Abstract
Fabry disease is a lysosomal storage disease caused by mutations in the GLA gene that encodes alpha-galactosidase (AGAL). The disease causes abnormal globotriaosylceramide (Gb3) storage in the lysosomes. Variants responsible for the genotypic spectrum of Fabry disease include mutations that abolish enzymatic activity and those that cause protein instability. The latter can be successfully treated with small molecules that either bind and stabilize AGAL or indirectly improve its cellular activity. This paper describes the first attempt to reposition curcumin, a nutraceutical, to treat Fabry disease. We tested the efficacy of curcumin in a cell model and found an improvement in AGAL activity for 80% of the tested mutant genotypes (four out of five tested). The fold-increase was dependent on the mutant and ranged from 1.4 to 2.2. We produced evidence that supports a co-chaperone role for curcumin when administered with AGAL pharmacological chaperones (1-deoxygalactonojirimycin and galactose). The combined treatment with curcumin and either pharmacological chaperone was beneficial for four out of five tested mutants and showed fold-increases ranging from 1.1 to 2.3 for DGJ and from 1.1 to 2.8 for galactose. Finally, we tested a long-term treatment on one mutant (L300F) and detected an improvement in Gb3 clearance and lysosomal markers (LAMP-1 and GAA). Altogether, our findings confirmed the necessity of personalized therapies for Fabry patients and paved the way to further studies and trials of treatments for Fabry disease.
1. Introduction
In the last 20 years, the keyword “curcumin” increased its presence from 0 to 0.2% of the total publication volume in biochemistry, medicine, and pharmacology. Roughly 2000 papers on curcumin were published in 2021 (as represented by English articles found on Scopus belonging to the BIOC, MEDI, and PHARM subject areas). Curcumin is a turmeric-derived compound, the interest in which in Western medicine is based on its historical usage in Chinese medicine. In fact, turmeric (Curcuma longa L.) has a long tradition as a treatment for different illnesses that range from oxidative stress-related pathogenesis to anorexia [1,2,3,4].
The long history of turmeric usage allowed the U.S. Food and Drug Administration (FDA) to classify curcumin as Generally Recognized As Safe (GRAS). The wide spectrum of its biological activities justifies the interest of the scientific community in this unique and interesting molecule [5]. Many pathologies such as cancer and chronic diseases, diabetes, Alzheimer’s disease, autoimmune diseases, and rare diseases have been discovered to benefit from curcumin treatment, often in combination with other drugs [6,7,8,9,10,11,12,13,14,15,16,17,18]. Its pleiotropic effects are related to its actions in different pathways that include the PI3K, Akt, mTOR, ERK5, AP-1, TGF-β, Wnt, β-catenin, Shh, PAK1, Rac1, STAT3, PPARγ, EBPα, NLRP3 inflammasome, p38MAPK, Nrf2, Notch-1, AMPK, TLR-4, and MyD-88 pathways. In addition, curcumin modulates autophagy and endoplasmic reticulum (ER) stress [19]. The main issue associated with the clinical use of curcumin is its poor bioavailability. Given the high biomedical interest, great effort is being put in identifying new ways to improve this aspect using many different types of formulations [5,20,21,22].
Fabry disease (FD) is an LSD characterized by abnormal globotriaosylceramide (Gb3) storage in the lysosomes. Such abnormality is caused by the deficiency of the enzyme acidic alpha-galactosidase (AGAL) [23,24,25,26]. This protein, which is encoded by the GLA gene, is synthesized in precursor form, imported into the ER for glycosylation, and then targeted to the lysosomes to catalyze glycosphingolipid hydrolysis [27]. Whereas mutations in the GLA gene that either prevent synthesis or produce truncated variants of AGAL give rise to the most severe genotypes, missense mutations can have a more variable effect based on the location of the mutation. For example, the mutation could fall within the active site or cause a substitution that prevents folding, thereby strongly impacting protein function. Alternatively, the mutation could occur at flexible exposed sites and destabilize the protein. Variants belonging to the second subgroup are intrinsically active but are cleared by the quality-control system. In these cases, the total AGAL activity is insufficient for the correct clearance of Gb3. In addition to the effect on AGAL activity, it was recently demonstrated that missense mutations caused ER stress [28]. It is worth noting that none of the many (>2000) mutations in GLA associated with FD were prevalent among the patients [29,30,31,32,33].
Depending on the genotype, two therapeutic options are available for FD patients. In the most severe cases—patients unable to produce AGAL—enzyme replacement therapy (ERT) is mandatory. In all the other cases where at least a functional protein is still synthesized, the patient can undergo pharmacological chaperone therapy (PCT) [30,34]. In PCT, the patient is given pharmacological chaperones (PCs), which are small molecules that specifically bind and stabilize proteins. While PCT and ERT are both lifelong measures, the former is less expensive, eligible for oral administration, and reaches the central nervous system (CNS). Furthermore, while all FD patients can benefit from ERT regardless of the genotype severity, the quality of life of less severe cases was significantly improved if treated with PCT. Thus, a massive effort was put in place to predict responsive mutations and test PCs on hundreds of genotypes using either fibroblasts or leucocytes derived from patients or transiently transfected COS or HEK cells as cellular models [32,35,36]. In addition, particular attention has been focused on the clinical identification of putatively responsive patients via many tools and educational resources for students and physicians [37,38,39].
At the time of writing, the only PC approved for FD was 1-deoxygalactonojirimycin (DGJ) [40,41,42]. However, this molecule has the undesirable characteristic of being a substrate analog for AGAL, meaning it can inhibit the enzyme, particularly because it also binds the protein at an acidic pH [43,44]. Therefore, the FDA requires a precise posology to balance the inhibiting and chaperoning activities. Such a balance is achieved by using an intermittent administration regimen [42,45,46,47].
This paper describes the first attempt to use a nutraceutical (curcumin) to treat FD. We tested the hypothesis that it could be used to enhance AGAL activity in FD cells both alone or in synergy with the two PCs (DGJ or galactose).
