Quantitative Proteomics of Medium-Sized Extracellular Vesicle-Enriched Plasma of Lacunar Infarction for the Discovery of Prognostic Biomarkers
Abstract
:1. Introduction
2. Results
2.1. Quality Control of Plasma-Derived MEV
2.2. Adverse Outcome Is Associated with an Increase in the Total Number of Altered Proteins
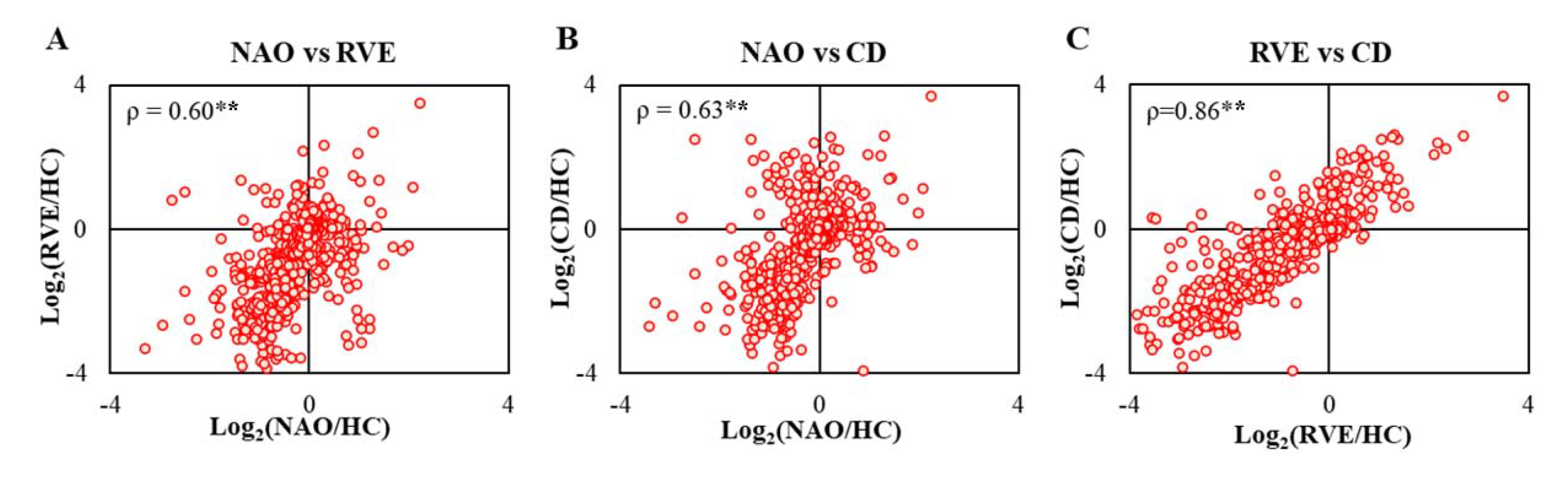
2.3. Proteome-Wide Correlation Analysis Reveals Differing Patterns Depending on Outcome
2.4. Convalescent Plasma MEV—Preferred Fraction for Biomarker Discovery
2.5. Plasma MEV Contain Disease-Specific Signatures of Key Pathological Events
2.6. Majority of Adverse Outcome Predictors Are Not Linked to Coagulation or Inflammation
2.7. MEV-Proteome Is Qualitatively and Quantitatively Different from SEV Proteome
3. Discussion
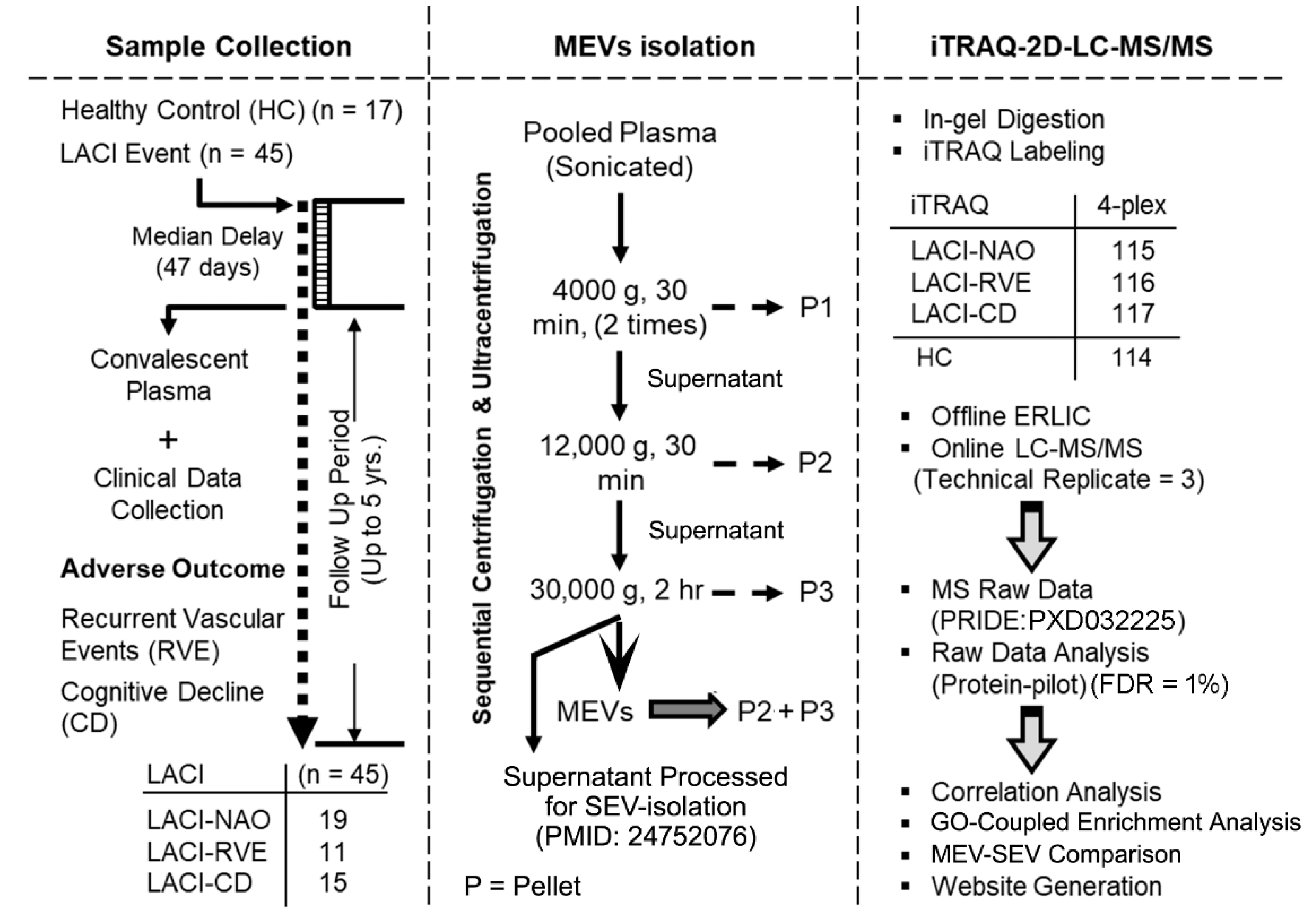
4. Materials and Methods
4.1. Reagents
4.2. Sample Collection and Patient Information
4.3. Experimental Design Guided by Outcome Measures
4.4. Proteomics Sample Preparation
4.4.1. Isolation of MEV-Enriched Fraction by Sequential Centrifugation and Ultracentrifugation
4.4.2. In-Gel Tryptic Digestion and Isobaric Labeling
4.4.3. Offline ERLIC and LC-MS/MS
4.4.4. MS Raw Data Analysis
4.5. GO Analysis
4.6. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, C.M. Lacunar strokes and infarcts: A review. Neurology 1982, 32, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Gunarathne, A.; Patel, J.V.; Gammon, B.; Gill, P.S.; Hughes, E.A.; Lip, G.Y.H. Ischemic stroke in south asians: A review of the epidemiology, pathophysiology, and ethnicity-related clinical features. Stroke 2009, 40, e415–e423. [Google Scholar] [CrossRef] [Green Version]
- Turin, T.C.; Kita, Y.; Rumana, N.; Nakamura, Y.; Takashima, N.; Ichikawa, M.; Sugihara, H.; Morita, Y.; Hirose, K.; Okayama, A.; et al. Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988–2004. Stroke 2010, 41, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Steinke, W.; Ley, S.C. Lacunar stroke is the major cause of progressive motor deficits. Stroke 2002, 33, 1510–1516. [Google Scholar] [CrossRef] [Green Version]
- Grau-Olivares, M.; Arboix, A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: A forerunner of vascular dementia? Expert Rev. Neurother. 2009, 9, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Galliazzo, S.; Grazioli, S.; Guasti, L.; Ageno, W.; Squizzato, A. Epidemiology and secondary prevention of ischemic stroke in patients on antiplatelet drug: A retrospective cohort study. J. Thromb. Thrombolysis 2019, 48, 336–344. [Google Scholar] [CrossRef]
