Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia
Abstract
1. Introduction
2. Results
2.1. MAM Rats Exhibit Sex-Specific Differences in Baseline Neuronal Activity
2.2. Drug-Induced Effects on Neuronal Activity in Male Control Rat Cortical Neurons
2.3. Drug-Induced Effects on Neuronal Activity in Female Control Rat Cortical Neurons
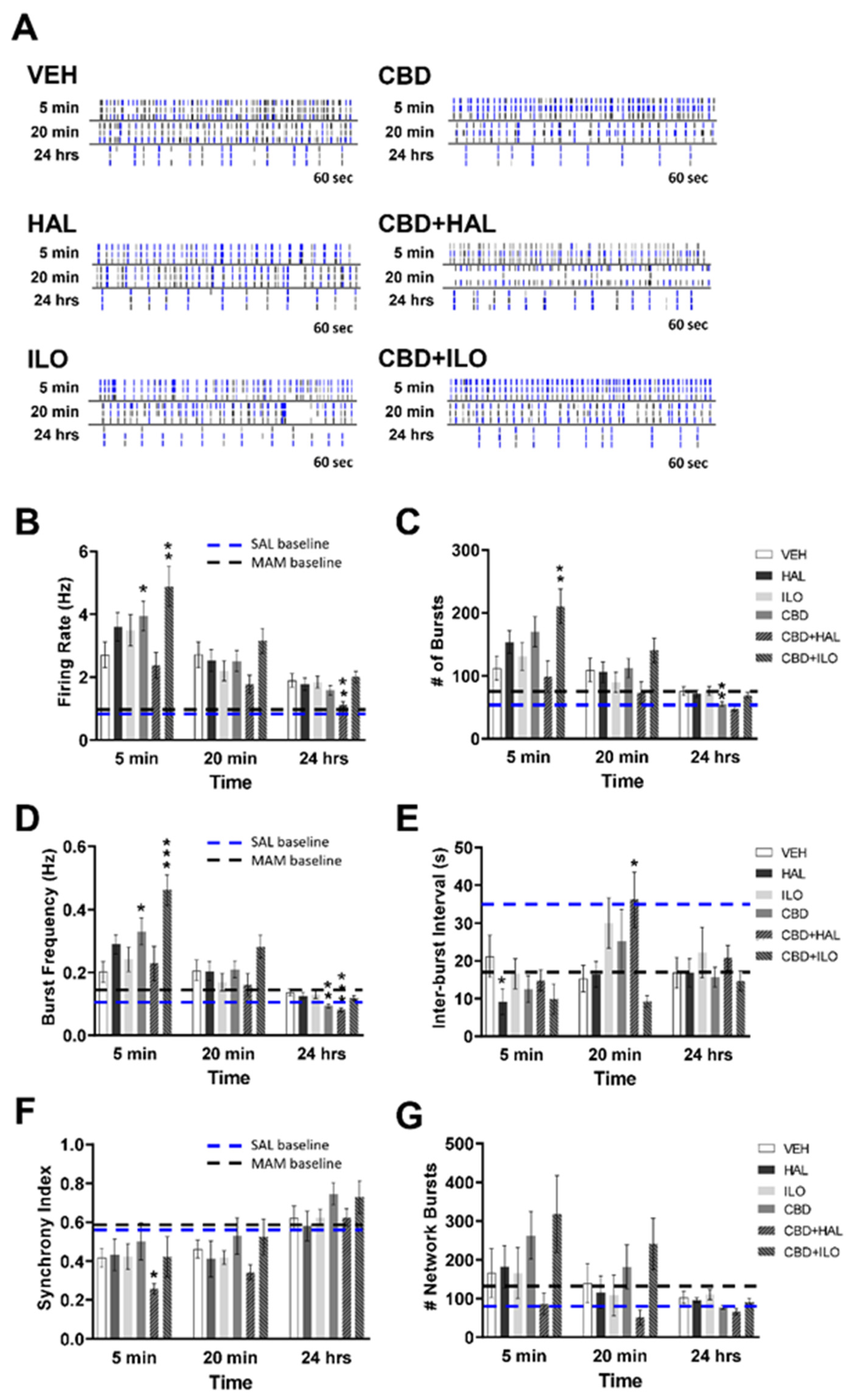
2.4. Drug-Induced Effects on Neuronal Activity in Male MAM Rat Cortical Neurons
2.5. Drug-Induced Effects on Neuronal Activity in Female MAM Rat Cortical Neurons
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. Drugs and Treatment of Cell Cultures
4.4. MEA Recordings
4.5. Immunocytochemistry
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| HAL | Haloperidol |
| ILO | Iloperidone |
| MAM | Methylazoxymethanol Acetate |
| SAL | Saline |
| SZ | Schizophrenia |
| VEH | Vehicle |
References
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Schizophrenia and other psychotic disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Nielsen, R.E.; Levander, S.; Kjaersdam Telleus, G.; Jensen, S.O.; Ostergaard Christensen, T.; Leucht, S. Second-generation antipsychotic effect on cognition in patients with schizophrenia—A meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015, 131, 185–196. [Google Scholar] [CrossRef]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.H.; Fiedosewicz, H.; Patel, C.; Klegon, D.A.; Bayog, R.; Berman, I. Treatment response and gender in patients with schizophrenia and schizoaffective disorder. Schizophr. Res. 2001, 49, 232. [Google Scholar]
- Uhlhaas, P.J.; Pipa, G.; Lima, B.; Melloni, L.; Neuenschwander, S.; Nikolic, D.; Singer, W. Neural synchrony in cortical networks: History, concept and current status. Front. Integr. Neurosci. 2009, 3, 17. [Google Scholar] [CrossRef]
- Vittala, A.; Murphy, N.; Maheshwari, A.; Krishnan, V. Understanding cortical dysfunction in schizophrenia with TMS/EEG. Front. Neurosci. 2020, 14, 554. [Google Scholar] [CrossRef]
- Oertel, V.; Knochel, C.; Rotarska-Jagiela, A.; Schonmeyer, R.; Lindner, M.; van de Ven, V.; Haenschel, C.; Uhlhaas, P.; Maurer, K.; Linden, D.E. Reduced laterality as a trait marker of schizophrenia—Evidence from structural and functional neuroimaging. J. Neurosci. 2010, 30, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.V.; Hong, L.E. High vs low frequency neural oscillations in schizophrenia. Schizophr. Bull. 2011, 37, 659–663. [Google Scholar] [CrossRef]
- Lodge, D.J.; Behrens, M.M.; Grace, A.A. A Loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009, 29, 2344–2354. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Lewis, D.A. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008, 34, 944–961. [Google Scholar] [CrossRef]
- Schmiedt, C.; Brand, A.; Hildebrandt, H.; Basar-Eroglu, C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Cogn. Brain Res. 2005, 25, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, J.; Winterer, G.; Herrmann, C.S.; Senkowski, D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin. Neurophysiol. 2004, 115, 1863–1874. [Google Scholar] [CrossRef]
- Basar-Eroglu, C.; Brand, A.; Hildebrandt, H.; Karolina Kedzior, K.; Mathes, B.; Schmiedt, C. Working memory related gamma oscillations in schizophrenia patients. Int. J. Psychophysiol. 2007, 64, 39–45. [Google Scholar] [CrossRef]