2. Results and Discussion
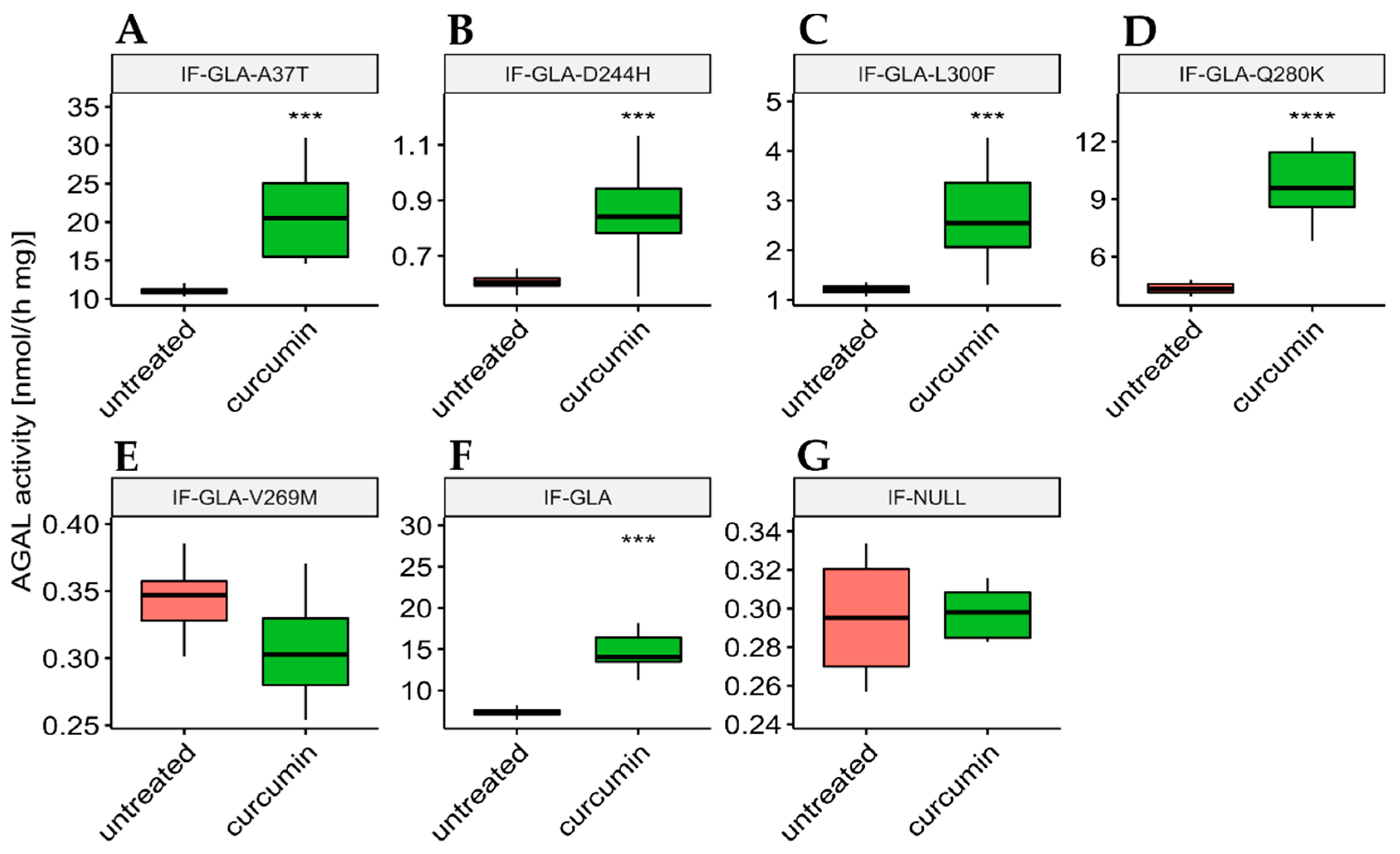
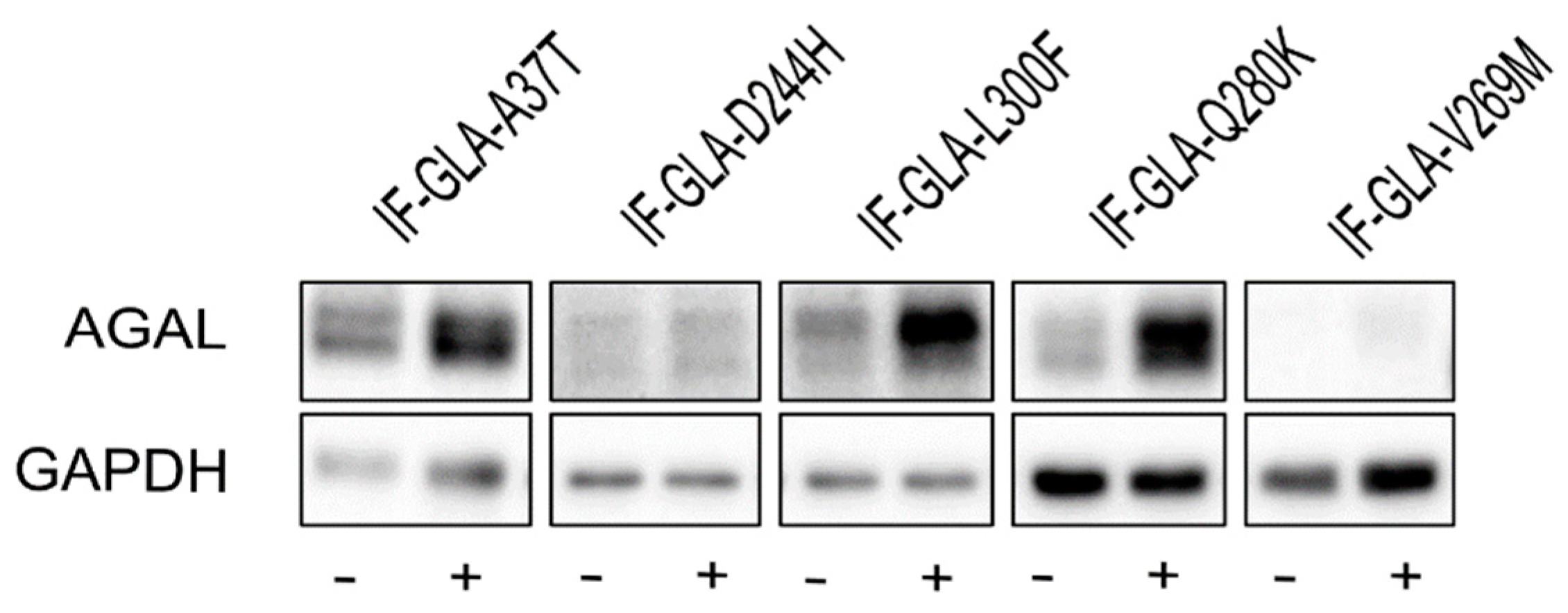
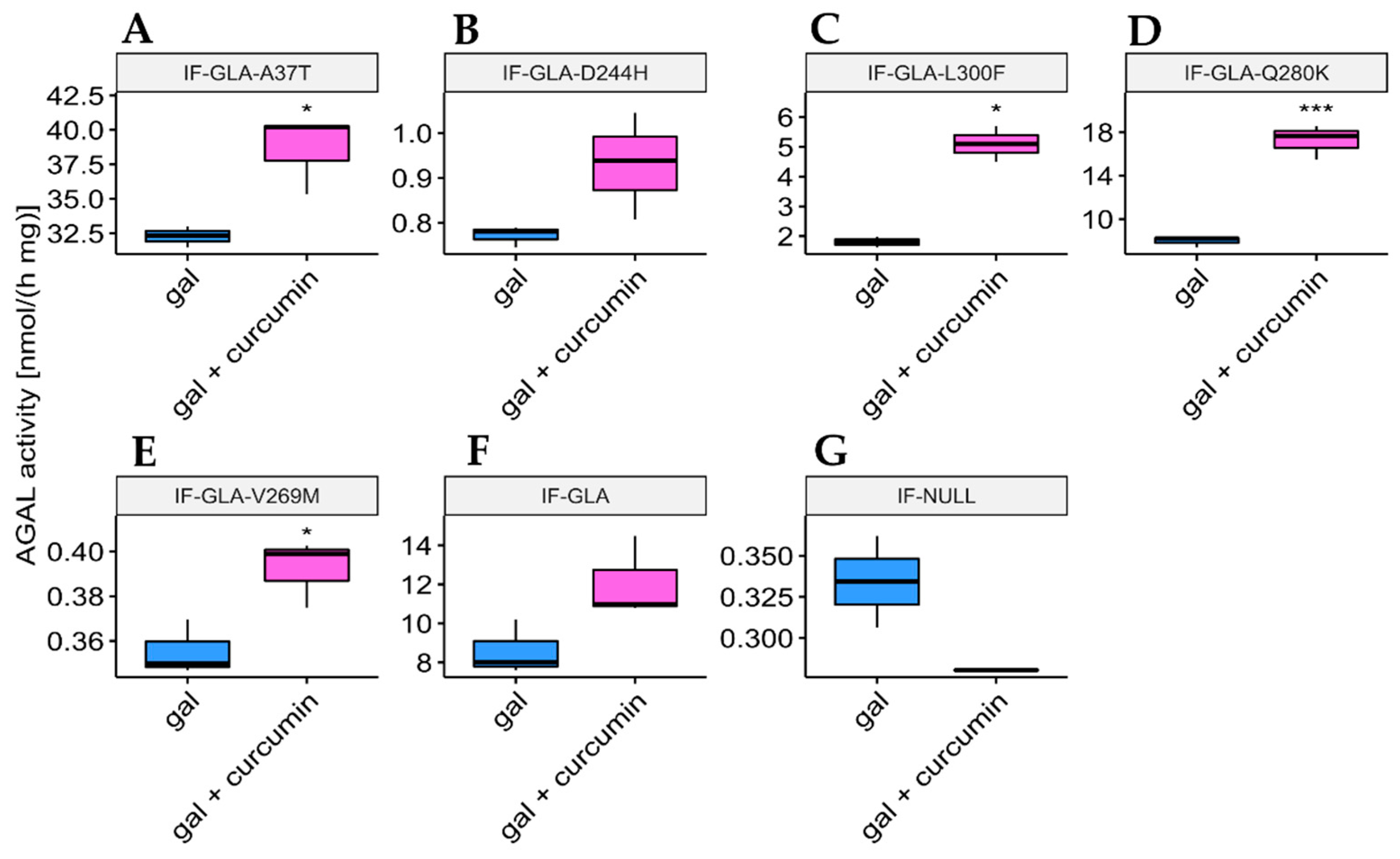
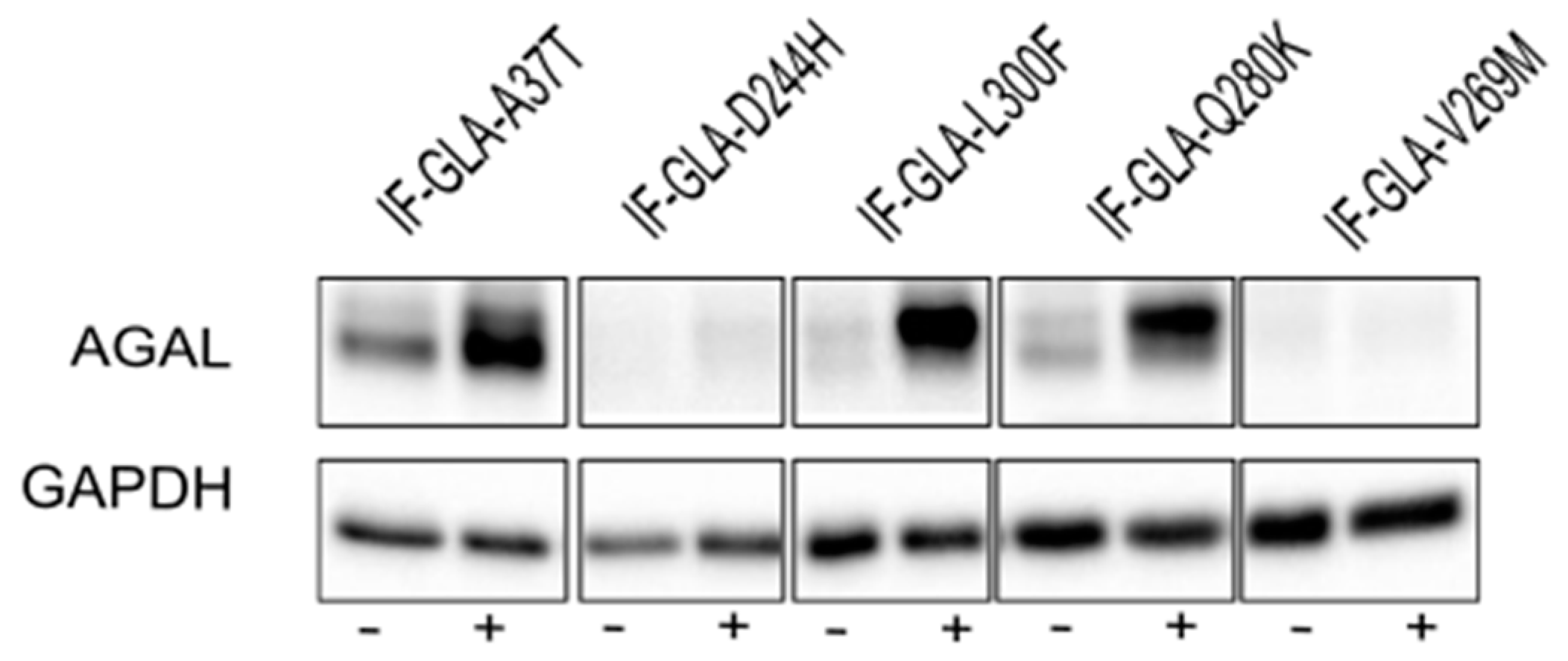
In this study, we tested the effect of curcumin treatment on AGAL activity over a panel of five mutants; specifically: c.109G>A (p.Ala37Thr, A37T), c.730G>C (p.Asp244His, D244H), c.898C>T (p.Leu300Phe, L300F), c.838C>A (p.Gln280Lys, Q280K), and c.805G>A (p.Val269Met, V269M). In particular, p.A37T causes the atypical renal-dominant phenotype [48], while p.D244H, p.Q280K, and p.V269M are related to the classic phenotype [49,50,51]; no information was given on the phenotype associated with p.L300F [52]. Immortalized fibroblasts transfected with individual pCMV6-AC plasmids carrying the five GLA mutants (IF-GLA-MUTs), immortalized fibroblasts transfected with the pCMV6-AC plasmid carrying wt-GLA (IF-GLA), and immortalized fibroblasts transfected with the empty vector (IF-NULL) obtained as described in [28] were treated with 20 μM curcumin or with no drug for 48 h. The time and dose of curcumin were selected based on the data available in literature, particularly those regarding cytotoxicity on fibroblasts [53,54,55]. After treatment, the AGAL-specific activity was tested on cell protein