- Gómez-Choco, M.; Mena, L.; Font, M.; Mengual, J.J.; Garcia-Sanchez, S.M.; Avellaneda, C.; Montull, C.; Castrillo, L.; Blanch, P.; Lleixa, M.; et al. NT-proBNP, cerebral small vessel disease and cardiac function in patients with a recent lacunar infarct. J. Hum. Hypertens. 2022, 1–6. [Google Scholar] [CrossRef]
- Wiseman, S.; Marlborough, F.; Doubal, F.; Webb, D.J.; Wardlaw, J. Blood Markers of Coagulation, Fibrinolysis, Endothelial Dysfunction and Inflammation in Lacunar Stroke versus Non-Lacunar Stroke and Non-Stroke: Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2014, 37, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef] [Green Version]
- Lundström, A.; Mobarrez, F.; Rooth, E.; Thålin, C.; von Arbin, M.; Henriksson, P.; Gigante, B.; Laska, A.C.; Wallén, H. Prognostic Value of Circulating Microvesicle Subpopulations in Ischemic Stroke and TIA. Transl. Stroke Res. 2020, 11, 708–719. [Google Scholar] [CrossRef]
- Jansen van Vuuren, J.; Pillay, S.; Naidoo, A. Circulating Biomarkers in Long-Term Stroke Prognosis: A Scoping Review Focusing on the South African Setting. Cureus 2022, 14, e23971. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Doubal, F.; Armitage, P.; Chappell, F.; Carpenter, T.; Muñoz Maniega, S.; Farrall, A.; Sudlow, C.; Dennis, M.; Dhillon, B. Lacunar stroke is associated with diffuse Blood-Brain barrier dysfunction. Ann. Neurol. 2009, 65, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Colombo, L.; Magni, G.; Viganó, F.; Boccazzi, M.; Deli, M.A.; Sperlágh, B.; Abbracchio, M.P.; Kittel, A. Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem. Int. 2011, 59, 259–271. [Google Scholar] [CrossRef]
- Babu, M.; Singh, N.; Datta, A. In Vitro Oxygen Glucose Deprivation Model of Ischemic Stroke: A Proteomics-Driven Systems Biological Perspective. Mol Neurobiol 2022, 59, 2363–2377. [Google Scholar] [CrossRef]
- Mitaki, S.; Wada, Y.; Sheikh, A.M.; Yamaguchi, S.; Nagai, A. Proteomic analysis of extracellular vesicles enriched serum associated with future ischemic stroke. Sci. Rep. 2021, 11, 24024. [Google Scholar] [CrossRef]
- Qin, C.; Zhao, X.L.; Ma, X.T.; Zhou, L.Q.; Wu, L.J.; Shang, K.; Wang, W.; Tian, D.S. Proteomic profiling of plasma biomarkers in acute ischemic stroke due to large vessel occlusion. J. Transl. Med. 2019, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhao, M.; Liu, Y.; Jin, H.; Cui, W.; Fan, C.; Teng, Y.; Zheng, L.; Huang, Y. Apolipoprotein C-III in the high-density lipoprotein proteome of cerebral lacunar infarction patients impairs its anti-inflammatory function. Int. J. Mol. Med. 2018, 41, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, A.; Chen, C.P.; Sze, S.K. Discovery of Prognostic Biomarker Candidates of Lacunar Infarction by Quantitative Proteomics of Microvesicles Enriched Plasma. PLoS ONE 2014, 9, e94663. [Google Scholar] [CrossRef]
