In Vitro and In Vivo Pharmacological Characterization of a Novel TRPM8 Inhibitor Chemotype Identified by Small-Scale Preclinical Screening
Abstract
:1. Introduction
2. Results
2.1. Virtual Screening
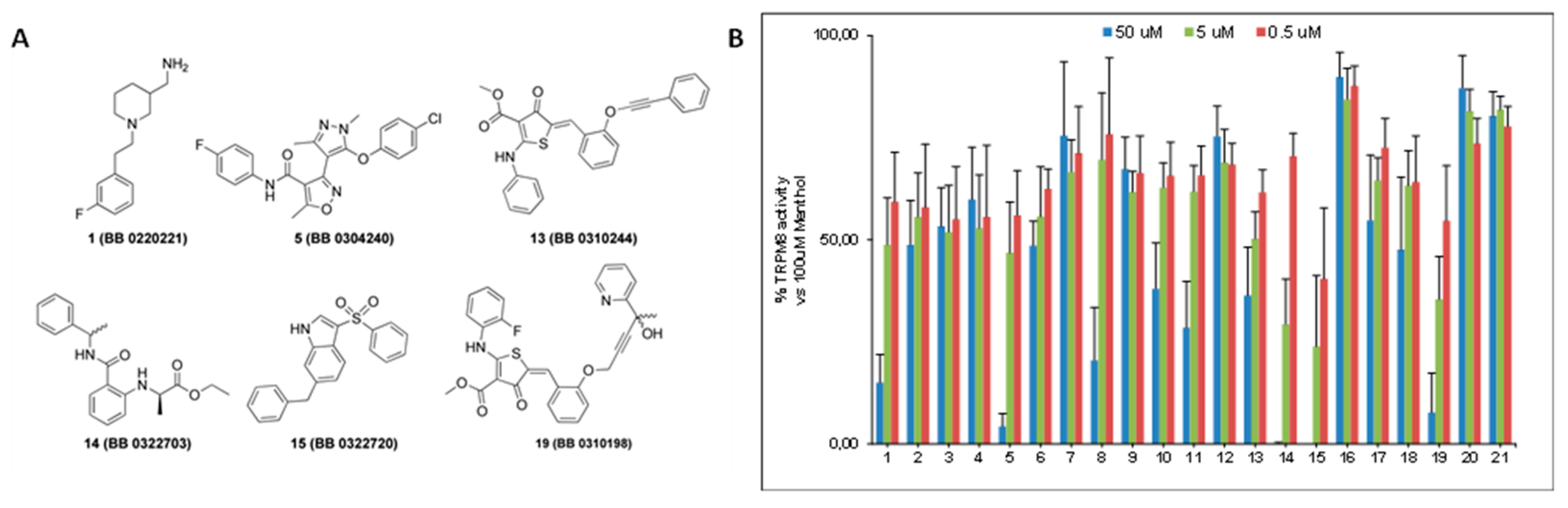
2.2. Screening by Ca2+-Microfluorometric Assay
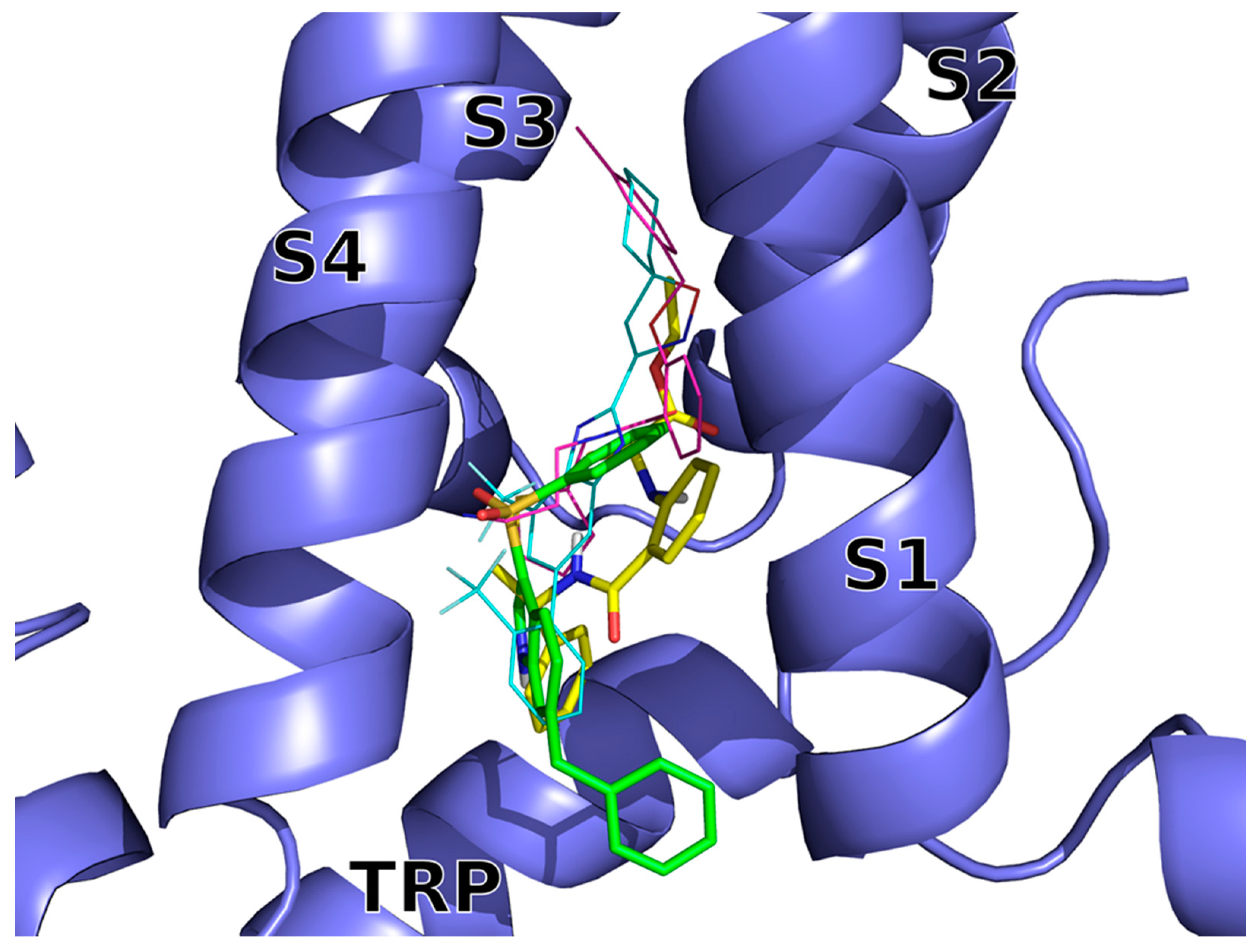
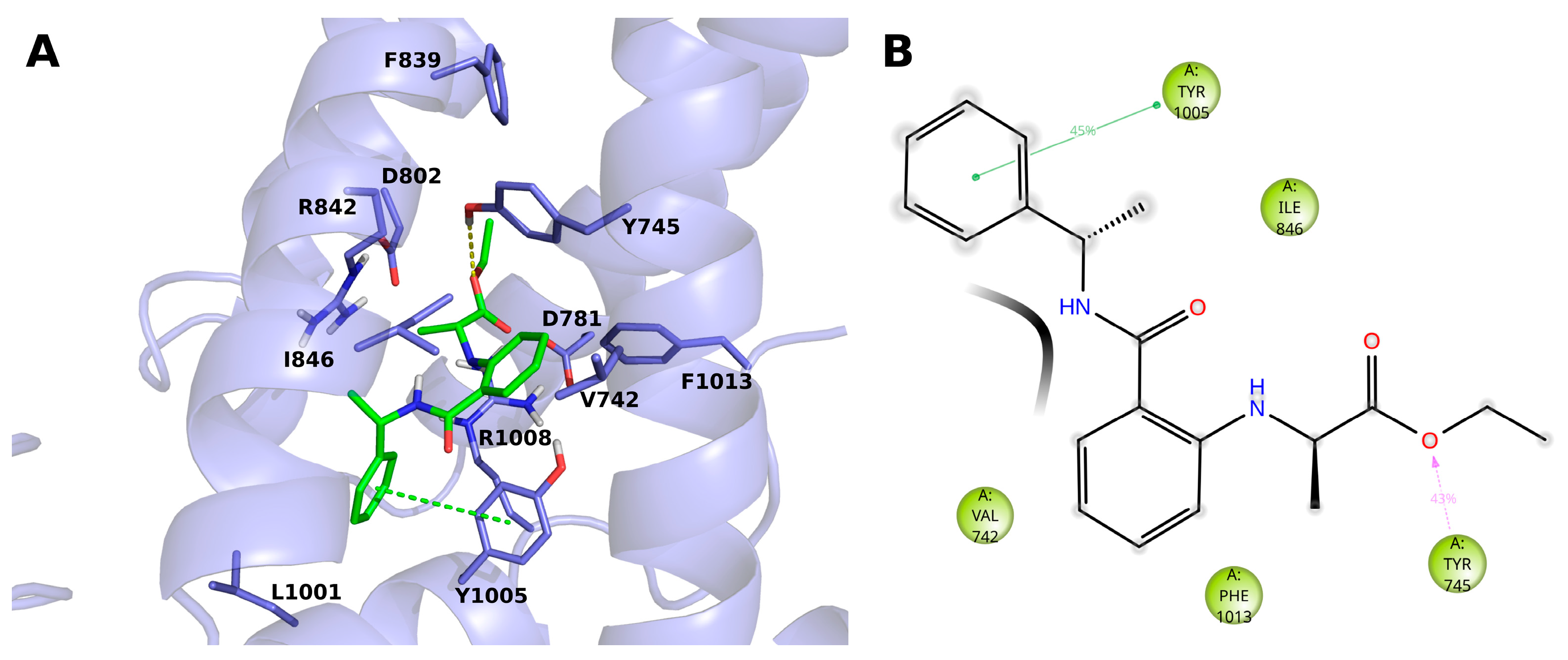
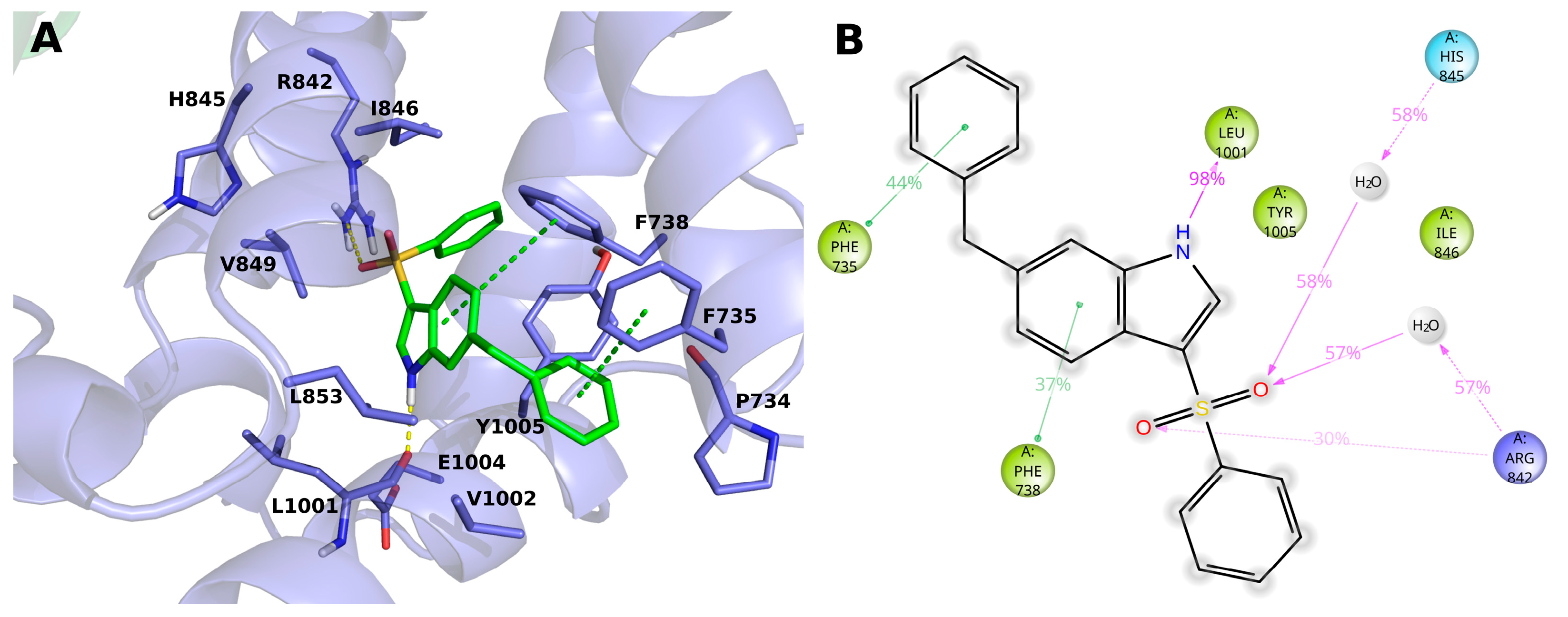
2.3. Molecular Modeling
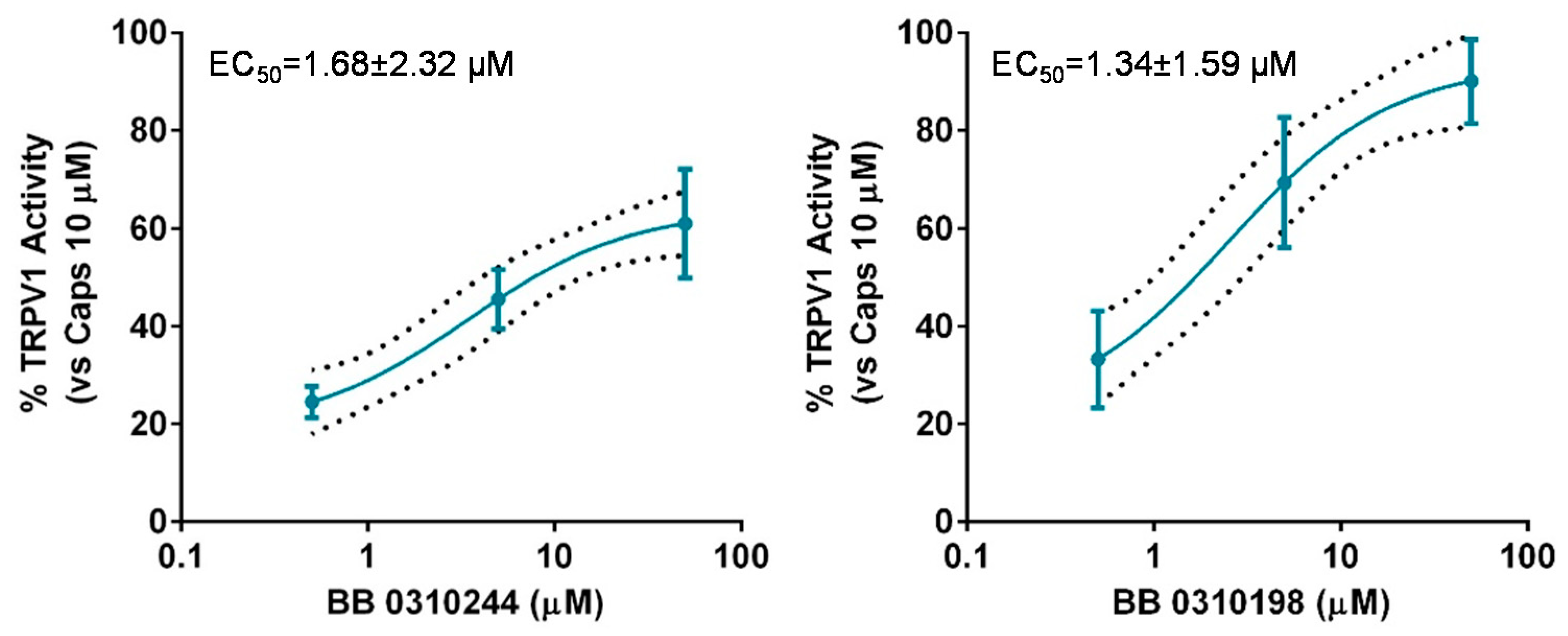
2.4. Selectivity Studies
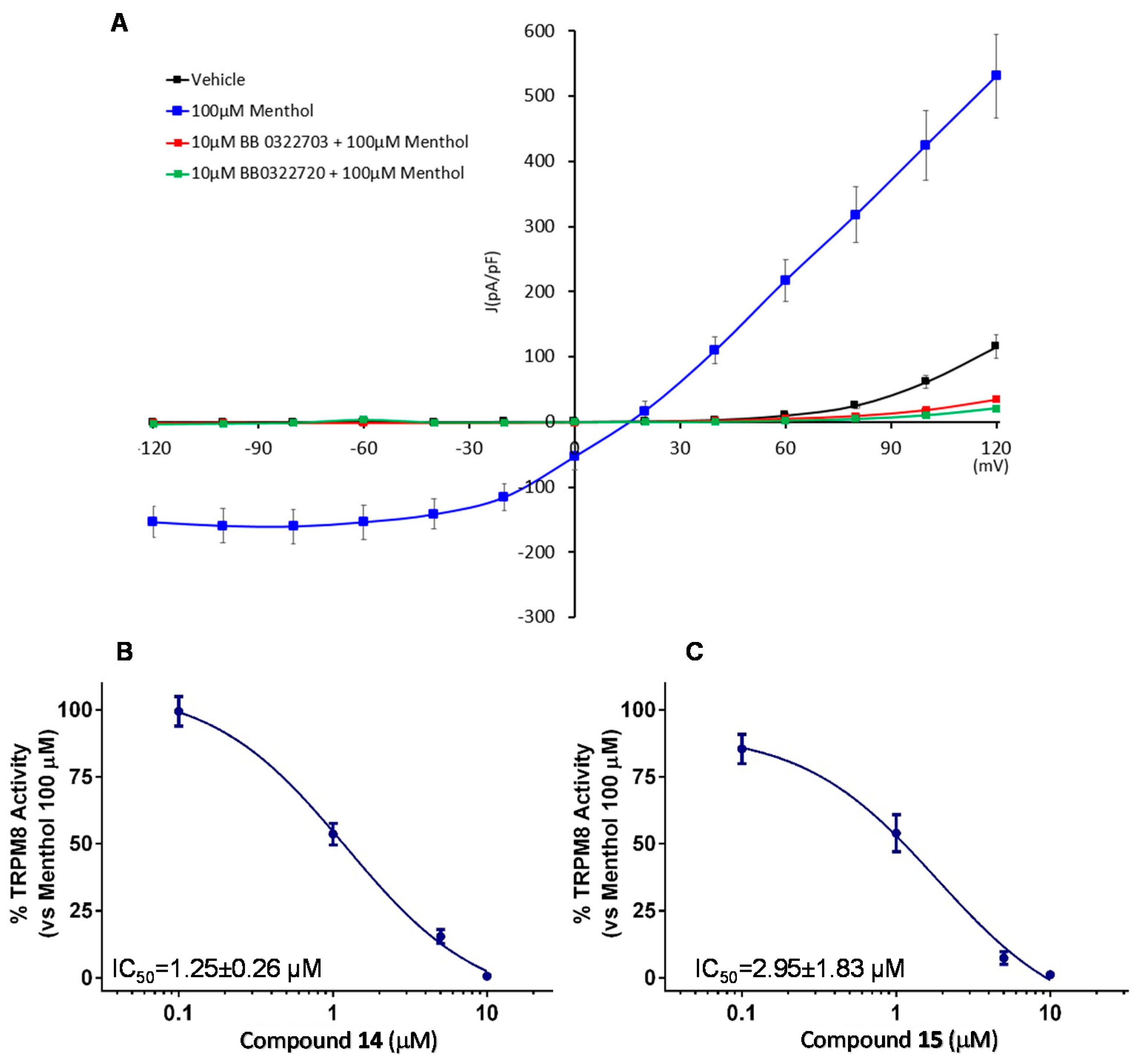
2.5. Patch-Clamp Electrophysiology Assays
2.6. In Vivo Assays
3. Discussion
4. Materials and Methods
4.1. Chemistry

4.2. In Vitro Biological Assays
4.2.1. Cell Cultures
4.2.2. Fluorometric Assays
4.2.3. Patch-Clamp Experiments
4.3. In Vivo Studies
4.3.1. Animals
4.3.2. Drugs