extracts. As shown in Figure 1, the wild type and all mutants except one (IF-GLA-V269M) showed a significant AGAL improvement upon curcumin treatment. An immunoblot reflected the enzyme activity results (Figure 2). The activity fold-increase varied between 1.4 (IF-GLA-D244H) and 2.2 (IF-GLA-L300F and IF-GLA-Q280K).
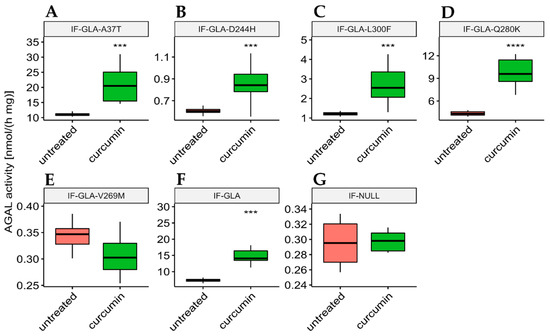
Figure 1.
Curcumin treatment enhanced AGAL activity. IF-GLA and IF-GLA-MUTs were treated with 20 µM curcumin for 48 h. The AGAL-specific activity was then tested on cell protein extracts. Wild-type cells and four mutants showed a significant AGAL improvement upon curcumin treatment (two-tailed unpaired t-test, *** = p < 1 × 10−3, **** = p < 1 × 10−4). (A) IF-GLA-A37T: n = 9, p. 1.85 × 10−4; (B) IF-GLA-D244H: n = 12, p. 1.02 × 10−4 (C) IF-GLA-L300F: n = 10, p. 1.43 × 10−4; (D) IF-GLA-Q280K: n = 6, p. 9.68 × 10−5; (E) IF-GLA-V269M: n = 6, p. 1.08 × 10−1 (F) IF-GLA: n = 7, p. 5.72 × 10−4. IF-NULL cells were used as a negative control (G).
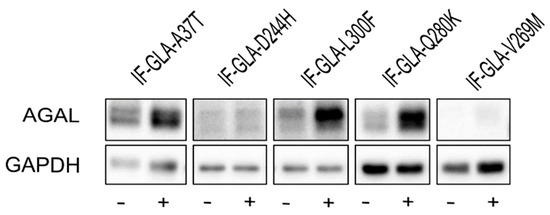
Figure 2.
Curcumin treatment increased AGAL. IF-GLA and IF-GLA-MUTs were treated with 20 µM curcumin for 48 h. AGAL was visualized via immunoblotting on cell protein extracts. The figure shows IF-GLA-MUTs treated with (+) or without (−) 20 µM curcumin.