- Datta, A.; Sze, S.K. Data for iTRAQ profiling of micro-vesicular plasma specimens: In search of potential prognostic circulatory biomarkers for Lacunar infarction. Data Brief 2015, 4, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Halkes, P.H.; van Gijn, J.; Kappelle, L.J.; Koudstaal, P.J.; Algra, A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): Randomised controlled trial. Lancet 2006, 367, 1665–1673. [Google Scholar] [PubMed]
- Narasimhalu, K.; Ang, S.; De Silva, D.A.; Wong, M.C.; Chang, H.M.; Chia, K.S.; Auchus, A.P.; Chen, C.P. The prognostic effects of poststroke cognitive impairment no dementia and domain-specific cognitive impairments in nondisabled ischemic stroke patients. Stroke 2011, 42, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Narasimhalu, K.; Ang, S.; De Silva, D.A.; Wong, M.C.; Chang, H.M.; Chia, K.S.; Auchus, A.P.; Chen, C. Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology 2009, 73, 1866–1872. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Wardlaw, J.M.; Sandercock, P.A.G.; Dennis, M.S.; Starr, J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003, 34, 806–811. [Google Scholar] [CrossRef]
- Wada, M.; Nagasawa, H.; Iseki, C.; Takahashi, Y.; Sato, H.; Arawaka, S.; Kawanami, T.; Kurita, K.; Daimon, M.; Kato, T. Cerebral small vessel disease and chronic kidney disease (CKD): Results of a cross-sectional study in community-based Japanese elderly. J. Neurol. Sci. 2008, 272, 36–42. [Google Scholar] [CrossRef]
- Pretnar-Oblak, J.; Sabovic, M.; Pogacnik, T.; Sebestjen, M.; Zaletel, M. Flow-mediated dilatation and intima-media thickness in patients with lacunar infarctions. Acta Neurol. Scand. 2006, 113, 273–277. [Google Scholar] [CrossRef]
- Datta, A.; Park, J.E.; Li, X.; Zhang, H.; Ho, Z.S.; Heese, K.; Lim, S.K.; Tam, J.P.; Sze, S.K. Phenotyping of an in vitro model of ischemic penumbra by iTRAQ-based shotgun quantitative proteomics. J. Prot. Res. 2010, 9, 472–484. [Google Scholar] [CrossRef]
- Grant, R.; Ansa-Addo, E.; Stratton, D.; Antwi-Baffour, S.; Jorfi, S.; Kholia, S.; Krige, L.; Lange, S.; Inal, J. A filtration-based protocol to isolate human Plasma Membrane-derived Vesicles and exosomes from blood plasma. J. Immunol. Methods 2011, 371, 143–151. [Google Scholar] [CrossRef]