- González-Hernández, J.A.; Cedeño, I.; Pita-Alcorta, C.; Galán, L.; Aubert, E.; Figueredo-RodríGuez, P. Induced oscillations and the distributed cortical sources during the Wisconsin card sorting test performance in schizophrenic patients: New clues to neural connectivity. Int. J. Psychophysiol. 2003, 48, 11–24. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Firl, A.J.; Yoon, J.H.; Gomes, G.C.; Reinking, C.; Carter, C.S. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010, 35, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Ehrlichman, R.S.; Gandal, M.J.; Maxwell, C.R.; Lazarewicz, M.T.; Finkel, L.H.; Contreras, D.; Turetsky, B.I.; Siegel, S.J. N-methyl-d-aspartic acid receptor antagonist–induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience 2009, 158, 705–712. [Google Scholar] [CrossRef]
- Alegre, M.; Molero, P.; Valencia, M.; Mayner, G.; Ortuño, F.; Artieda, J. Atypical antipsychotics normalize low-gamma evoked oscillations in patients with schizophrenia. Psychiatry Res. 2017, 247, 214–221. [Google Scholar] [CrossRef]
- Ahnaou, A.; Huysmans, H.; Van De Casteele, T.; Drinkenburg, W.H.I.M. Cortical high gamma network oscillations and connectivity: A translational index for antipsychotics to normalize aberrant neurophysiological activity. Transl. Psychiatry 2017, 7. [Google Scholar] [CrossRef]
- Anderson, P.M.; Pinault, D.; O'Brien, T.J.; Jones, N.C. Chronic administration of antipsychotics attenuates ongoing and ketamine-induced increases in cortical γ oscillations. Int. J. Neuropsychopharmacol. 2014, 17, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.C.; Reddy, M.; Anderson, P.; Salzberg, M.R.; O'Brien, T.J.; Pinault, D. Acute administration of typical and atypical antipsychotics reduces EEG gamma power, but only the preclinical compound LY379268 reduces the ketamine-induced rise in gamma power. Int. J. Neuropsychopharmacol. 2012, 15, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Foute Nelong, T.; Manduca, J.D.; Zonneveld, P.M.; Perreault, M.L. Asenapine maleate normalizes low frequency oscillatory deficits in a neurodevelopmental model of schizophrenia. Neurosci. Lett. 2019, 711, 134404. [Google Scholar] [CrossRef]
- Olszewski, M.; Piasecka, J.; Goda, S.A.; Kasicki, S.; Hunt, M.J. Antipsychotic compounds differentially modulate high-frequency oscillations in the rat nucleus accumbens: A comparison of first- and second-generation drugs. Int. J. Neuropsychopharmacol. 2013, 16, 1009–1020. [Google Scholar] [CrossRef]
- Haddad, P.; Brain, C.; Scott, J. Nonadherence with antipsychotic medication in schizophrenia: Challenges and management strategies. Patient Relat. Outcome Meas. 2014, 5, 43–62. [Google Scholar] [CrossRef]
- Takeuchi, H.; Suzuki, T.; Remington, G.; Bies, R.R.; Abe, T.; Graff-Guerrero, A.; Watanabe, K.; Mimura, M.; Uchida, H. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: An open-label, randomized, controlled, pilot study. Schizophr. Bull. 2013, 39, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.M.; Sharma, S.G. Adverse effects of atypical antipsychotics. CNS Drugs 2007, 21, 911–936. [Google Scholar] [CrossRef]