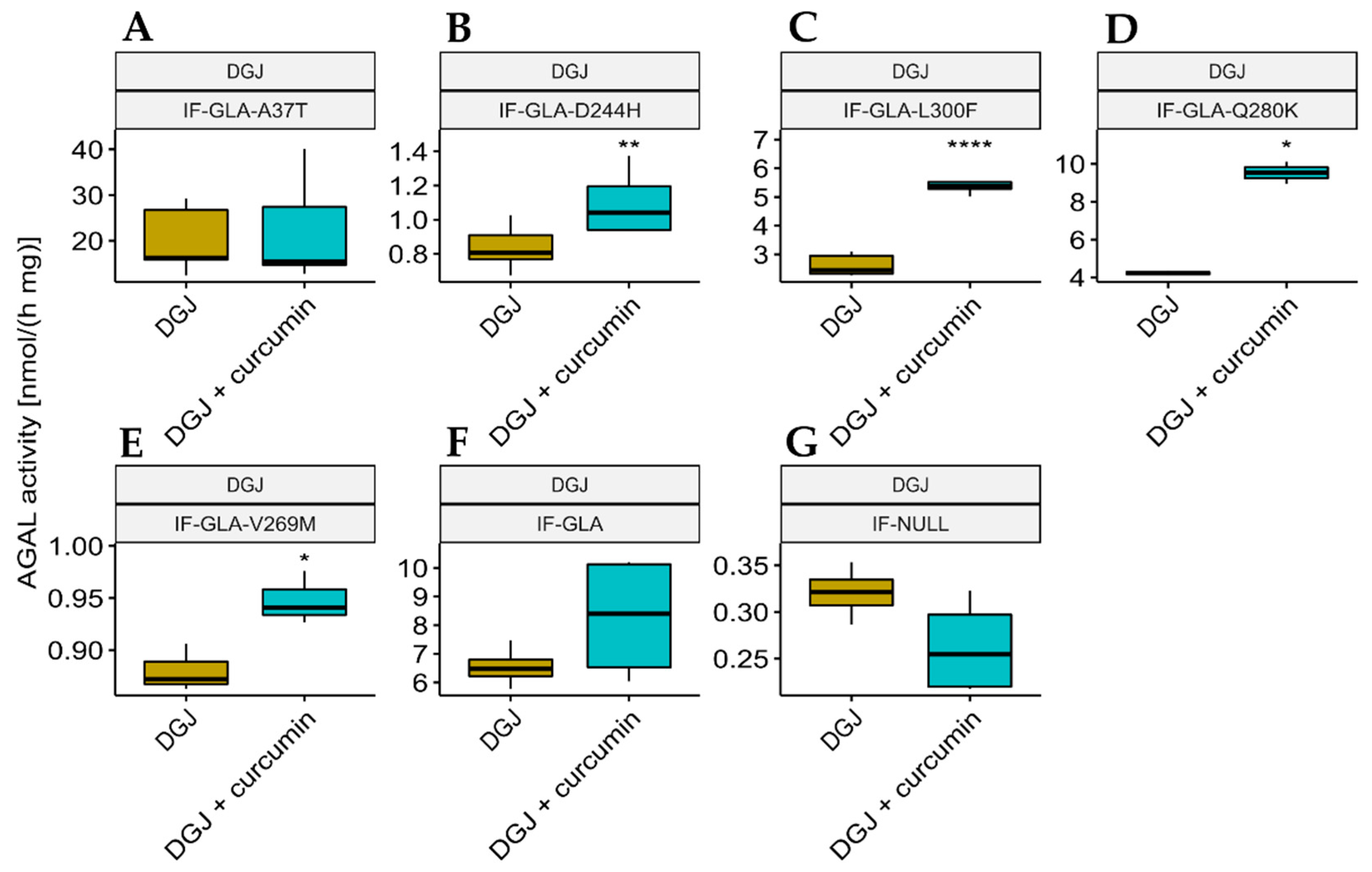
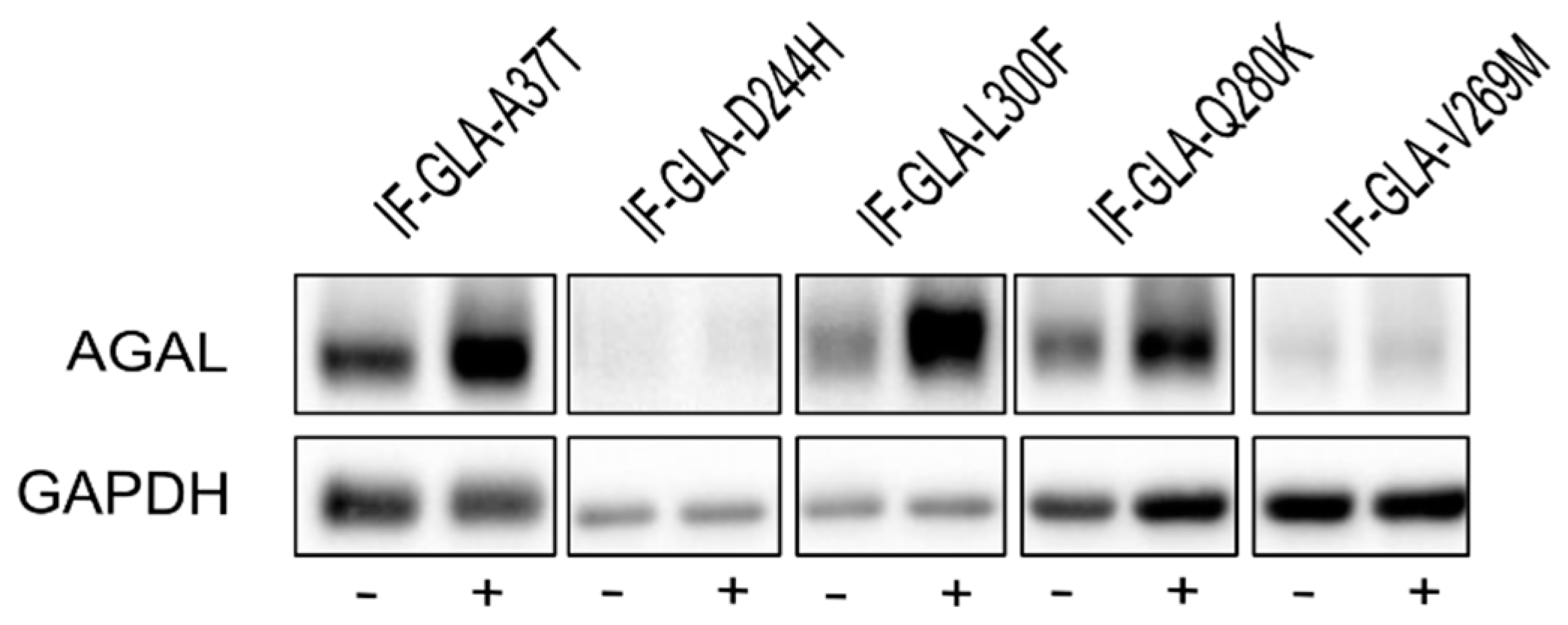
We also explored the potential of curcumin as a PC enhancer; i.e., a molecule able to strengthen the chaperoning effect of PCs. Cells were treated for 48 h with 10 μM DGJ either alone or in combination with 20 μM curcumin, and the protein extracts were analyzed. Figure 3 shows that the presence of curcumin improved AGAL stabilization induced by DGJ in four out of the five tested mutants (L300F, D244H, Q280K, and V269M) in a fold-change range of 1.1 (IF-GLA-V269M) to 2.3 (IF-GLA-L300F). As shown in Figure 4, immunoblots reflected the results.
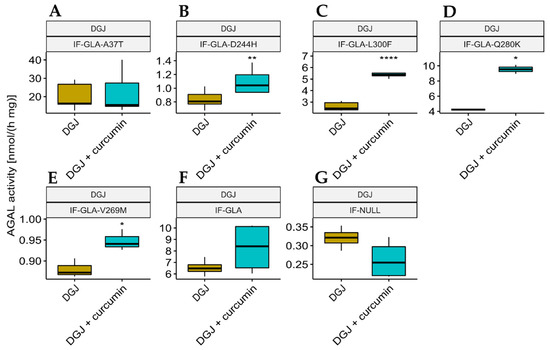
Figure 3.
Combined treatment with DGJ and curcumin enhanced AGAL activity. IF-GLA and IF-GLA-MUTs were treated for 48 h with 10 µM DGJ in the presence or absence of 20 µM curcumin. The AGAL-specific activity was then tested on cell protein extracts. The presence of curcumin improved AGAL stabilization induced by DGJ in four out of the five tested mutants (two-tailed unpaired t-test, * = p < 5 × 10−2, ** = p < 1 × 10−2, **** = p < 1 × 10−4). (A) IF-GLA-A37T: n = 5, p. 8.01 × 10−1; (B) IF-GLA-D244H: n = 9, p. 2.64 × 10−3; (C) IF-GLA-L300F: n = 6, p. 5.03 × 10−5; (D) IF-GLA-Q280K: n = 2, p. 1.22 × 10−2; (E) IF-GLA-V269M: n = 3, p. 2.66 × 10−2; (F) IF-GLA: n = 4, p. 1.89 × 10−1. IF-NULL cells were used as a negative control (G).
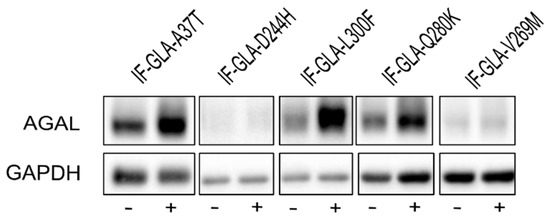
Figure 4.