- Wang, B.; Cai, W.; Zhang, Z.; Zhang, H.; Tang, K.; Zhang, Q.; Wang, X. Circulating microparticles in patients after ischemic stroke: A systematic review and meta-analysis. Rev. Neurosci. 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Bastos-Amador, P.; Royo, F.; Gonzalez, E.; Conde-Vancells, J.; Palomo-Diez, L.; Borras, F.E.; Falcon-Perez, J.M. Proteomic analysis of microvesicles from plasma of healthy donors reveals high individual variability. J. Proteom. 2012, 75, 3574–3584. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Chu, K.; Lee, S.T.; Park, H.K.; Bahn, J.J.; Kim, D.H.; Kim, J.H.; Kim, M.; Kun Lee, S.; Roh, J.K. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann. Neurol. 2009, 66, 191–199. [Google Scholar] [CrossRef]
- Kuriyama, N.; Nagakane, Y.; Hosomi, A.; Ohara, T.; Kasai, T.; Harada, S.; Takeda, K.; Yamada, K.; Ozasa, K.; Tokuda, T.; et al. Evaluation of factors associated with elevated levels of platelet-derived microparticles in the acute phase of cerebral infarction. Clin. Appl. Thromb. Hemost. 2010, 16, 26–32. [Google Scholar] [CrossRef]
- Connor, D.E.; Ly, K.; Aslam, A.; Boland, J.; Low, J.; Jarvis, S.; Muller, D.W.; Joseph, J.E. Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease. Platelets 2016, 27, 805–811. [Google Scholar] [CrossRef]
- Kotur-Stevuljević, J.; Vekić, J.; Stefanović, A.; Zeljković, A.; Ninić, A.; Ivanišević, J.; Miljković, M.; Sopić, M.; Munjas, J.; Mihajlović, M.; et al. Paraoxonase 1 and atherosclerosis-related diseases. Biofactors 2020, 46, 193–205. [Google Scholar] [CrossRef]
- Vogelsang, P.; Giil, L.M.; Lund, A.; Vedeler, C.A.; Parkar, A.P.; Nordrehaug, J.E.; Kristoffersen, E.K. Reduced glucose transporter-1 in brain derived circulating endothelial cells in mild Alzheimer's disease patients. Brain Res. 2018, 1678, 304–309. [Google Scholar] [CrossRef]
- Datta, A.; Jingru, Q.; Khor, T.H.; Teo, M.T.; Heese, K.; Sze, S.K. Quantitative neuroproteomics of an in vivo rodent model of focal cerebral ischemia/reperfusion injury reveals a temporal regulation of novel pathophysiological molecular markers. J. Prot. Res. 2011, 10, 5199–5213. [Google Scholar] [CrossRef]
- Xu, C.; Yu, B.; Zhao, X.; Lin, X.; Tang, X.; Liu, Z.; Gao, P.; Ge, J.; Wang, S.; Li, L. Valosin Containing Protein as a Specific Biomarker for Predicting the Development of Acute Coronary Syndrome and Its Complication. Front. Cardiovasc. Med. 2022, 9, 803532. [Google Scholar] [CrossRef]
- Yu, B.; Xu, C.; Tang, X.; Liu, Z.; Lin, X.; Meng, H.; Shi, C.; Ma, K.; Xiao, B.; Li, L. Endoplasmic reticulum stress-related secretory proteins as biomarkers of early myocardial ischemia-induced sudden cardiac deaths. Int. J. Leg. Med. 2022, 136, 159–168. [Google Scholar] [CrossRef]
