ODI, SF-36 | MCs were not associated with post-operative PROs |
| Masala et al. [52] (2014) | To evaluate the effectiveness of vertebral augmentation with calcium sulfate and hydroxyapatite resorbable cement in patients with LBP due to MC-I | Prospective | Vertebroplasty with calcium sulfate and hydroxyapatite resorbable bone cement | 12 | VAS back, ODI | All patients experienced improvement in pain and disability |
| Ohtori et al. [53] (2010) | To examine the relationship between LBP after discectomy for disc herniation and MC 1 | Prospective | Discectomy | 24 | VAS back, ODI, JOA | All scores improved from baseline. MC were not associated with post-operative PROs or fusion rates |
| Rahme et al. [54] (2010) | To study the impact of surgery on the natural history of MC | Retrospective | Discectomy | 60 | ODI, patient satisfaction, presence of symptoms, work status | MC were not associated with post-operative clinical outcomes |
| Blondel et al. [55] (2011) | To analyze the influence of MC on the clinical results of lumbar total disc arthroplasty | Prospective | Disc replacement | 30 | VAS back, VAS leg, ODI | All groups improved in all outcomes at final follow-up. Patients with MC1 had the greatest improvement in ODI and radicular pain by final follow-up |
| Gautschi et al. [56] (2016) | To determine the relationship of radiological grading scales of lumbar DDD with postoperative pain intensity, functional impairment, and health-related quality of life | Prospective | Microdiscecomty, decompression, TLIF, PLIF, or XLIF | 24 | ODI, RMDI, SF-12, PCS-12, and EQ-5D index | No significant difference in improvement in clinical outcome between patients with or without MC |
| Hellum et al. [57] (2012) | To evaluate predictors of outcome in patients treated with disc prosthesis or multidisciplinary rehabilitation | Prospective | Disc replacement | 24 | ODI | Patients with MC-I or MC-II has significantly better ODI outcomes after disc replacement |
| Kwon et al. [58] (2009) | To investigate the efficacy of PLIF with cages in chronic DDD with MCs | Retrospective | PLIF w/cage | 59.8 | Fusion rate, Prolo’s scale for symptomatic improvement, VAS Back | Patients with MC-III had lower fusion rate and PROs in symptoms and pain compared to those with other subtypes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrechts, M.J.; Brush, P.; Issa, T.Z.; Toci, G.R.; Heard, J.C.; Syal, A.; Schilken, M.M.; Canseco, J.A.; Kepler, C.K.; Vaccaro, A.R. Evaluating the Impact of Modic Changes on Operative Treatment in the Cervical and Lumbar Spine: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 10158. https://doi.org/10.3390/ijerph191610158
Lambrechts MJ, Brush P, Issa TZ, Toci GR, Heard JC, Syal A, Schilken MM, Canseco JA, Kepler CK, Vaccaro AR. Evaluating the Impact of Modic Changes on Operative Treatment in the Cervical and Lumbar Spine: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(16):10158. https://doi.org/10.3390/ijerph191610158
Chicago/Turabian StyleLambrechts, Mark J., Parker Brush, Tariq Z. Issa, Gregory R. Toci, Jeremy C. Heard, Amit Syal, Meghan M. Schilken, Jose A. Canseco, Christopher K. Kepler, and Alexander R. Vaccaro. 2022. "Evaluating the Impact of Modic Changes on Operative Treatment in the Cervical and Lumbar Spine: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 16: 10158. https://doi.org/10.3390/ijerph191610158
APA StyleLambrechts, M. J., Brush, P., Issa, T. Z., Toci, G. R., Heard, J. C., Syal, A., Schilken, M. M., Canseco, J. A., Kepler, C. K., & Vaccaro, A. R. (2022). Evaluating the Impact of Modic Changes on Operative Treatment in the Cervical and Lumbar Spine: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(16), 10158. https://doi.org/10.3390/ijerph191610158