- Acosta, F.J.; Bosch, E.; Sarmiento, G.; Juanes, N.; Caballero-Hidalgo, A.; Mayans, T. Evaluation of noncompliance in schizophrenia patients using electronic monitoring (MEMS®) and its relationship to sociodemographic, clinical and psychopathological variables. Schizophr. Res. 2009, 107, 213–217. [Google Scholar] [CrossRef]
- Löffler, W.; Kilian, R.; Toumi, M.; Angermeyer, M.C. Schizophrenic patients' subjective reasons for compliance and noncompliance with neuroleptic treatment. Pharmacopsychiatry 2003, 36, 105–112. [Google Scholar] [CrossRef]
- Liu-Seifert, H.; Osuntokun, O.O.; Feldman, P.D. Factors associated with adherence to treatment with olanzapine and other atypical antipsychotic medications in patients with schizophrenia. Compr. Psychiatry 2012, 53, 107–115. [Google Scholar] [CrossRef]
- Bumb, J.M.; Enning, F.; Leweke, F.M. Drug repurposing and emerging adjunctive treatments for schizophrenia. Expert Opin. Pharmacother. 2015, 16, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Bhattacharyya, S. Cannabidiol as a potential treatment for psychosis. Ther. Adv. Psychopharmacol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, A.; Malone, D.T. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr. Res. 2016, 176, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Mcguire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Makiol, C.; Kluge, M. Remission of severe, treatment-resistant schizophrenia following adjunctive cannabidiol. Aust. New Zealand J. Psychiatry 2019, 53, 262. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Schnakenberg Martin, A.M.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 2018, 235, 1923–1932. [Google Scholar] [CrossRef]
- Kathuria, A.; Lopez-Lengowski, K.; Watmuff, B.; Mcphie, D.; Cohen, B.M.; Karmacharya, R. Synaptic deficits in iPSC-derived cortical interneurons in schizophrenia are mediated by NLGN2 and rescued by N-acetylcysteine. Transl. Psychiatry 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Schrode, N.; Ho, S.-M.; Yamamuro, K.; Dobbyn, A.; Huckins, L.; Matos, M.R.; Cheng, E.; Deans, P.J.M.; Flaherty, E.; Barretto, N.; et al. Synergistic effects of common schizophrenia risk variants. Nat. Genet. 2019, 51, 1475–1485. [Google Scholar] [CrossRef]
- Wang, X.; Ye, F.; Wen, Z.; Guo, Z.; Yu, C.; Huang, W.-K.; Rojas Ringeling, F.; Su, Y.; Zheng, W.; Zhou, G.; et al. Structural interaction between DISC1 and ATF4 underlying transcriptional and synaptic dysregulation in an iPSC model of mental disorders. Mol. Psychiatry 2019, 26, 1346–1360. [Google Scholar] [CrossRef]
- Sarkar, A.; Mei, A.; Paquola, A.C.M.; Stern, S.; Bardy, C.; Klug, J.R.; Kim, S.; Neshat, N.; Kim, H.J.; Ku, M.; et al. Efficient generation of CA3 neurons from human pluripotent stem cells enables modeling of hippocampal connectivity in vitro. Cell Stem Cell 2018, 22, 684–697.e689. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, E.J.; Charlesworth, P.; Coba, M.P.; Grant, S.G.N. Knockdown of mental disorder susceptibility genes disrupts neuronal network physiology in vitro. Mol. Cell. Neurosci. 2011, 47, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, E.; Deranieh, R.M.; Artimovich, E.; Lee, I.S.; Siegel, A.J.; Levy, D.L.; Nestor, M.W.; Brennand, K.J. Patient-derived hiPSC neurons with heterozygous CNTNAP2 deletions display altered neuronal gene expression and network activity. NPJ Schizophr. 2017, 3. [Google Scholar] [CrossRef]