- Ito, Y.; Nakachi, K.; Imai, K.; Hashimoto, S.; Watanabe, Y.; Inaba, Y.; Tamakoshi, A.; Yoshimura, T. Stability of frozen serum levels of insulin-like growth factor-I, insulin-like growth factor-II, insulin-like growth factor binding protein-3, transforming growth factor beta, soluble Fas, and superoxide dismutase activity for the JACC study. J. Epidemiol. 2005, 15 (Suppl. I), S67–S73. [Google Scholar] [CrossRef]
- Alegre, E.; Varo, N.; Fernández-Calle, P.; Calleja, S.; González, Á. Impact of ultra-low temperature long-term storage on the preanalytical variability of twenty-one common biochemical analytes. Clin. Chem. Lab. Med. 2022, 60, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Menne, F.; Schipke, C.G.; Clark, C.; Popp, J. Long-term stability and age-dependence of six regulatory serum proteins. Biomark Med. 2022, 16, 511–521. [Google Scholar] [CrossRef]
- Yin, Z.G.; Wang, Q.S.; Yu, K.; Wang, W.W.; Lin, H.; Yang, Z.H. Sex differences in associations between blood lipids and cerebral small vessel disease. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 28–34. [Google Scholar] [CrossRef]
- Dykstra-Aiello, C.; Sharp, F.R.; Jickling, G.C.; Hull, H.; Hamade, F.; Shroff, N.; Durocher, M.; Cheng, X.; Zhan, X.; Liu, D.; et al. Alternative Splicing of Putative Stroke/Vascular Risk Factor Genes Expressed in Blood Following Ischemic Stroke Is Sexually Dimorphic and Cause-Specific. Front. Neurol. 2020, 11, 584695. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Akatsu, H.; Heese, K.; Sze, S.K. Quantitative Clinical Proteomic Study of Autopsied Human Infarcted Brain Specimens to Elucidate the Deregulated Pathways in Ischemic Stroke Pathology. J. Proteom. 2013, 91, 556–568. [Google Scholar] [CrossRef]
- Datta, A.; Yang, C.R.; Salhadar, K.; Park, E.; Chou, C.L.; Raghuram, V.; Knepper, M.A. Phosphoproteomic identification of vasopressin-regulated protein kinases in collecting duct cells. Br. J. Pharm. 2021, 178, 1426–1444. [Google Scholar] [CrossRef]
- De Schryver, E.L.L.M. Design of ESPRIT: An international randomized trial for secondary prevention after non-disabling cerebral ischaemia of arterial origin. Cerebrovasc. Dis. 2000, 10, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bonita, R.; Beaglehole, R. Modification of rankin scale: Recovery of motor function after stroke. Stroke 1988, 19, 1497–1500. [Google Scholar] [CrossRef] [Green Version]
- Diagnostic and Statistical Manual of Mental Disorders-IV; American Psychiatric Association: Washington, DC, USA, 1994.
