Metformin Preserves VE–Cadherin in Choroid Plexus and Attenuates Hydrocephalus via VEGF/VEGFR2/p-Src in an Intraventricular Hemorrhage Rat Model
Abstract
:1. Introduction
2. Results
2.1. Ventricular Injection of Homologous Blood Caused Hydrocephalus and Downregulation of VE–Cadherin Expression in Choroid Plexus
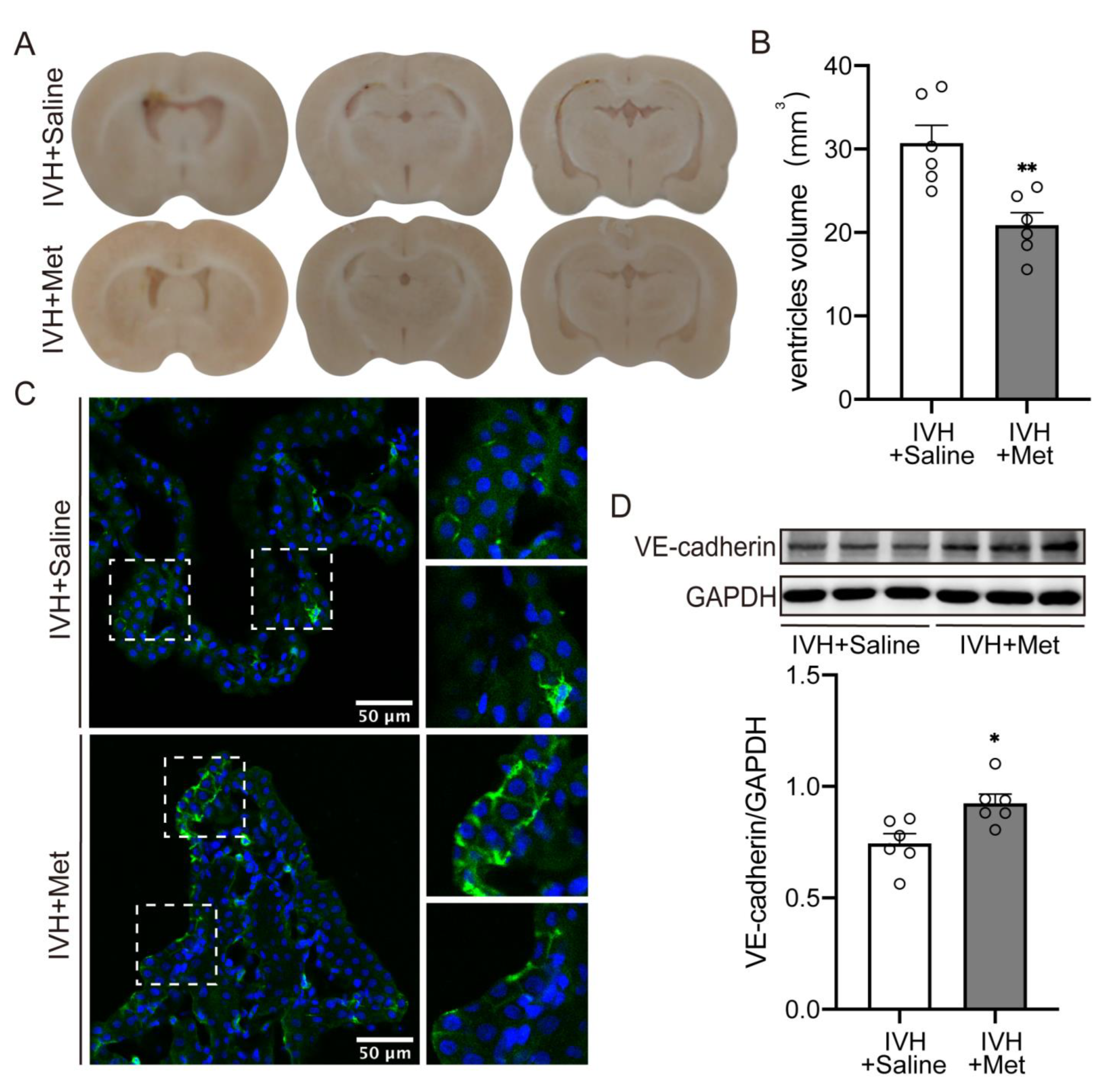
2.2. Metformin Attenuated the Hydrocepralus and Increased VE–Cadherin Expression in Choroid Plexus after IVH
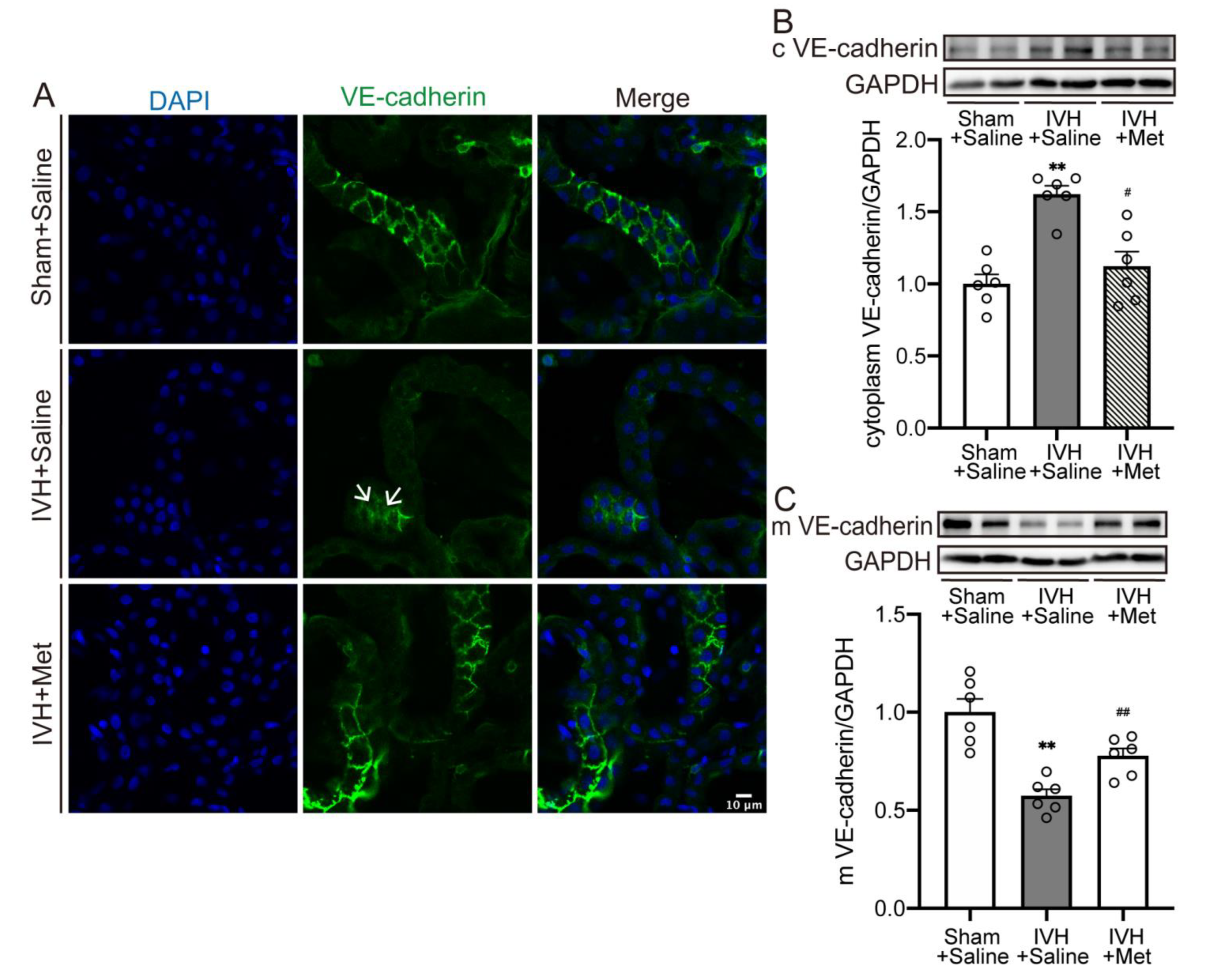
2.3. Metformin Downregulated the Internalization of VE–Cadherin in Choroid Plexus after IVH
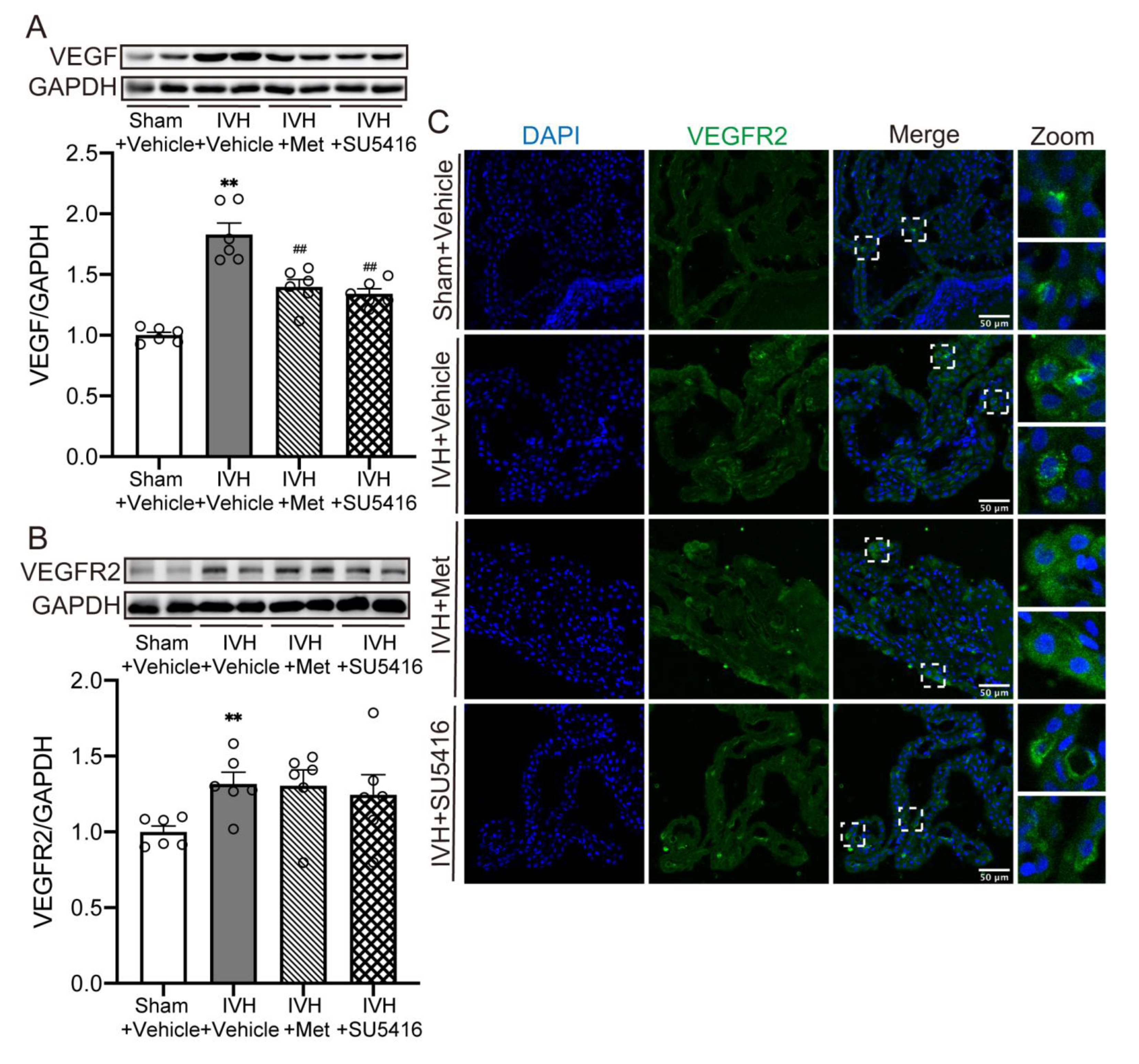
2.4. Metformin Diminished the Upregulation of VEGF Expression after IVH
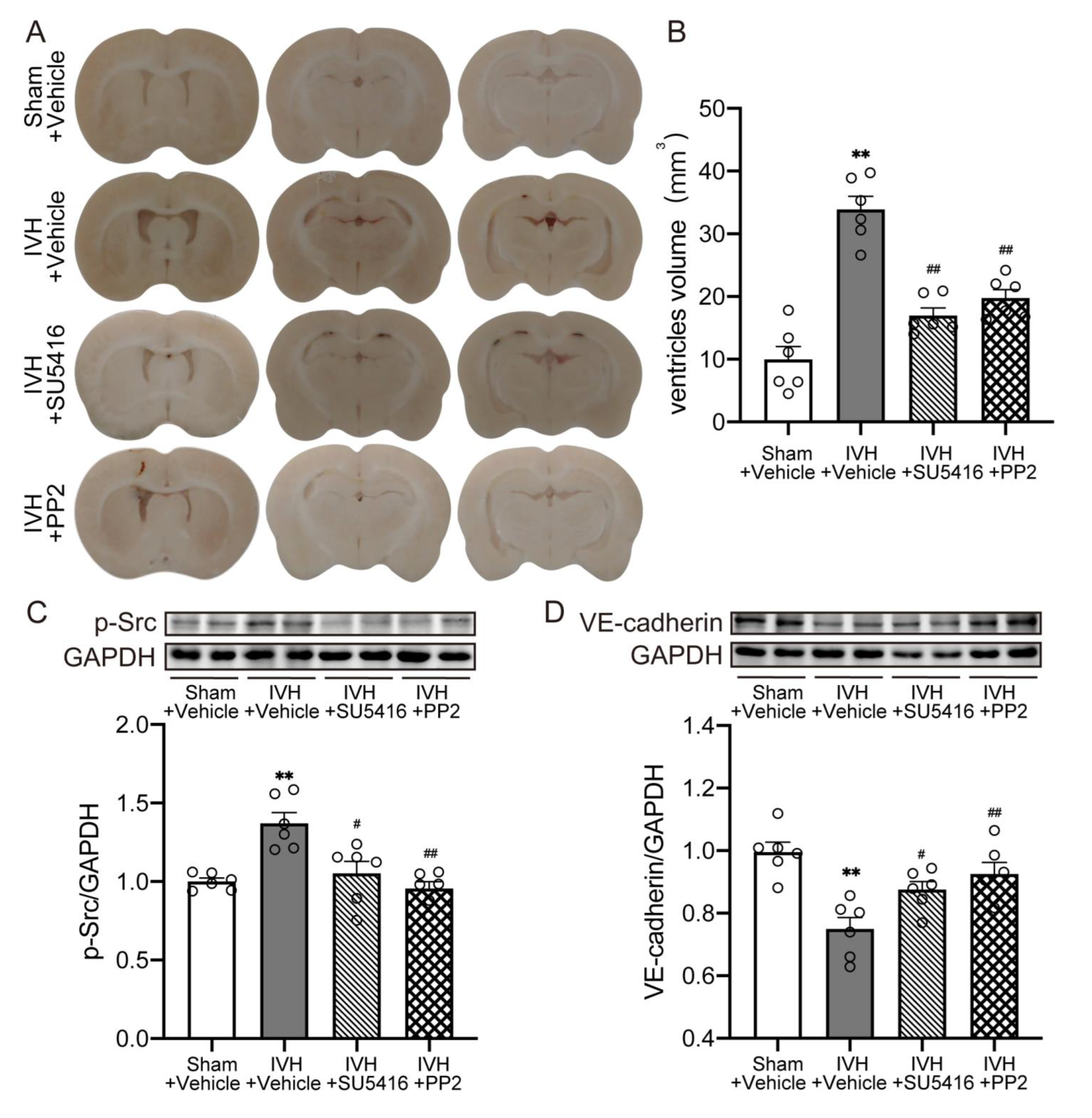
2.5. Inhibition of VEGFR2/p-Src Attenuated IVH-Induced Hydrocephalus and Escalated the Level of VE–Cadherin
3. Discussion
4. Materials and Methods
4.1. Animals Model
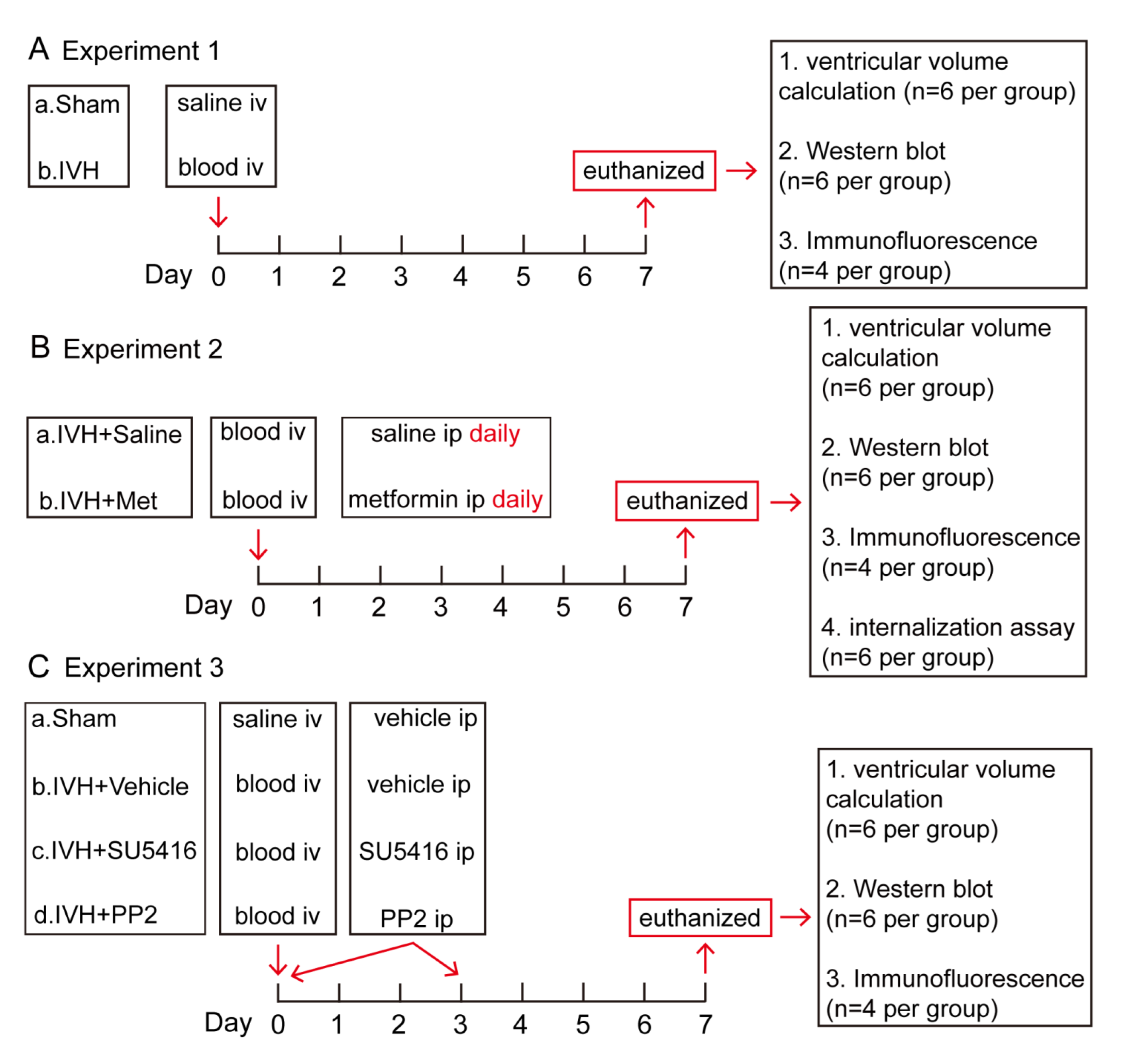
4.2. Experimental Groups
4.3. Ventricular Volume Analysis
4.4. Immunofluorescence Staining
4.5. Western Blot
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanley, D.F. Intraventricular hemorrhage: Severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 2009, 40, 1533–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifan, G.; Arshi, B.; Testai, F.D. Intraventricular Hemorrhage Severity as a Predictor of Outcome in Intracerebral Hemorrhage. Front. Neurol. 2019, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Jabbarli, R.; Reinhard, M.; Roelz, R.; Shah, M.; Niesen, W.D.; Kaier, K.; Taschner, C.; Weyerbrock, A.; Velthoven, V.V. The predictors and clinical impact of intraventricular hemorrhage in patients with aneurysmal subarachnoid hemorrhage. Int. J. Stroke 2016, 11, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Chen, M.; Gao, T.; Wang, X.; Li, X.; Gao, F. Mechanisms of hydrocephalus after intraventricular haemorrhage in adults. Stroke Vasc Neurol 2016, 1, 23–27. [Google Scholar] [CrossRef]