- Ramsey, J.M.; Schwarz, E.; Guest, P.C.; Van Beveren, N.J.M.; Leweke, F.M.; Rothermundt, M.; Bogerts, B.; Steiner, J.; Bahn, S. Distinct molecular phenotypes in male and female schizophrenia patients. PLoS ONE 2013, 8, e78729. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Cherkerzian, S.; Tsuang, M.T.; Petryshen, T.L. Sex differences in the genetic risk for schizophrenia: History of the evidence for sex-specific and sex-dependent effects. Am. J. Med. Genet. Part B Neuropsych. Genet. 2013, 162, 698–710. [Google Scholar] [CrossRef]
- Aleman, A.; Kahn, R.S.; Selten, J.-P. Sex differences in the risk of schizophrenia. Arch. Gen. Psychiatry 2003, 60, 565. [Google Scholar] [CrossRef]
- Usall, J.; Suarez, D.; Haro, J.M. Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Res. 2007, 153, 225–231. [Google Scholar] [CrossRef]
- Seeman, M.V. Men and women respond differently to antipsychotic drugs. Neuropharmacology 2020, 163, 107631. [Google Scholar] [CrossRef]
- Hui, C.W.; St-Pierre, A.; El Hajj, H.; Remy, Y.; Hébert, S.S.; Luheshi, G.N.; Srivastava, L.K.; Tremblay, M.-È. Prenatal immune challenge in mice leads to partly sex-dependent behavioral, microglial, and molecular abnormalities associated with schizophrenia. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef]
- Chalkiadaki, K.; Velli, A.; Kyriazidis, E.; Stavroulaki, V.; Vouvoutsis, V.; Chatzaki, E.; Aivaliotis, M.; Sidiropoulou, K. Development of the MAM model of schizophrenia in mice: Sex similarities and differences of hippocampal and prefrontal cortical function. Neuropharmacology 2019, 144, 193–207. [Google Scholar] [CrossRef]
- Bristow, G.C.; Bostrom, J.A.; Haroutunian, V.; Sodhi, M.S. Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizophr. Res. 2015, 167, 57–63. [Google Scholar] [CrossRef]
- Kaar, S.J.; Angelescu, I.; Marques, T.R.; Howes, O.D. Pre-frontal parvalbumin interneurons in schizophrenia: A meta-analysis of post-mortem studies. J. Neural. Transm. 2019, 126, 1637–1651. [Google Scholar] [CrossRef]
- Perez, S.M.; Donegan, J.J.; Lodge, D.J. Effect of estrous cycle on schizophrenia-like behaviors in MAM exposed rats. Behav. Brain Res. 2019, 362, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.D.; Craft, R.M. Sex-dependent effects of cannabis and cannabinoids: A translational perspective. Neuropsychopharmacology 2018, 43, 34–51. [Google Scholar] [CrossRef]
- Anderson, D.E.; Madhavan, D.; Swaminathan, A. Global brain network dynamics predict therapeutic responsiveness to cannabidiol treatment for refractory epilepsy. Brain Commun. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Falkenberg, I.; Martin-Santos, R.; Atakan, Z.; Crippa, J.A.; Giampietro, V.; Brammer, M.; Mcguire, P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology 2015, 40, 1343–1352. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R.L. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef]