- Mead, G.E.; Lewis, S.C.; Wardlaw, J.M.; Dennis, M.S.; Warlow, C.P. How well does the Oxfordshire community stroke project classification predict the site and size of the infarct on brain imaging? J. Neurol. Neurosurg. Psychiatry 2000, 68, 558–562. [Google Scholar] [CrossRef] [PubMed]




| Gene Symbol | Accession | Protein Name | Identification Parameters | Quantitation Ratios 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Protein Score 1 | %Cov(95) | Peptides (95%) 2 | Log2(NAO/HC) | Log2(RVE/HC) | Log2(CD/HC) | |||
| FLII | Q13045 | Protein flightless-1 homolog | 4.6 | 1.9 | 2 | −0.58 | −2.11 | −2.86 |
| DYNC1H1 | Q14204 | Cytoplasmic dynein 1 heavy chain 1 | 6.9 | 0.4 | 2 | −0.40 | −0.98 | −1.04 |
| AP2B1 | P63010-2 | Isoform 2 of AP-2 complex subunit beta | 7.1 | 2.7 | 3 | −0.57 | −2.13 | −1.01 |
| BCHE | P06276 | Cholinesterase | 7.2 | 7.0 | 3 | −0.12 | 2.18 | 2.39 |
| UBA52 | P62987 | Ubiquitin-60S ribosomal protein L40 | 7.4 | 29.7 | 3 | −0.36 | −1.54 | −0.53 |
| MYLK | Q15746-5 | Isoform 4 of myosin light chain kinase, smooth muscle | 8.0 | 2.2 | 4 | −0.47 | −1.67 | −1.77 |
| CANX | P27824-2 | Isoform 2 of calnexin | 8.3 | 8.0 | 4 | −0.44 | −2.39 | −2.03 |
| KLKB1 | H0YAC1 | Plasma kallikrein (fragment) | 8.4 | 6.7 | 4 | −0.58 | −0.50 | 1.09 |
| KIF2A | O00139-4 | Isoform 4 of kinesin-like protein KIF2A | 8.4 | 6.5 | 4 | −0.53 | −1.99 | −1.38 |
| SLC2A1 | P11166 | Solute carrier family 2, facilitated glucose transporter member 1 | 10.6 | 13.2 | 6 | 1.04 | −2.75 | −1.00 |
| DIAPH1 | A0A0G2JH68 | Protein diaphanous homolog 1 | 10.9 | 5.0 | 5 | −0.33 | −2.10 | −1.74 |
| IQGAP2 | Q13576 | Ras GTPase-activating-like protein IQGAP2 | 10.9 | 1.8 | 3 | 0.25 | −1.10 | −2.02 |
| PIGR | P01833 | Polymeric immunoglobulin receptor | 11.3 | 10.9 | 6 | −0.88 | 1.14 | 1.70 |
| PECAM1 | P16284-6 | Isoform Delta15 of platelet endothelial cell adhesion molecule | 11.6 | 10.0 | 5 | −0.45 | −1.21 | −1.61 |
| GANAB | Q14697 | Neutral alpha-glucosidase AB | 11.7 | 9.7 | 6 | −0.53 | −1.25 | −1.50 |
| PROS1 | P07225 | Vitamin K-dependent protein S | 11.9 | 10.8 | 6 | −0.13 | 1.16 | 1.24 |
| HPR | A0A0A0MRD9 | Haptoglobin-related protein | 12.3 | 55.5 | 39 | −2.50 | 1.04 | 2.50 |
| HSP90B1 | P14625 | Endoplasmin | 13.4 | 9.2 | 6 | −0.68 | −2.68 | −1.59 |
| ADD1 | P35611-3 | Isoform 3 of alpha-adducin | 13.4 | 9.8 | 6 | 0.39 | −1.85 | 0.00 |