Combined treatment with DGJ and curcumin treatment increased AGAL. IF-GLA and IF-GLA-MUTs were treated for 48 h with 10 µM DGJ in the presence or absence of 20 µM curcumin. AGAL was visualized via immunoblotting on cell protein extracts. The figure shows IF-GLA-MUTs treated with 10 µM DGJ in the presence (+) or the absence (−) of 20 µM curcumin.
In addition to DGJ, which is approved for therapeutical use, galactose is a low-affinity PC for AGAL, thus it requires a high dosage for patients to receive a beneficial effect. Investigation of the potential use of galactose supplementation showed very promising results for other rare diseases (mainly congenital disorders of glycosylation) [56,57,58,59,60]. Therefore, we tested whether the presence of curcumin could improve its stabilizing effect. We analyzed protein extracts derived from cells treated for 48 h with 100 mM galactose either alone or in combination with 20 μM curcumin. As Figure 5 shows, galactose potentiation benefited four out of the five tested mutants in a fold-change range of 1.1 (IF-GLA-V269M) to 2.8 (IF-GLA-L300F). Immunoblots reflecting the enzyme activity results are shown in Figure 6. Interestingly, different mutants showed different behaviors when moving to the combined therapies with PCs. For example, mutant IF-GLA-A37T did not show DGJ potentiation upon curcumin treatment (Figure 3). On the contrary, its activity improved upon combined treatment with galactose and curcumin with respect to galactose monotherapy (Figure 5). The opposite behavior was detected for IF-GLA-D244H, which was responsive to the combination of DGJ and curcumin but not to galactose and curcumin. These results were particularly interesting in a disease that requires personalized therapies.
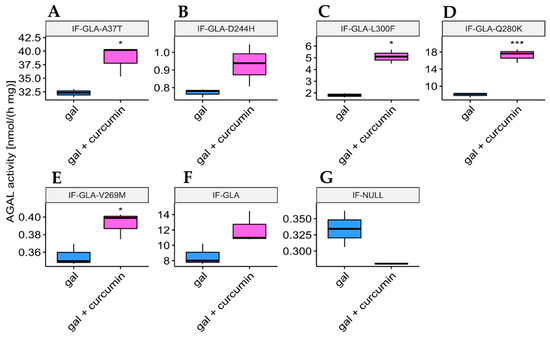
Figure 5.
AGAL activity increased upon combined treatment with galactose and curcumin. IF-GLA and IF-GLA-MUTs were treated for 48 h with 100 mM galactose in the presence or the absence of 20 µM curcumin. The AGAL-specific activity was then tested on cell protein extracts. The effect of galactose potentiation was appreciated in four out of the five tested mutants (two-tailed unpaired t-test, * = p < 5 × 10−2, *** = p < 1 × 10−3). (A) IF-GLA-A37T: n = 3, p. 2.06 × 10−2; (B) IF-GLA-D244H: n = 3, p. 8.61 × 10−2; (C) IF-GLA-L300F: n = 2, p. 3.41 × 10−2; (D) IF-GLA-Q280K: n = 3, p. 6.31 × 10−4; (E) IF-GLA-V269M: n = 3, p. 3.1 × 10−2; (F) IF-GLA: n = 3, p. 7.36 × 10−2. IF-NULL cells were used as a negative control (G).
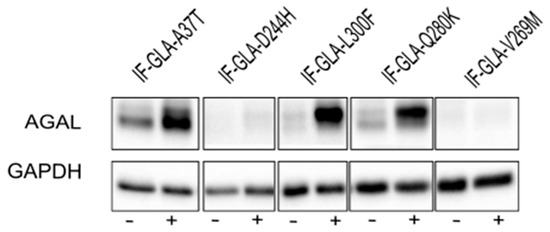
Figure 6.
Combined treatment with galactose and curcumin treatment increased AGAL. IF-GLA and IF-GLA-MUTs were treated for 48 h with 100 mM galactose in the presence or absence of 20 µM curcumin. AGAL was visualized via immunoblotting on cell protein extracts. The figure shows IF-GLA-MUTs treated with 100 mM galactose in the presence (+) or the absence (−) of 20 µM curcumin.
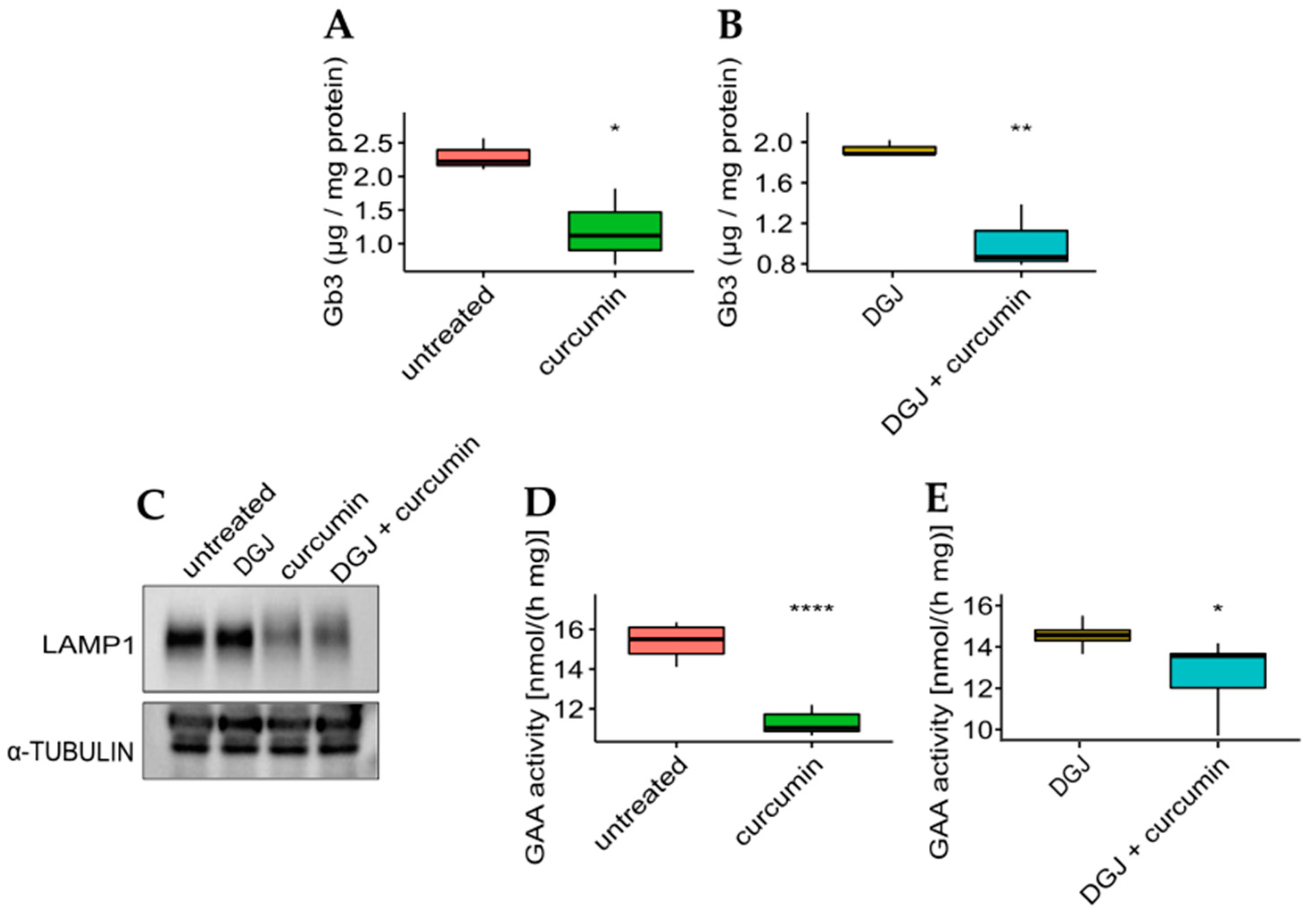
The effectiveness of curcumin was then evaluated concerning the phenotypic response to the monotherapy or combined therapy on the mutant with the highest fold-increase for all the treatments. The IF-GLA-L300F mutant was treated for up to 50 days with drug administration every seven days. At the end of the long-term treatments, cells were lysed, lipid extraction was accomplished following a described protocol, and Gb3 content was evaluated via LC-MS/MS [61,62]. As shown in Figure 7, Gb3 clearance was significantly improved upon curcumin treatment both in monotherapy (panel A) or combined therapy with DGJ (panel B). The Gb3 quantity in treated cells (Figure 7A,B) was comparable to that of IF-GLA (Supplementary Figure S1A).