- Rosen, D.S.; Macdonald, R.L.; Huo, D.; Goldenberg, F.D.; Novakovic, R.L.; Frank, J.I.; Rosengart, A.J. Intraventricular hemorrhage from ruptured aneurysm: Clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J. Neurosurg. 2007, 107, 261–265. [Google Scholar] [CrossRef]
- Klebe, D.; McBride, D.; Krafft, P.R.; Flores, J.J.; Tang, J.; Zhang, J.H. Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: Established mechanisms and proposed pathways. J. Neurosci. Res. 2020, 98, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strahle, J.M.; Garton, T.; Bazzi, A.A.; Kilaru, H.; Garton, H.J.; Maher, C.O.; Muraszko, K.M.; Keep, R.F.; Xi, G. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery 2014, 75, 696–705; discussion 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Gao, C.; Hua, Y.; Keep, R.F.; Muraszko, K.; Xi, G. Role of iron in brain injury after intraventricular hemorrhage. Stroke 2011, 42, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.Y.; Wang, J.; Qian, Z.M.; Yang, Q.W. Iron and intracerebral hemorrhage: From mechanism to translation. Transl. Stroke Res. 2014, 5, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Feng, Z.; Tan, Q.; Guo, J.; Tang, J.; Tan, L.; Feng, H.; Chen, Z. Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. J. Neurol. Sci. 2017, 375, 220–230. [Google Scholar] [CrossRef]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.D.; Le, C.S.; Zhang, H.M.; Shang, D.S.; Tong, L.S.; Gao, F. Thrombin disrupts vascular endothelial-cadherin and leads to hydrocephalus via protease-activated receptors-1 pathway. CNS Neurosci. Ther. 2019, 25, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Vestweber, D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Manaenko, A.; Chen, S.; Klebe, D.; Ma, Q.; Caner, B.; Fujii, M.; Zhou, C.; Zhang, J.H. Role of SCH79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke 2013, 44, 1410–1417. [Google Scholar] [CrossRef] [Green Version]
- Rand, D.; Ravid, O.; Atrakchi, D.; Israelov, H.; Bresler, Y.; Shemesh, C.; Omesi, L.; Liraz-Zaltsman, S.; Gosselet, F.; Maskrey, T.S.; et al. Endothelial Iron Homeostasis Regulates Blood-Brain Barrier Integrity via the HIF2alpha-Ve-Cadherin Pathway. Pharmaceutics 2021, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Gavard, J.; Gutkind, J.S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006, 8, 1223–1234. [Google Scholar] [CrossRef]