| FLOT2 | E7EMK3 | Flotillin-2 | 14.2 | 18.0 | 7 | −0.35 | −1.54 | −0.62 |
| APOH | P02749 | Beta-2-glycoprotein 1 | 14.5 | 28.1 | 12 | −0.27 | −2.76 | −1.75 |
| VASP | P50552 | Vasodilator-stimulated phosphoprotein | 15.0 | 19.7 | 7 | −0.64 | −3.36 | −1.42 |
| VTN | P04004 | Vitronectin | 17.5 | 20.9 | 13 | −0.23 | −1.82 | −0.78 |
| CAT | P04040 | Catalase | 17.6 | 22.8 | 8 | 0.61 | −0.23 | 0.16 |
| ATP5A1 | P25705 | ATP synthase subunit alpha, mitochondrial | 18.3 | 21.2 | 9 | −0.31 | −1.81 | −1.90 |
| PZP | P20742 | Pregnancy zone protein | 18.6 | 16.8 | 126 | −1.04 | 0.01 | 1.53 |
| FCGBP | Q9Y6R7 | IgGFc-binding protein | 19.0 | 3.1 | 9 | −1.37 | 1.37 | 2.48 |
| HPX | P02790 | Hemopexin | 19.3 | 21.9 | 10 | 0.82 | −0.90 | 0.11 |
| TFRC | P02786 | Transferrin receptor protein 1 | 19.5 | 14.2 | 9 | −1.77 | −0.27 | 0.01 |
| PON1 | P27169 | Serum paraoxonase/arylesterase 1 | 20.0 | 39.7 | 11 | 0.82 | −0.39 | −0.29 |
| KNG1 | P01042-2 | Isoform LMW of kininogen-1 | 23.1 | 28.6 | 12 | 0.69 | −1.02 | 0.24 |
| APOL1 | O14791 | Apolipoprotein L1 | 25.3 | 34.7 | 14 | −1.33 | 0.25 | 1.91 |
| CD5L | O43866 | CD5 antigen-like | 25.6 | 41.2 | 16 | −0.69 | 0.74 | 1.99 |
| STOM | P27105 | Erythrocyte band 7 integral membrane protein | 27.0 | 50.0 | 20 | −0.07 | −0.82 | −1.34 |
| GP5 | P40197 | Platelet glycoprotein V | 27.7 | 33.6 | 17 | −0.45 | −2.15 | −1.90 |
| C4BPA | P04003 | C4b-binding protein alpha chain | 28.1 | 28.3 | 19 | −0.23 | 1.22 | 1.42 |
| EPB42 | P16452 | Erythrocyte membrane protein band 4.2 | 29.5 | 19.0 | 16 | 0.96 | −1.53 | −0.72 |
| MSN | P26038 | Moesin | 29.6 | 28.4 | 17 | −0.17 | −1.73 | −0.44 |
| ITGB3 | P05106 | Integrin beta-3 | 32.8 | 22.8 | 21 | −0.56 | −2.26 | −2.13 |
| LGALS3BP | Q08380 | Galectin-3-binding protein | 35.9 | 34.4 | 23 | −1.12 | 1.10 | 2.03 |
| APOA1 | P02647 | Apolipoprotein A-I | 39.2 | 56.9 | 23 | −0.80 | −1.40 | 0.96 |
| EPB41 | P11171-2 | Isoform 2 of protein 4.1 | 39.8 | 30.6 | 21 | 0.98 | −2.48 | −0.60 |
| APOE | P02649 | Apolipoprotein E | 40.1 | 68.4 | 25 | 0.13 | −0.72 | −0.94 |
| VCP | P55072 | Transitional endoplasmic reticulum ATPase | 43.9 | 36.6 | 21 | −0.61 | 0.09 | 0.08 |
| SERPINA1 | P01009 | Alpha-1-antitrypsin | 45.2 | 55.5 | 32 | 0.33 | −1.47 | −0.09 |
| LPA | P08519 | Apolipoprotein(a) | 46.5 | 29.2 | 38 | 1.36 | 0.05 | −0.48 |
| F5 | A0A0A0MRJ7 | Coagulation factor V | 47.3 | 11.9 | 22 | −0.29 | −2.13 | −1.29 |