4.3.3. Oxaliplatin-Induced Neuropathic Pain
4.3.4. Cold Sensitivity
4.3.5. Statistical Analysis
4.4. In Silico-Studies
4.4.1. Models Building
4.4.2. Molecular Dynamics Simulations of 14 (BB 0322703) and 15 (BB 0322720) in Complex with hTRPM8723–1013
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izquierdo, C.; Martín-Martínez, M.; Gómez-Monterrey, I.; González-Muñiz, R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int. J. Mol. Sci. 2021, 22, 8502. [Google Scholar] [CrossRef] [PubMed]
- Diver, M.M.; Cheng, Y.; Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science 2019, 365, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Brauchi, S.; Orio, P.; Latorre, R. Latorre. Clues to understanding cold sensation: Thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc. Natl. Acad. Sci. USA 2004, 101, 15494–15499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Wang, C.; Yu, Y.-H.; Ren, Y.-Y.; Xie, K.-L.; Wang, G.-L. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Alarcón-Alarcón, D.; Cabañero, D.; de Andrés-López, J.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. Androgenic TRPM8 activity drives sexual dimorphism in a murine model of chronic migraine. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006, 16, 1591–1605. [Google Scholar] [CrossRef] [Green Version]
- Andersson, K.-E.; Gratzke, C.; Hedlund, P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int. 2010, 106, 1114–1127. [Google Scholar] [CrossRef]
- Grolez, G.P.; Gkika, D. TRPM8 Puts the Chill on Prostate Cancer. Pharmaceuticals 