- Shim, J.W.; Sandlund, J.; Madsen, J.R. VEGF: A potential target for hydrocephalus. Cell Tissue Res. 2014, 358, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.W.; Madsen, J.R. VEGF Signaling in Neurological Disorders. Int. J. Mol. Sci. 2018, 19, 275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lolansen, S.D.; Rostgaard, N.; Oernbo, E.K.; Juhler, M.; Simonsen, A.H.; MacAulay, N. Inflammatory Markers in Cerebrospinal Fluid from Patients with Hydrocephalus: A Systematic Literature Review. Dis. Markers 2021, 2021, 8834822. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dombrowski, S.M.; Deshpande, A.; Krajcir, N.; Luciano, M.G. VEGF/VEGFR-2 changes in frontal cortex, choroid plexus, and CSF after chronic obstructive hydrocephalus. J. Neurol. Sci. 2010, 296, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Tarnaris, A.; Toma, A.K.; Pullen, E.; Chapman, M.D.; Petzold, A.; Cipolotti, L.; Kitchen, N.D.; Keir, G.; Lemieux, L.; Watkins, L.D. Cognitive, biochemical, and imaging profile of patients suffering from idiopathic normal pressure hydrocephalus. Alzheimers Dement. 2011, 7, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.W.; Sandlund, J.; Han, C.H.; Hameed, M.Q.; Connors, S.; Klagsbrun, M.; Madsen, J.R.; Irwin, N. VEGF, which is elevated in the CSF of patients with hydrocephalus, causes ventriculomegaly and ependymal changes in rats. Exp. Neurol. 2013, 247, 703–709. [Google Scholar] [CrossRef]
- Park, M.J.; Moon, S.J.; Baek, J.A.; Lee, E.J.; Jung, K.A.; Kim, E.K.; Kim, D.S.; Lee, J.H.; Kwok, S.K.; Min, J.K.; et al. Metformin Augments Anti-Inflammatory and Chondroprotective Properties of Mesenchymal Stem Cells in Experimental Osteoarthritis. J. Immunol. 2019, 203, 127–136. [Google Scholar] [CrossRef]
- Sun, S.; Gong, F.; Liu, P.; Miao, Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene 2018, 664, 50–57. [Google Scholar] [CrossRef]