| FCN3 | O75636 | Ficolin-3 | 48.3 | 64.9 | 87 | 0.27 | −1.00 | −0.93 |
| VWF | P04275 | von Willebrand factor | 48.7 | 9.7 | 28 | −0.43 | −1.09 | 1.49 |
| IGHA1 | P01876 | Immunoglobulin heavy constant alpha 1 | 52.9 | 56.9 | 68 | −0.85 | −0.07 | 1.16 |
| IGKC | P01834 | Immunoglobulin kappa constant | 54.5 | 91.6 | 123 | −0.28 | 0.40 | 1.94 |
| FGG | P02679 | Fibrinogen gamma chain | 69.2 | 61.6 | 99 | −0.49 | −2.56 | 0.43 |
| HP | P00738 | Haptoglobin | 73.8 | 64.5 | 92 | 0.29 | 2.33 | 2.22 |
| TF | P02787 | Serotransferrin | 75.2 | 52.6 | 57 | 0.58 | −1.95 | 0.07 |
| SLC4A1 | P02730 | Band 3 anion transport protein | 98.1 | 41.8 | 99 | 1.05 | −3.16 | −1.05 |
| FN1 | P02751-15 | Isoform 15 of fibronectin | 101.6 | 27.5 | 67 | −0.85 | 0.76 | 1.54 |
| FGA | P02671 | Fibrinogen alpha chain | 105.5 | 42.8 | 152 | −0.19 | −3.56 | 0.33 |
| FGB | P02675 | Fibrinogen beta chain | 110.1 | 81.9 | 118 | −0.48 | −3.48 | 0.28 |
| ANK1 | P16157-14 | Isoform Er13 of ankyrin-1 | 145.5 | 45.4 | 110 | 0.94 | −2.25 | −0.41 |
| IGHM | P01871 | Immunoglobulin heavy constant mu | 192.7 | 69.1 | 390 | −4.07 | 1.30 | 2.62 |
| SPTB | P11277-2 | Isoform 2 of spectrin beta chain, erythrocytic | 200.6 | 50.1 | 129 | 0.80 | −3.20 | −0.53 |
| SPTA1 | P02549 | Spectrin alpha chain, erythrocytic 1 | 230.4 | 57.0 | 138 | 0.73 | −2.96 | −0.36 |
| ALB | P02768 | Serum albumin | 258.4 | 85.5 | 399 | 1.21 | −2.75 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datta, A.; Chen, C.; Gao, Y.-G.; Sze, S.K. Quantitative Proteomics of Medium-Sized Extracellular Vesicle-Enriched Plasma of Lacunar Infarction for the Discovery of Prognostic Biomarkers. Int. J. Mol. Sci. 2022, 23, 11670. https://doi.org/10.3390/ijms231911670
Datta A, Chen C, Gao Y-G, Sze SK. Quantitative Proteomics of Medium-Sized Extracellular Vesicle-Enriched Plasma of Lacunar Infarction for the Discovery of Prognostic Biomarkers. International Journal of Molecular Sciences. 2022; 23(19):11670. https://doi.org/10.3390/ijms231911670
Chicago/Turabian StyleDatta, Arnab, Christopher Chen, Yong-Gui Gao, and Siu Kwan Sze. 2022. "Quantitative Proteomics of Medium-Sized Extracellular Vesicle-Enriched Plasma of Lacunar Infarction for the Discovery of Prognostic Biomarkers" International Journal of Molecular Sciences 23, no. 19: 11670. https://doi.org/10.3390/ijms231911670