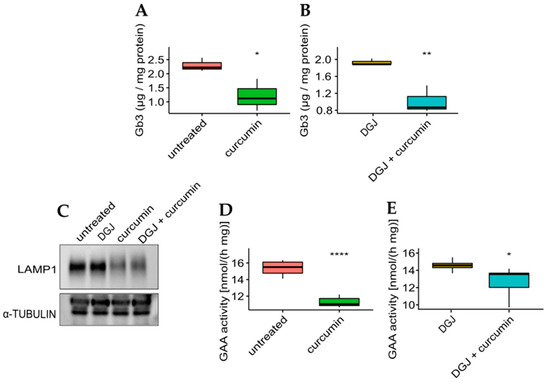
Figure 7.
Curcumin treatment improved Gb3 clearance and lysosomal markers. (A,B) IF-GLA-L300F cells were treated for 50 days with drug administration once a week. At the end of the treatment (day 50), cells were collected and lipids were extracted through a methanol:chloroform:water protocol. Lipid content was measured via LC-MS/MS. (A) IF-GLA-L300F treated with 20 µM curcumin or with no drug showed improved Gb3 clearance upon curcumin treatment (two-tailed unpaired t-test, * = p < 5 × 10−2, n = 3, p. 3.79 × 10−2). (B) IF-GLA-L300F treated with 10 µM DGJ and 20 µM curcumin showed Gb3 clearance improved with respect to DGJ monotherapy (two-tailed unpaired t-test; ** = p < 1 × 10−2, n = 3, p. 8.96 × 10−3). (C–E) IF-GLA-L300F cells were treated for 15 days with 10 µM DGJ, 20 µM curcumin, or with both drugs. At the end of the treatment, the immunoblot showed a reduction in the levels of lysosome-associated membrane glycoprotein 1 (LAMP-1) upon curcumin treatment with or without DGJ (C). In addition, a reduction in GAA activity was determined via an enzyme activity assay both in monotherapy (D) (two-tailed unpaired t-test, **** = p < 1 × 10−4, n = 6, p. 3.95 × 10−6) or in combined therapy with DGJ (E) (two-tailed unpaired t-test * = p < 5 × 10−2, n = 6, p. 3.3 × 10−2).
Jehn et al. [63] recently described alterations in the lysosomal pathway in the context of FD. In particular, they reported an increased expression in lysosomal hydrolases that was potentially due to Gb3 accumulation in lysosomes. Pereira et al. [64] described higher levels of lysosome-associated membrane protein 1 (LAMP-1) in FD lymphocytes compared to healthy controls, and we were able to confirm this upregulation in our FD cell model (Supplementary Figure S1B). The IF-NULL cells also showed higher levels of acidic α-glucosidase (GAA) than IF-GLA (Supplementary Figure S1C).
To evaluate the effect of curcumin treatment on potential lysosomal biomarkers, the IF-L300F cell line was treated with curcumin with or without DGJ. As shown in Figure 7, curcumin treatment resulted in a reduction in LAMP-1 levels (panel C), which had been previously described upon ERT treatment [64,65]. In addition, GAA activity is reduced upon curcumin treatment, in curcumin monotherapy (panel D), or in combined therapy (panel E). These results highlighted the beneficial effect of curcumin treatment on the FD cell model.
Curcumin has been described to disrupt Hsp90’s molecular function [66,67,68]. The inhibition mechanism is not fully understood; one hypothesis is the disruption of p210 bcr/abl with the Hsp90/p23 complex [66]. Sang et al. demonstrated the major role of Hsp90 in the curcumin-mediated protective effect in an Alzheimer’s disease cell model [69]. In particular, silencing Hsp90 significantly attenuated the rescuing effect of curcumin, while Hsp90 overexpression facilitated its effect. Jehn et al. previously demonstrated that AGAL rescue in an FD model had a prominent effect on Hsp90 expression, thus suggesting a role of the molecular chaperon in AGAL folding [63]. We hypothesized that Hsp90 has a prominent role in mediating curcumin effects in FD, thereby allowing AGAL precursor stabilization. Its action on the exosome/microvesicle secretion pathway has also been observed, which suggests its potential role in the treatments of LSDs [70]. Further studies will be needed both to explore the mechanism of action of curcumin in FD and to focus on a wider panel of mutations and precisely analyze their responsiveness to the mono- or combined therapies. We are aware that curcumin preparations might contain curcuminoid contaminants and would be better described as curcuminoid extracts.
FD patients show multiple clinical phenotypes. The classical form of FD usually presents symptoms such as neuropathic pain, cornea verticillata, and angiokeratoma, as well as long-term manifestations such as hypertrophic cardiomyopathy, cardiac rhythm disturbances, progressive renal failure, and strokes. In nonclassical FD, also known as late-onset or atypical FD, patients have residual enzyme activity and lower levels of the deacetylated substrate. This disease form is characterized by milder manifestations that affect just a single organ [71]. However, nonclassical FD patients may experience the same long-term effects as in the classical form [72].
The central role of dysregulated autophagy in LSDs was proposed many years ago [73], and its predominance in FD was recently highlighted. In fact, autophagy is among the mechanisms that underlie the FD phenotype together with overall lysosomal dysfunction, lipid dysmetabolism, and inflammation [74]. Tens of different biological effects of curcumin have been described over the years, and more applications are still being recorded yearly [22]. These mainly concern human health and mostly refer to the improvement of common pathological conditions that include both mild or severe conditions. Molecular mechanisms have already been studied [5,21]. It is worth noting that the beneficial effects of curcumin on cardiac and kidney function have been associated with the direct action of the molecule on autophagy and inflammatory pathways [75,76,77,78,79,80,81,82].
The beneficial effects of curcumin also have been described in cases of rare diseases such as Niemann–Pick type C disease (NPC) [17], neuronal ceroid lipofuscinosis (NCL) [83], and Tay–Sachs disease [18]. It is of utmost importance that for NPC, a triple combination therapy using miglustat, curcumin, and ibuprofen was investigated that resulted in a greater neuroprotective benefit compared with single and dual therapies [84].
Herein, we did demonstrate in an FD cell model that both curcumin or curcumin combined with DGJ produced an improvement in the phenotype of FD. Heart failure and renal involvement are significant issues for FD patients. Thus, the action of curcumin on different molecular mechanisms that leads to a general improvement might underlie its possible benefits in a large cohort of patients with different phenotypes.
These results represent a preliminary study, and further research will be needed to fully address the feasibility of moving from in vitro models to patients. Nevertheless, our results are promising because they open the field to using curcumin for both classic and non-classic FD phenotypes. Of course, the number of genotypes analyzed will need to be widened, and the effects of curcumin will have to be tested in different models (mainly patients’ fibroblasts or lymphoblasts carrying different mutations). In addition, the timing and dosage of curcumin will have to be defined.
3. Materials and Methods
3.1. Materials
The RPMI, fetal bovine serum, and reagents for cell cultures were purchased from Euroclone (Milan, Italy).
The curcumin was from BDH Chemicals Ltd., Poole, England (product number: 20031).
The DGJ was from Cayman Chemicals (Ann Arbor, Michigan, USA, product number: 17179). The cell lysis buffer (Roche product number: 4719956001), 4-methylumbelliferyl α-D-galactopyranoside (product number: M7633), 4-methylumbelliferone (product number: M1381), N-acetyl-D-galactosamine (product number: A2795), and lactosylsphingosine (product number: 42137) were purchased from Merck (Milan, Italy). The 4-methylumbelliferyl-α-D-glucopyranoside (product number: 69591) was from Biochemika (Merck, Milan, Italy). The Bradford dye (product number: 5000205), 30% acrylamide/bis soln. 37.5:1 (product number: 1610159), Clarity Western ECL (product number: 1705060), PVDF membranes (product number: 1620175), and Precision Plus Protein All Blue Standards (product number: 1610373) were purchased from Bio-Rad (Milan, Italy).
The Alpha-Galactosidase Polyclonal Antibody (product number: PA5-27349) and GAPDH Loading Control Monoclonal Antibody (product number: MA5-15738) were purchased from ThermoFisher Scientific (Milan, Italy). The lysosome-associated membrane glycoprotein 1 antibody (product number: H4A3, DHSB) was a kind gift from the Telethon Institute of Genetics and Medicine (TIGEM). All of the materials were used without further purification.