2016, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, Y.; Shuai, S.; Ding, D.; Li, R.; Luo, R. TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumor. Biol. 2014, 35, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S.; Brown, R.D.; Lee, M.S.; Zhou, W.; Jensen, C.; Gerke, H.; Yee, R.K. TRPM8 ion channel is aberrantly expressed and required for preventing replicative senescence in pancreatic adenocarcinoma. Cancer Biol. Ther. 2014, 13, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wu, H.; Wei, Z.; Wang, X.; Shen, P.; Wang, S.; Wang, A.; Chen, W.; Lu, Y. TRPM8: A potential target for cancer treatment. J. Cancer Res. Clin. Oncol. 2016, 142, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Peabody, N.C.; Pohl, J.B.; Diao, F.; Vreede, A.P.; Sandstrom, D.J.; Wang, H.; Zelensky, P.K.; White, B.H. Characterization of the Decision Network for Wing Expansion in Drosophila Using Targeted Expression of the TRPM8 Channel. J. Neurosci. 2009, 29, 3343–3353. [Google Scholar] [CrossRef] [Green Version]
- Pertusa, M.; Rivera, B.; González, A.; Ugarte, G.; Madrid, R. Critical role of the pore domain in the cold response of TRPM8 channels identified by ortholog functional comparison. J. Biol. Chem. 2018, 293, 12454–12471. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lu, X.; Wang, Y.; Xu, L.; Chen, X.; Yang, F.; Lai, R. A paradigm of thermal adaptation in penguins and elephants by tuning cold activation in TRPM8. Proc. Natl. Acad. Sci. USA 2020, 117, 8633–8638. [Google Scholar] [CrossRef] [Green Version]
- Press Release: The Nobel Prize in Physiology or Medicine 2021. Available online: https://www.nobelprize.org/prizes/medicine/2021/summary/ (accessed on 30 December 2021).