- Hallak, J.E.C.; Machado-De-Sousa, J.P.; Crippa, J.A.S.; Sanches, R.F.; Trzesniak, C.; Chaves, C.; Bernardo, S.A.; Regalo, S.C.; Zuardi, A.W. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev. Bras. Psiquiatr. 2010, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, A.; Taylor, D.A.; Malone, D.T. Cannabidiol and clozapine reverse MK-801-induced deficits in social interaction and hyperactivity in Sprague–Dawley rats. J. Psychopharmacol. 2012, 26, 1317–1332. [Google Scholar] [CrossRef]
- Gomes, F.V.; Llorente, R.; Del Bel, E.A.; Viveros, M.-P.; López-Gallardo, M.; Guimarães, F.S. Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr. Res. 2015, 164, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.L.; Solowij, N.; Babic, I.; Huang, X.-F.; Weston-Green, K. Improved social interaction, recognition and working memory with cannabidiol treatment in a prenatal infection (poly I:C) rat model. Neuropsychopharmacology 2017, 42, 1447–1457. [Google Scholar] [CrossRef]
- Long, L.E.; Chesworth, R.; Huang, X.-F.; Wong, A.; Spiro, A.; Mcgregor, I.S.; Arnold, J.C.; Karl, T. Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuregulin 1 mutant mice. PLoS ONE 2012, 7, e34129. [Google Scholar] [CrossRef]
- Görtz, P.; Henning, U.; Theiss, S.; Lange-Asschenfeldt, C. Effect fingerprints of antipsychotic drugs on neural networks in vitro. J. Neural Transm. 2019, 126, 1363–1371. [Google Scholar] [CrossRef]
- Dzyubenko, E.; Juckel, G.; Faissner, A. The antipsychotic drugs olanzapine and haloperidol modify network connectivity and spontaneous activity of neural networks in vitro. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Gottschling, C.; Geissler, M.; Springer, G.; Wolf, R.; Juckel, G.; Faissner, A. First and second generation antipsychotics differentially affect structural and functional properties of rat hippocampal neuron synapses. Neuroscience 2016, 337, 117–130. [Google Scholar] [CrossRef]
- Nordström, A.-L.; Farde, L.; Halldin, C. Time course of D2-dopamine receptor occupancy examined by PET after single oral doses of haloperidol. Psychopharmacology 1992, 106, 433–438. [Google Scholar] [CrossRef]
- Tauscher, J.; Jones, C.; Remington, G.; Zipursky, R.B.; Kapur, S. Significant dissociation of brain and plasma kinetics with antipsychotics. Mol. Psychiatry 2002, 7, 317–321. [Google Scholar] [CrossRef][Green Version]
- Kapur, S.; Arenovich, T.; Agid, O.; Zipursky, R.; Lindborg, S.; Jones, B. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am. J. Psychiatry 2005, 162, 939–946. [Google Scholar] [CrossRef]
- Huang, X.-F.; Song, X. Effects of antipsychotic drugs on neurites relevant to schizophrenia treatment. Med. Res. Rev. 2019, 39, 386–403. [Google Scholar] [CrossRef]
- Critchlow, H.M.; Maycox, P.R.; Skepper, J.N.; Krylova, O. Clozapine and haloperidol differentially regulate dendritic spine formation and synaptogenesis in rat hippocampal neurons. Mol. Cell. Neurosci. 2006, 32, 356–365. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, C.H.; Cho, H.Y.; Seo, M.K.; Lee, J.G.; Lee, B.J.; Seol, W.; Kee, B.S.; Kim, Y.H. Effects of antipsychotic drugs on the expression of synaptic proteins and dendritic outgrowth in hippocampal neuronal cultures. Synapse 2013, 67, 224–234. [Google Scholar] [CrossRef]