3.2. Cell Cultures
Immortalized patient-derived fibroblasts carrying a large deletion in GLA exons 3 and 4 were obtained from the Telethon Biobank and stably transfected as described in Monticelli et al. [62]. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 0.5 mg/mL penicillin, 0.5 mg/mL streptomycin, and non-essential amino acids at 37 °C in 5% humidified CO2. Geneticin 0.1 mg/mL was used to maintain the selection. Treatments with drugs were performed in the absence of geneticin. Drugs were dissolved in dimethyl sulfoxide, which represented the control for the untreated samples.
3.3. Enzymatic Activity Assays
Fibroblasts from a 90% confluent 20 cm2 plate were collected in Roche M cOmplete lysis buffer then centrifuged at 14,000× g for 10 min. An AGAL enzymatic activity assay was performed as described in [85] with the modifications described in Monticelli et al. [62]. Briefly, 40 µg of protein extracts were incubated with 0.4 mM 4-methylumbelliferyl-galactopyranoside and 8.7 mM N-acetylgalactosamine at 37 °C for 60 min in McIlvaine pH 4.4 buffer. A GAA enzymatic activity assay was performed similarly via incubation of a total of 20 µg of protein with 1.3 mM 4-methylumbelliferyl-α-D-glucopyranoside at 37 °C for 60 min in McIlvaine buffer pH 4.4. The reactions were stopped by the addition of GlyNaOH 1 M pH 10.5, and the fluorescence at 365/460 nm ex/em was read. 4-Methylumbelliferone was used for the calibration curves.
3.4. Gb3 Extraction
The extraction was accomplished according to the protocol outlined by Bligh and Dyer [61] with a few modifications as described in [62]. Briefly, cell pellets were lysed via resuspension in water and freezing–thawing, the soluble proteins were measured, and then lactosylsphingosine was added as an internal standard (2.5 ng of standard/µg of protein). Lipid extraction was performed with chloroform, methanol, water, and hydrochloric acid up to a final condition of (1:1:1:0.05) added in the following order: (i) chloroform/methanol (1:2); (ii) HCl; (iii) chloroform; (iv) water. Centrifuging (1500× g, 45 min at 20 °C) allowed us to obtain upper and lower phases. The samples were eventually dried under nitrogen and then analyzed via liquid chromatography–tandem mass spectrometry. For the UPLC-MS/MS analysis of Gb3, see Monticelli et al. [62].
3.5. Miscellaneous
The protein concentration was determined using the Bradford method with BSA as the standard [86]. Immunoblotting was performed under standard conditions as given in [40]. The data handling, analysis, and visualization were performed using the R environment for statistical computing (v4.2.1) with the tidyverse collection of packages (v1.3.1) [87,88] for the data handling and the rstatix (v0.7.0) [89] and ggpubr (v0.4.0) [90] packages for the statistical analysis and visualization, respectively. We performed unpaired two-tailed t-tests using the rstatix::t-test() function. All of the experiments were performed at least in biological duplicate; each biological duplicate was analyzed at least in technical duplicate. Biological replicates were considered in the statistical analysis.
4. Conclusions
This paper presented the results of a pilot study aimed at improving therapeutic approaches to FD. An AGAL increase in cells is highly beneficial for FD patients regardless of the mechanism leading to this effect. This is demonstrated in the approved therapies (ERT and PCT) [91] based on very different approaches but with the same outcome. We explored the possibility of obtaining this effect in an FD cell model upon curcumin treatment by analyzing the improvement in AGAL activity and the AGAL increase via immunoblotting.
The wide spectrum of biological activities makes curcumin a very unique and interesting molecule in the biomedical field [5]. For this reason, there is a large amount of literature in the field that will certainly benefit the Fabry community. In particular, data on the absorption, distribution, metabolism, and excretion (ADME) of curcumin have been collected over several decades that shows that curcumin bioavailability poses a severe limitation to its application for therapeutic purposes. To overcome this issue, different formulations to enhance curcumin bioavailability have already been widely investigated and clinical outcomes are also available. These research outputs include the combination of curcumin with adjuvants, the discovery of its structural analogues, its nanoformulations, and its inclusion in liposomes or phospholipid complexes [20,21,22]. A recent review on the topic that was published in 2021 highlighted the urgency of clinical trials to analyze the efficiency of nanoforms of curcumin as well as its derivative analogues [21]. Remarkably, one bio-enhanced derivative of curcumin was been successfully tested in a cell model of a rare variant form of Gaucher disease (GD) caused by mutations in the prosaposin gene (PSAP) [92].
Drug development and approval are multi-step processes that require significant investments and have poor chances of successful results [93]. These limitations imply a significant difficulty in improving new drugs for rare diseases. In this context, drug repositioning—using previously approved drugs for new therapeutic purposes—is a strategy for overcoming some problems related to de novo drug discovery. Repositioning reduces the time “between bench and bedside” while keeping research-related costs low. Most importantly, it reduces the risk of failure from more than 95% to around 45% [94,95,96,97,98,99]. Therefore, drug repurposing is a highly recommended practice in the scientific community, particularly for rare diseases [93].
The results described herein represent a case of nutraceutical repositioning for FD that pave the way to improving personalized therapies. We believe that this approach would be highly beneficial for patients, increase the advantages of PCT, and lead to the broadening of the target audience treatable with oral therapy.
Our proposal for FD is even more promising when considering that combined therapeutic approaches for rare diseases have been proposed [100,101,102,103,104] and that most importantly, one of them was recently approved by the FDA for the treatment of cystic fibrosis [105].
In conclusion, we believe that our results pave a new way toward the improvement of FD therapies, notwithstanding the limitations of our study and the further research needed for the translation to a medical approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24021095/s1.
Author Contributions
Conceptualization, M.M., B.H.M., M.A., M.V.C. and G.A.; validation, J.L., M.C.M., M.V.C. and G.A.; formal analysis, M.M. and B.H.M.; investigation, M.M., M.A., L.L. and E.M.; data curation, B.H.M.; writing—original draft preparation, M.M. and B.H.M.; writing—review and editing, J.L., M.V.C. and G.A.; visualization, M.M. and B.H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and supplementary material.
Acknowledgments
We thank the “Cell Line and DNA Biobank from Patients Affected by Genetic Diseases”, a member of the Telethon Network of Genetic Biobanks, which provided us with specimens (project no. GGP12108). We thank the M.A. program at the DISTABIF, Università degli Studi della Campania “Luigi Vanvitelli”, for the fellowship POR Campania FSE 2014/2020 “Dottorati di Ricerca Con Caratterizzazione Industriale”. We thank Ryan C. Vignogna from the Department of Molecular Biology and Genetics, Weill Institute for Cell and Molecular Biology, Cornell University, New York, NY, USA, for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AGAL: acidic alpha-galactosidase; CNS: central nervous system; DGJ: 1-deoxygalactonojirimycin; ER: endoplasmic reticulum; ERT: enzyme replacement therapy; FD: Fabry disease; FDA: Food and Drug Administration; GAA: acidic alpha-glucosidase; Gb3: globotriaosylceramide; IF-GLA: immortalized fibroblasts transfected with individual pCMV6-AC plasmid carrying wt-GLA; IF-GLA-MUTs: immortalized fibroblasts transfected with individual pCMV6-AC plasmids carrying GLA mutants; IF-NULL: immortalized fibroblasts transfected with the empty vector; LAMP-1: lysosomal-associated membrane protein 1; LSD: lysosomal storage disease; NPC: Niemann–Pick type C disease; PC: pharmacological chaperone; PCT: pharmacological chaperone therapy.