- Xu, L.; Han, Y.; Chen, X.; Aierken, A.; Wen, H.; Zheng, W.; Wang, H.; Lu, X.; Zhao, Z.; Ma, C.; et al. Molecular mechanisms underlying menthol binding and activation of TRPM8 ion channel. Nat. Commun. 2020, 11, 3790. [Google Scholar] [CrossRef]
- Andersson, D.A.; Chase, H.W.N.; Bevan, S.J. TRPM8 Activation by Menthol, Icilin, and Cold Is Differentially Modulated by Intracellular pH. J. Neurosci. 2004, 24, 5364–5369. [Google Scholar] [CrossRef]
- Weil, A.; Moore, S.E.; Waite, N.J.; Randall, A.; Gunthorpe, M.J. Conservation of Functional and Pharmacological Properties in the Distantly Related Temperature Sensors TRPV1 and TRPM8. Mol. Pharmacol. 2005, 68, 518–527. [Google Scholar] [CrossRef]
- Meseguer, V.; Karashima, Y.; Talavera, K.; D’Hoedt, D.; Donovan-Rodríguez, T.; Viana, F.; Nilius, B.; Voets, T. Transient Receptor Potential Channels in Sensory Neurons Are Targets of the Antimycotic Agent Clotrimazole. J. Neurosci. 2008, 28, 576–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, G.; Droogmans, G.; Nilius, B. Multiple effects of SK&F 96365 on ionic currents and intracellular calcium in human endothelial cells. Cell Calcium. 1994, 15, 45–54. [Google Scholar] [PubMed]
- Aierken, A.; Xie, Y.; Dong, W.; Apaer, A.; Lin, J.; Zhao, Z.; Yang, S.; Xu, Z.; Yang, F. Rational Design of a Modality-Specific Inhibitor of TRPM8 Channel against Oxaliplatin-Induced Cold Allodynia. Adv. Sci. 2021, 8, 2101717. [Google Scholar] [CrossRef]
- Beccari, A.R.; Gemei, M.; Monte, M.L.; Menegatti, N.; Fanton, M.; Pedretti, A.; Bovolenta, S.; Nucci, C.; Molteni, A.; Rossignoli, A.; et al. Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand- and structure-based virtual screening approach. Sci. Rep. 2017, 7, 10999. [Google Scholar] [CrossRef] [Green Version]
- Knowlton, W.M.; Daniels, R.; Palkar, R.; McCoy, D.D.; McKemy, D.D. Pharmacological Blockade of TRPM8 Ion Channels Alters Cold and Cold Pain Responses in Mice. PLoS ONE 2011, 6, e25894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertamino, A.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Soldovieri, M.V.; Iraci, N.; Carvajal, A.F.; de la Torre-Martinez, R.; et al. Tryptamine-Based Derivatives as Transient Receptor Potential Melastatin Type 8 (TRPM8) Channel Modulators. J. Med. Chem. 2016, 59, 2179–2191. [Google Scholar] [CrossRef] [Green Version]
- Bertamino, A.; Iraci, N.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Pepe, G.; Sala, M.; Soldovieri, M.V.; et al. Identification of a Potent Tryptophan-Based TRPM8 Antagonist With in Vivo Analgesic Activity. J. Med. Chem. 2018, 61, 6140–6152. [Google Scholar] [CrossRef]
- Bertamino, A.; Ostacolo, C.; Medina, A.; Di Sarno, V.; Lauro, G.; Ciaglia, T.; Vestuto, V.; Pepe, G.; Basilicata, M.G.; Musella, S.; et al. Exploration of TRPM8 Binding Sites by β-Carboline-Based Antagonists and Their In Vitro Characterization and In Vivo Analgesic Activities. J. Med. Chem. 2020, 63, 9672–9694. [Google Scholar] [CrossRef]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of New TRPM8 Modulators in Pain Perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef] [Green Version]
- Di Donato, M.; Ostacolo, C.; Giovannelli, P.; Di Sarno, V.; Monterrey, I.M.G.; Campiglia, P.; Migliaccio, A.; Bertamino, A.; Castoria, G. Therapeutic potential of TRPM8 antagonists in prostate cancer. Sci. Rep. 2021, 11, 23232. [Google Scholar] [CrossRef]
- Bechelane-Maia, E.H.; Assis, L.C.; Alves de Oliveira, T.; Marques da Silva, A.; Gutterres Taranto, A. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front. Chem. 2020, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Talarico, C.; Gervasoni, S.; Manelfi, C.; Pedretti, A.; Vistoli, G.; Beccari, A.R. Combining Molecular Dynamics and Docking Simulations to Develop Targeted Protocols for Performing Optimized Virtual Screening Campaigns on the hTRPM8 Channel. Int. J. Mol. Sci. 2020, 21, 2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wu, X.; Zhang, L.; Mao, A.; Ma, X.; He, D. Menthol relieves acid reflux inflammation by regulating TRPV1 in esophageal epithelial cells. Biochem. Biophys. Res. Commun. 