- Yi, Q.Y.; Deng, G.; Chen, N.; Bai, Z.S.; Yuan, J.S.; Wu, G.H.; Wang, Y.W.; Wu, S.J. Metformin inhibits development of diabetic retinopathy through inducing alternative splicing of VEGF-A. Am. J. Transl. Res. 2016, 8, 3947–3954. [Google Scholar] [PubMed]
- Amable, G.; Martinez-Leon, E.; Picco, M.E.; Nemirovsky, S.I.; Rozengurt, E.; Rey, O. Metformin inhibition of colorectal cancer cell migration is associated with rebuilt adherens junctions and FAK downregulation. J. Cell Physiol. 2020, 235, 8334–8344. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, X.; Zheng, J.; Wang, H.; Liu, J. Effects of metformin treatment on glioma-induced brain edema. Am J. Transl Res. 2016, 8, 3351–3363. [Google Scholar]
- Fatemi, I.; Saeed-Askari, P.; Hakimizadeh, E.; Kaeidi, A.; Esmaeil-Moghaddam, S.; Pak-Hashemi, M.; Allahtavakoli, M. Long-term metformin therapy improves neurobehavioral functions and antioxidative activity after cerebral ischemia/reperfusion injury in rats. Brain Res. Bull. 2020, 163, 65–71. [Google Scholar] [CrossRef]
- Tao, L.; Li, D.; Liu, H.; Jiang, F.; Xu, Y.; Cao, Y.; Gao, R.; Chen, G. Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-kappaB and MAPK signaling pathway. Brain Res. Bull. 2018, 140, 154–161. [Google Scholar] [CrossRef]
- Rahimi, S.; Ferdowsi, A.; Siahposht-Khachaki, A. Neuroprotective effects of metformin on traumatic brain injury in rats is associated with the AMP-activated protein kinase signaling pathway. Metab. Brain Dis. 2020, 35, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S. Neonatal posthemorrhagic hydrocephalus from prematurity: Pathophysiology and current treatment concepts. J. Neurosurg. Pediatr. 2012, 9, 242–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.S.; Shen, Y.Q.; Zhang, X.D.; Zhao, L.B.; Wei, X.; Xiong, X.; Xie, X.F.; Li, R.; Deng, L.; Li, X.H.; et al. Hydrocephalus Growth: Definition, Prevalence, Association with Poor Outcome in Acute Intracerebral Hemorrhage. Neurocrit. Care 2021, 35, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Hua, Y.; Keep, R.F.; Xi, G.; Garton, H.J.L. CD47 blocking antibody accelerates hematoma clearance and alleviates hydrocephalus after experimental intraventricular hemorrhage. Neurobiol. Dis. 2021, 155, 105384. [Google Scholar] [CrossRef]