- Bowling, H.; Zhang, G.; Bhattacharya, A.; Perez-Cuesta, L.M.; Deinhardt, K.; Hoeffer, C.A.; Neubert, T.A.; Gan, W.-B.; Klann, E.; Chao, M.V. Antipsychotics activate mTORC1-dependent translation to enhance neuronal morphological complexity. Sci. Signal. 2014, 7, ra4. [Google Scholar] [CrossRef]
- Krichmar, J.L.; Nasuto, S.J.; Scorcioni, R.; Washington, S.D.; Ascoli, G.A. Effects of dendritic morphology on CA3 pyramidal cell electrophysiology: A simulation study. Brain Res. 2002, 941, 11–28. [Google Scholar] [CrossRef]
- Zhu, G.; Du, L.; Jin, L.; Offenhäusser, A. Effects of morphology constraint on electrophysiological properties of cortical neurons. Sci. Rep. 2016, 6, 23086. [Google Scholar] [CrossRef] [PubMed]
- Van Elburg, R.A.J.; Van Ooyen, A. Impact of dendritic size and dendritic topology on burst firing in pyramidal cells. PLoS Comput. Biol. 2010, 6, e1000781. [Google Scholar] [CrossRef]
- Elsworth, J.D.; Morrow, B.A.; Hajszan, T.; Leranth, C.; Roth, R.H. Phencyclidine-induced loss of asymmetric spine synapses in rodent prefrontal cortex is reversed by acute and chronic treatment with olanzapine. Neuropsychopharmacology 2011, 36, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- De Bartolomeis, A.; Buonaguro, E.F.; Latte, G.; Rossi, R.; Marmo, F.; Iasevoli, F.; Tomasetti, C. Immediate-early genes modulation by antipsychotics: Translational implications for a putative gateway to drug-induced long-term brain changes. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, G.M.; Cousins, L.; Deakin, J.; Lennox, B.R.; Yolken, R.; Jones, P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry 2015, 2, 258–270. [Google Scholar] [CrossRef]
- Na, K.-S.; Jung, H.-Y.; Kim, Y.-K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-Analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Scarante, F.F.; Joca, S.R.L.; Sales, A.J.; Gomes, F.V.; Sonego, A.B.; Rodrigues, N.S.; Galve-Roperh, I.; Guimarães, F.S. Plastic and neuroprotective mechanisms Involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Kato, T.; Monji, A.; Hashioka, S.; Kanba, S. Risperidone significantly inhibits interferon-γ-induced microglial activation in vitro. Schizophr. Res. 2007, 92, 108–115. [Google Scholar] [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Obuchowicz, E.; Bielecka-Wajdman, A.M.; Paul-Samojedny, M.; Nowacka, M. Different influence of antipsychotics on the balance between pro- and anti-inflammatory cytokines depends on glia activation: An in vitro study. Cytokine 2017, 94, 37–44. [Google Scholar] [CrossRef]
- Sugino, H.; Futamura, T.; Mitsumoto, Y.; Maeda, K.; Marunaka, Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2009, 33, 303–307. [Google Scholar] [CrossRef]
- Monji, A.; Kato, T.A.; Mizoguchi, Y.; Horikawa, H.; Seki, Y.; Kasai, M.; Yamauchi, Y.; Yamada, S.; Kanba, S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 42, 115–121. [Google Scholar] [CrossRef]
- Prieto, G.A.; Cotman, C.W. Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Growth Factor Rev. 2017, 34, 27–33. [Google Scholar] [CrossRef]
- Prieto, G.A.; Tong, L.; Smith, E.D.; Cotman, C.W. TNFα and IL-1β but not IL-18 suppresses hippocampal long-term potentiation directly at the synapse. Neurochem. Res. 2019, 44, 49–60. [Google Scholar] [CrossRef]