2020, 525, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, K.J.; Grant, E.R.; Wu, G.; Hachicha, M.; Schmid, L.; Tafesse, L.; Sun, Q.; Rotshteyn, Y.; Francis, J.; Limberis, J.; et al. N-(4-Tertiarybutylphenyl)-4-(3chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. In Vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 2003, 306, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.Z.; Ando, H.; Unno, S.; Masuda, Y.; Kitagawa, J. Activation of TRPV1 and TRPM8 Channels in the Larynx and Associated Laryngopharyngeal Regions Facilitates the Swallowing Reflex. Int. J. Mol. Sci. 2018, 19, 4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereiter, D.A.; Rahman, M.; Thompson, R.; Stephenson, P.; Saito, H. TRPV1 and TRPM8 Channels and Nocifensive Behavior in a Rat Model for Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3739–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Faginas, P.; Aranda, M.T.; de la Torre-Martínez, R.; Quirce, S.; Fernández-Carvajal, A.; Ferrer-Montiel, R.; González-Muñiz, R. New transient receptor potential TRPV1, TRPM8 and TRPA1 channel antagonists from a single linear β,γ-diamino ester scaffold. RCS Adv. 2016, 6, 6868–6877. [Google Scholar]
- Weyer, A.D.; Lehto, S.G. Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine. Pharmaceuticals 2017, 10, 37. [Google Scholar] [CrossRef]
- González-Muñiz, R.; Bonache, M.A.; Martín-Escura, C.; Gómez-Monterrey, I. Recent Progress in TRPM8 Modulation: An Update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef] [Green Version]
- Winchester, W.; Gore, K.; Glatt, S.; Petit, W.; Gardiner, J.C.; Conlon, K.; Postlethwaite, M.; Saintot, P.-P.; Roberts, S.; Gosset, J.R.; et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in human. J. Pharmacol. Exp. Ther. 2022, 380, jpet.114.216010. [Google Scholar] [CrossRef] [Green Version]
- Kurkin, A.V.; Bernovskaya, A.A.; Yurovskaya, M.A. Synthesis of N-alkylanthranilamides with a chiral substituent at the nitrogen atom. Tetrahedron Asymmetry 2010, 21, 2100–2107. [Google Scholar] [CrossRef]
- Jiang, H.; Sha, S.-C.; Jeong, S.A.; Manor, B.C.; Walsh, P.J. Ni(NIXANTPHOS)-Catalyzed Mono-Arylation of Toluenes with Aryl Chlorides and Bromides. Org. Lett. 2019, 21, 1735–1739. [Google Scholar] [CrossRef]
- Cordero-Sánchez, C.; Mudarra-Fraguas, I.; Fernández-Carvajal, A. Fluorescence-Based Functional Assays for Ca2+-Permeable ThermoTRP Channels. Methods Mol. Biol. 2019, 1987, 99–110. [Google Scholar] [PubMed]
- Zimmermann, M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol. Scand. Suppl. 1986, 554, 221–233. [Google Scholar] [PubMed]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the Role of the Crystal Environment in Determining Protein Side-chain Conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Sancineto, L.; Iraci, N.; Massari, S.; Attanasio, V.; Corazza, G.; Barreca, M.L.; Sabatini, S.; Manfroni, G.; Avanzi, N.R.; Cecchetti, V.; et al. Computer-Aided Design, Synthesis and Validation of 2-Phenylquinazolinone Fragments as CDK9 Inhibitors with Anti-HIV-1 Tat-Mediated Transcription Activity. ChemMedChem 2013, 8, 1941–1953. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Schrödinger LLC. Schrödinger Release 2019-1: LigPrep; Schrödinger LLC: New York, NY, USA, 2019. [Google Scholar]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, A.; Iraci, N.; Sabatini, S.; Barreca, M.; Cecchetti, V. p38α MAPK and Type I Inhibitors: Binding Site Analysis and Use of Target Ensembles in Virtual Screening. Molecules 2015, 20, 15842–15861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, M.L.; Iraci, N.; Manfroni, G.; Gaetani, R.; Guercini, C.; Sabatini, S.; Tabarrini, O.; Cecchetti, V. Accounting for Target Flexibility and Water Molecules by Docking to Ensembles of Target Structures: The HCV NS5B Palm Site I Inhibitors Case Study. J. Chem. Inf. Model. 2013, 54, 481–497. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters, In Proceedings of the SC’06: 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006.