- Purohit, D.; Finkel, D.A.; Malfa, A.; Liao, Y.; Ivanova, L.; Kleinman, G.M.; Hu, F.; Shah, S.; Thompson, C.; Joseph, E.; et al. Human Cord Blood Derived Unrestricted Somatic Stem Cells Restore Aquaporin Channel Expression, Reduce Inflammation and Inhibit the Development of Hydrocephalus After Experimentally Induced Perinatal Intraventricular Hemorrhage. Front Cell Neurosci. 2021, 15, 633185. [Google Scholar] [CrossRef]
- Mahaney, K.B.; Buddhala, C.; Paturu, M.; Morales, D.; Limbrick, D.D., Jr.; Strahle, J.M. Intraventricular Hemorrhage Clearance in Human Neonatal Cerebrospinal Fluid: Associations With Hydrocephalus. Stroke 2020, 51, 1712–1719. [Google Scholar] [CrossRef]
- Bosche, B.; Mergenthaler, P.; Doeppner, T.R.; Hescheler, J.; Molcanyi, M. Complex Clearance Mechanisms After Intraventricular Hemorrhage and rt-PA Treatment—A Review on Clinical Trials. Transl. Stroke Res. 2020, 11, 337–344. [Google Scholar] [CrossRef]
- Liddelow, S.A. Development of the choroid plexus and blood-CSF barrier. Front. Neurosci. 2015, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benarroch, E.E. Choroid plexus—CSF system: Recent developments and clinical correlations. Neurology 2016, 86, 286–296. [Google Scholar] [CrossRef]
- Dejana, E.; Vestweber, D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog. Mol. Biol. Transl. Sci. 2013, 116, 119–144. [Google Scholar] [PubMed]
- Del Bigio, M.R. Ependymal cells: Biology and pathology. Acta Neuropathol. 2010, 119, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bellido, D.; Serrano-Saenz, S.; Fernandez-Cortes, M.; Oliver, F.J. Vasculogenic mimicry signaling revisited: Focus on non-vascular VE-cadherin. Mol. Cancer 2017, 16, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Padhan, N.; Sjostrom, E.O.; Roche, F.P.; Testini, C.; Honkura, N.; Sainz-Jaspeado, M.; Gordon, E.; Bentley, K.; Philippides, A.; et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat. Commun. 