- Clarkson, B.D.S.; Kahoud, R.J.; Mccarthy, C.B.; Howe, C.L. Inflammatory cytokine-induced changes in neural network activity measured by waveform analysis of high-content calcium imaging in murine cortical neurons. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Araque, A.; Navarrete, M. Glial cells in neuronal network function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2375–2381. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef]
- Béchade, C.; Cantaut-Belarif, Y.; Bessis, A. Microglial control of neuronal activity. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- York, E.M.; Bernier, L.-P.; MacVicar, B.A. Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol. 2018, 78, 593–603. [Google Scholar] [CrossRef]
- Stellwagen, D.; Malenka, R.C. Synaptic scaling mediated by glial TNF-α. Nature 2006, 440, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Metna-Laurent, M.; Marsicano, G. Rising stars: Modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia 2015, 63, 353–364. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 2008, 57, 883–893. [Google Scholar] [CrossRef]
- D'Addario, C.; Micale, V.; Di Bartolomeo, M.; Stark, T.; Pucci, M.; Sulcova, A.; Palazzo, M.; Babinska, Z.; Cremaschi, L.; Drago, F.; et al. A preliminary study of endocannabinoid system regulation in psychosis: Distinct alterations of CNR1 promoter DNA methylation in patients with schizophrenia. Schizophr. Res. 2017, 188, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Flaum, M.; Swayze, V.W., 2nd; O’Leary, D.S.; Yuh, W.T.; Ehrhardt, J.C.; Arndt, S.V.; Andreasen, N.C. Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am. J. Psychiatry 1995, 152, 704–714. [Google Scholar] [CrossRef]
- Agid, O.; Seeman, P.; Kapur, S. The “delayed onset” of antipsychotic action—An idea whose time has come and gone. J. Psychiatry Neurosci. 2006, 31, 93–100. [Google Scholar] [PubMed]
- Drysdale, A.J.; Ryan, D.; Pertwee, R.G.; Platt, B. Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology 2006, 50, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.L.; Hasbi, A.; Alijaniaram, M.; O’Dowd, B.F.; George, S.R. Reduced striatal dopamine D1–D2 receptor heteromer expression and behavioural subsensitivity in juvenile rats. Neuroscience 2012, 225, 130–139. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thériault, R.-K.; St-Denis, M.; Hewitt, T.; Khokhar, J.Y.; Lalonde, J.; Perreault, M.L. Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia. Int. J. Mol. Sci. 2021, 22, 5511. https://doi.org/10.3390/ijms22115511
Thériault R-K, St-Denis M, Hewitt T, Khokhar JY, Lalonde J, Perreault ML. Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia. International Journal of Molecular Sciences. 2021; 22(11):5511. https://doi.org/10.3390/ijms22115511
Chicago/Turabian StyleThériault, Rachel-Karson, Myles St-Denis, Tristen Hewitt, Jibran Y. Khokhar, Jasmin Lalonde, and Melissa L. Perreault. 2021. "Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia" International Journal of Molecular Sciences 22, no. 11: 5511. https://doi.org/10.3390/ijms22115511
APA StyleThériault, R.-K., St-Denis, M., Hewitt, T., Khokhar, J. Y., Lalonde, J., & Perreault, M. L. (2021). Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia. International Journal of Molecular Sciences, 22(11), 5511. https://doi.org/10.3390/ijms22115511