- Lomize, M.A.; Lomize, A.L.; Pogozheva, I.D.; Mosberg, H.I. OPM: Orientations of Proteins in Membranes database. Bioinformatics 2006, 22, 623–625. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]

| Compound | XP GlideScore | VS Rank a |
|---|---|---|
| 1 (BB 0220221) | −9.315 | 57 |
| 2 (BB 0301246) | −9.556 | 34 |
| 3 (BB 0301259) | −9.621 | 29 |
| 4 (BB 0301261) | −9.382 | 49 |
| 5 (BB 0304240) | −9.019 | 85 |
| 6 (BB 0304398) | −9.432 | 39 |
| 7 (BB 0304425) | −9.245 | 64 |
| 8 (BB 0305409) | −9.786 | 22 |
| 9 (BB 0305411) | −9.004 | 87 |
| 10 (BB 0305430) | −9.528 | 35 |
| 11 (BB 0310197) | −9.400 | 43 |
| 12 (BB 0310217) | −9.349 | 51 |
| 13 (BB 0310244) | −9.589 | 31 |
| 14 (BB 0322703) | −9.242 | 66 |
| 15 (BB 0322720) | −9.063 | 81 |
| 16 (BB 0323219) | −9,092 | 79 |
| 17 (BB 0323225) | −9.106 | 77 |
| 18 (BB 0301235) | −10.104 | 14 |
| 19 (BB 0310198) | −10.235 | 10 |
| 20 (BB 0310207) | −10.694 | 6 |
| 21 (BB 0237332) | −8.964 | 90 |
| Compound | IC50 (µM) | Efficacy a |
|---|---|---|
| 1 (BB 0220221) | 10.21 ± 1.31 | 85 |
| 5 (BB 0304240) | 11.10 ± 1.32 | 95 |
| 13 (BB 0310244) | 1.01 ± 0.51 | 90 |
| 14 (BB 0322703) | 0.25 ± 0.15 | 100 |
| 15 (BB 0322720) | 0.22 ± 0.10 | 100 |
| 19 (BB 0310198) | 5.52 ± 1.45 | 90 |
| AMTB | 7.15 ± 1.24 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iraci, N.; Ostacolo, C.; Medina-Peris, A.; Ciaglia, T.; Novoselov, A.M.; Altieri, A.; Cabañero, D.; Fernandez-Carvajal, A.; Campiglia, P.; Gomez-Monterrey, I.; et al. In Vitro and In Vivo Pharmacological Characterization of a Novel TRPM8 Inhibitor Chemotype Identified by Small-Scale Preclinical Screening. Int. J. Mol. Sci. 2022, 23, 2070. https://doi.org/10.3390/ijms23042070
Iraci N, Ostacolo C, Medina-Peris A, Ciaglia T, Novoselov AM, Altieri A, Cabañero D, Fernandez-Carvajal A, Campiglia P, Gomez-Monterrey I, et al. In Vitro and In Vivo Pharmacological Characterization of a Novel TRPM8 Inhibitor Chemotype Identified by Small-Scale Preclinical Screening. International Journal of Molecular Sciences. 2022; 23(4):2070. https://doi.org/10.3390/ijms23042070
Chicago/Turabian StyleIraci, Nunzio, Carmine Ostacolo, Alicia Medina-Peris, Tania Ciaglia, Anton M. Novoselov, Andrea Altieri, David Cabañero, Asia Fernandez-Carvajal, Pietro Campiglia, Isabel Gomez-Monterrey, and et al. 2022. "In Vitro and In Vivo Pharmacological Characterization of a Novel TRPM8 Inhibitor Chemotype Identified by Small-Scale Preclinical Screening" International Journal of Molecular Sciences 23, no. 4: 2070. https://doi.org/10.3390/ijms23042070