2016, 7, 11017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, K.; Garner, J.; Buckley, K.M.; Vincent, P.A.; Chiasson, C.M.; Dejana, E.; Faundez, V.; Kowalczyk, A.P. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell 2005, 16, 5141–5151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, N. Defenders and Challengers of Endothelial Barrier Function. Front. Immunol. 2017, 8, 1847. [Google Scholar] [CrossRef] [Green Version]
- Raab, S.; Plate, K.H. Different networks, common growth factors: Shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007, 113, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.S.; Walshe, T.E.; Saint-Geniez, M.; Venkatesha, S.; Maldonado, A.E.; Himes, N.C.; Matharu, K.S.; Karumanchi, S.A.; D’Amore, P.A. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J. Exp. Med. 2008, 205, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.; Storkebaum, E.; de Almodovar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular endothelial growth factor: A neurovascular target in neurological diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef]
- Wolburg, H.; Paulus, W. Choroid plexus: Biology and pathology. Acta Neuropathol. 2010, 119, 75–88. [Google Scholar] [CrossRef]
- Ange, M.; Castanares-Zapatero, D.; De Poortere, J.; Dufeys, C.; Courtoy, G.E.; Bouzin, C.; Quarck, R.; Bertrand, L.; Beauloye, C.; Horman, S. alpha1AMP-Activated Protein Kinase Protects against Lipopolysaccharide-Induced Endothelial Barrier Disruption via Junctional Reinforcement and Activation of the p38 MAPK/HSP27 Pathway. Int. J. Mol. Sci. 2020, 21, 5581. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Chen, Z.; Kim, K.T.; Sun, J.; Xue, M.; Chen, G.; Li, S.; Shen, Y.; Zhu, Z.; Wang, X.; et al. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy 2019, 15, 843–870. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Guigas, B.; Chauvin, C.; Batandier, C.; Fontaine, E.; Wiernsperger, N.; Leverve, X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 2005, 54, 2179–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgharzadeh, F.; Barneh, F.; Fakhraie, M.; Adel Barkhordar, S.L.; Shabani, M.; Soleimani, A.; Rahmani, F.; Ariakia, F.; Mehraban, S.; Avan, A.; et al. Metformin inhibits polyphosphate-induced hyper-permeability and inflammation. Int. Immunopharmacol. 2021, 99, 107937. [Google Scholar] [CrossRef]
- Takata, F.; Dohgu, S.; Matsumoto, J.; Machida, T.; Kaneshima, S.; Matsuo, M.; Sakaguchi, S.; Takeshige, Y.; Yamauchi, A.; Kataoka, Y. Metformin induces up-regulation of blood-brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2013, 433, 586–590. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, G.; Li, Y.; Wang, Y.; Chen, X.; Gu, X.; Zhang, Z.; Wang, Y.; Yang, G.Y. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflamm. 2014, 11, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, D.; Guo, J.; Wu, Y.; Chen, W.; Du, J.; Yang, L.; Wang, X.; Gong, K.; Dai, J.; Miao, S.; et al. Metformin-repressed miR-381-YAP-snail axis activity disrupts NSCLC growth and metastasis. J. Exp. Clin. Cancer Res. 2020, 39, 6. [Google Scholar] [CrossRef] [PubMed]
- Elia, E.M.; Quintana, R.; Carrere, C.; Bazzano, M.V.; Rey-Valzacchi, G.; Paz, D.A.; Pustovrh, M.C. Metformin decreases the incidence of ovarian hyperstimulation syndrome: An experimental study. J. Ovarian Res. 2013, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Kadry, H.; Noorani, B.; Bickel, U.; Abbruscato, T.J.; Cucullo, L. Comparative assessment of in vitro BBB tight junction integrity following exposure to cigarette smoke and e-cigarette vapor: A quantitative evaluation of the protective effects of metformin using small-molecular-weight paracellular markers. Fluids Barriers CNS 2021, 18, 28. [Google Scholar] [CrossRef]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Nakada, T.; Kwee, I.L.; Igarashi, H.; Suzuki, Y. Aquaporin-4 Functionality and Virchow-Robin Space Water Dynamics: Physiological Model for Neurovascular Coupling and Glymphatic Flow. Int. J. Mol. Sci. 2017, 18, 1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Zhu, L.; Liu, J.; Zhu, T.; Xie, Z.; Sun, X.; Zhang, H. Metformin Protects against Oxidative Stress Injury Induced by Ischemia/Reperfusion via Regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxid. Med. Cell Longev. 2019, 2019, 8768327. [Google Scholar] [CrossRef] [PubMed]
- Nilles, K.L.; Williams, E.I.; Betterton, R.D.; Davis, T.P.; Ronaldson, P.T. Blood-Brain Barrier Transporters: Opportunities for Therapeutic Development in Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 1898. [Google Scholar] [CrossRef] [PubMed]
- Mendel, D.B.; Schreck, R.E.; West, D.C.; Li, G.; Strawn, L.M.; Tanciongco, S.S.; Vasile, S.; Shawver, L.K.; Cherrington, J.M. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin. Cancer Res. 2000, 6, 4848–4858. [Google Scholar] [PubMed]
- Cai, H.; Ma, Y.; Jiang, L.; Mu, Z.; Jiang, Z.; Chen, X.; Wang, Y.; Yang, G.Y.; Zhang, Z. Hypoxia Response Element-Regulated MMP-9 Promotes Neurological Recovery via Glial Scar Degradation and Angiogenesis in Delayed Stroke. Mol. Ther. 2017, 25, 1448–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, D.; Ye, X.; Li, J.; Hao, X.; Jin, L.; Jin, Y.; Tong, L.; Gao, F. Metformin Preserves VE–Cadherin in Choroid Plexus and Attenuates Hydrocephalus via VEGF/VEGFR2/p-Src in an Intraventricular Hemorrhage Rat Model. Int. J. Mol. Sci. 2022, 23, 8552. https://doi.org/10.3390/ijms23158552
Shen D, Ye X, Li J, Hao X, Jin L, Jin Y, Tong L, Gao F. Metformin Preserves VE–Cadherin in Choroid Plexus and Attenuates Hydrocephalus via VEGF/VEGFR2/p-Src in an Intraventricular Hemorrhage Rat Model. International Journal of Molecular Sciences. 2022; 23(15):8552. https://doi.org/10.3390/ijms23158552
Chicago/Turabian StyleShen, Dan, Xianghua Ye, Jiawen Li, Xiaodi Hao, Luhang Jin, Yujia Jin, Lusha Tong, and Feng Gao. 2022. "Metformin Preserves VE–Cadherin in Choroid Plexus and Attenuates Hydrocephalus via VEGF/VEGFR2/p-Src in an Intraventricular Hemorrhage Rat Model" International Journal of Molecular Sciences 23, no. 15: 8552. https://doi.org/10.3390/ijms23158552