References
- Gautam, S.C.; Gao, X.; Dulchavsky, S. Immunomodulation by curcumin. Adv. Exp. Med. Biol. 2007, 595, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Han, R. Recent progress in the study of anticancer drugs originating from plants and traditional medicines in China. Chin. Med. Sci. J. 1994, 9, 61–69. [Google Scholar] [PubMed]
- Araújo, C.A.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Racz, L.Z.; Racz, C.P.; Pop, L.-C.; Tomoaia, G.; Mocanu, A.; Barbu, I.; Sárközi, M.; Roman, I.; Avram, A.; Tomoaia-Cotisel, M.; et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules 2022, 27, 6854. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.; Corno, C.; Colombo, D.; Perego, P. Curcumin and Related Compounds in Cancer Cells: New Avenues for Old Molecules. Front. Pharmacol. 2022, 13, 889816. [Google Scholar] [CrossRef]
- Surma, S.; Sahebkar, A.; Urbanski, J.; Penson, P.E.; Banach, M. Curcumin—The Nutraceutical with Pleiotropic Effects? Which Cardiometabolic Subjects Might Benefit the Most? Front. Nutr. 2022, 9, 865497. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guan, B.; Lin, L.; Wang, Y. Improvement of intestinal barrier function, gut microbiota, and metabolic endotoxemia in type 2 diabetes rats by curcumin. Bioengineered 2021, 12, 11947–11958. [Google Scholar] [CrossRef] [PubMed]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.J. Curcumin and autoimmune disease. Adv. Exp. Med. Biol. 2007, 595, 425–451. [Google Scholar] [CrossRef]
- Yang, M.; Akbar, U.; Mohan, C. Curcumin in autoimmune and rheumatic diseases. Nutrients 2019, 11, 1004. [Google Scholar] [CrossRef]
- García-Seisdedos, D.; Babiy, B.; Lerma, M.; Casado, M.E.; Martínez-Botas, J.; Lasunción, M.A.; Pastor, Ó.; Busto, R. Curcumin stimulates exosome/microvesicle release in an in vitro model of intracellular lipid accumulation by increasing ceramide synthesis. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158638. [Google Scholar] [CrossRef]
- Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Garanina, E.E.; Salafutdinov, I.I.; Laikov, A.V.; Kursenko, V.V.; Chakrabarti, L.; Zakharova, E.Y.; Bukina, T.M.; et al. Serum cytokine profile, beta-hexosaminidase a enzymatic activity and gm2 ganglioside levels in the plasma of a tay-sachs disease patient after cord blood cell transplantation and curcumin administration: A case report. Life 2021, 11, 1007. [Google Scholar] [CrossRef]
- Patel, S.S.; Acharya, A.; Ray, R.S.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical applications and bioavailability of curcumin—An updated overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Simonetta, I.; Riolo, R.; Todaro, F.; Di Chiara, T.; Miceli, S.; Pinto, A. Pathogenesis and molecular mechanisms of anderson–fabry disease and possible new molecular addressed therapeutic strategies. Int. J. Mol. Sci. 2021, 22, 10088. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Hughes, D.A.; Adam, M.P.; Everman, D.B.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.H.; Gripp, K.W.; Amemiya, A. (Eds.) Fabry Disease. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Bernardes, T.P.; Foresto, R.D.; Kirsztajn, G.M. Fabry disease: Genetics, pathology, and treatment. Rev. Assoc. Med. Bras. 2020, 66, s10–s16. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Adam, D.N. A Review of Fabry Disease. Skin Therapy Lett. 2018, 23, 4–6. [Google Scholar] [PubMed]
- Lemansky, P.; Bishop, D.F.; Desnick, R.J.; Hasilik, A.; von Figura, K. Synthesis and processing of α-galactosidase A in human fibroblasts. Evidence for different mutations in Fabry disease. J. Biol. Chem. 1987, 262, 2062–2065. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Michaud, M.; Mauhin, W.; Belmatoug, N.; Garnotel, R.; Bedreddine, N.; Catros, F.; Ancellin, S.; Lidove, O.; Gaches, F. When and How to Diagnose Fabry Disease in Clinical Pratice. Am. J. Med. Sci. 2020, 360, 641–649. [Google Scholar] [CrossRef]
- Cairns, T.; Müntze, J.; Gernert, J.; Spingler, L.; Nordbeck, P.; Wanner, C. Hot topics in Fabry disease. Postgrad. Med. J. 2018, 94, 709–713. [Google Scholar] [CrossRef]
- Citro, V.; Cammisa, M.; Liguori, L.; Cimmaruta, C.; Lukas, J.; Vittoria, M.; Andreotti, G. The large phenotypic spectrum of fabry disease requires graduated diagnosis and personalized therapy: A Meta-Analysis can help to differentiate missense mutations. Int. J. Mol. Sci. 2016, 17, 2010. [Google Scholar] [CrossRef] [PubMed]
- Lukas, J.; Cimmaruta, C.; Liguori, L.; Pantoom, S.; Iwanov, K.; Petters, J.; Hund, C.; Bunschkowski, M.; Hermann, A.; Cubellis, M.V.; et al. Assessment of gene variant amenability for pharmacological chaperone therapy with 1-deoxygalactonojirimycin in fabry disease. Int. J. Mol. Sci. 2020, 21, 956. [Google Scholar] [CrossRef] [PubMed]
- Galafold Amenability Table. Available online: https://galafoldamenabilitytable.com/reference (accessed on 15 November 2022).
- Lenders, M.; Brand, E. Fabry Disease: The Current Treatment Landscape. Drugs 2021, 81, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.R.; Della Valle, M.C.; Wu, X.; Katz, E.; Pruthi, F.; Bond, S.; Bronfin, B.; Williams, H.; Yu, J.; Bichet, D.G.; et al. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet. Med. 2017, 19, 430–438. [Google Scholar] [CrossRef]
- Andreotti, G.; Citro, V.; Correra, A.; Cubellis, M.V. A thermodynamic assay to test pharmacological chaperones for Fabry disease. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 1214. [Google Scholar] [CrossRef] [PubMed]
- Cammisa, M.; Correra, A.; Andreotti, G.; Cubellis, M.V. Fabry-CEP: A tool to identify Fabry mutations responsive to pharmacological chaperones. Orphanet J. Rare Dis. 2013, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Cimmaruta, C.; Liguori, L.; Monticelli, M.; Andreotti, G.; Citro, V. E-learning for rare diseases: An example using Fabry disease. Int. J. Mol. Sci. 2017, 18, 2049. [Google Scholar] [CrossRef] [PubMed]
- Therapeutics, A. Galafold. Available online: https://www.galafold.com/hcp/amenability (accessed on 10 November 2022).
- Citro, V.; Peña-García, J.; Den-Haan, H.; Pérez-Sánchez, H.; Del Prete, R.; Liguori, L.; Cimmaruta, C.; Lukas, J.; Cubellis, M.V.; Andreotti, G. Identification of an allosteric binding site on human lysosomal alpha-galactosidase opens the way to new pharmacological chaperones for Fabry disease. PLoS ONE 2016, 11, e0165463. [Google Scholar] [CrossRef]
- Nowak, A.; Huynh-Do, U.; Krayenbuehl, P.A.; Beuschlein, F.; Schiffmann, R.; Barbey, F. Fabry disease genotype, phenotype, and migalastat amenability: Insights from a national cohort. J. Inherit. Metab. Dis. 2020, 43, 326–333. [Google Scholar] [CrossRef]
- McCafferty, E.H.; Scott, L.J. Migalastat: A Review in Fabry Disease. Drugs 2019, 79, 543–554. [Google Scholar] [CrossRef]
- Liguori, L.; Monticelli, M.; Allocca, M.; Mele, B.H.; Lukas, J.; Cubellis, M.V.; Andreotti, G. Pharmacological chaperones: A therapeutic approach for diseases caused by destabilizing missense mutations. Int. J. Mol. Sci. 2020, 21, 489. [Google Scholar] [CrossRef]
- Andreotti, G.; Monticelli, M.; Cubellis, M.V. Looking for protein stabilizing drugs with thermal shift assay. Drug Test. Anal. 2015, 7, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Pharmacoeconomic Review Report: Migalastat (Galafold): (Amicus Therapeutics): Indication: Fabry Disease; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018.
- Benjamin, E.R.; Flanagan, J.J.; Schilling, A.; Chang, H.H.; Agarwal, L.; Katz, E.; Wu, X.; Pine, C.; Wustman, B.; Desnick, R.J.; et al. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J. Inherit. Metab. Dis. 2009, 32, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Waldek, S.; Germain, D.P.; Nicholls, K.; Bichet, D.G.; Simosky, J.K.; Bragat, A.C.; Castelli, J.P.; Benjamin, E.R.; Boudes, P.F. A Phase 2 study of migalastat hydrochloride in females with Fabry disease: Selection of population, safety and pharmacodynamic effects. Mol. Genet. Metab. 2013, 109, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ouyang, Y.; Wang, Z.; Ren, H.; Shen, P.; Wang, W.; Xu, Y.; Ni, L.; Yu, X.; Chen, X.; et al. Genotype: A crucial but not unique factor affecting the clinical phenotypes in Fabry disease. PLoS ONE 2016, 11, e0161330. [Google Scholar] [CrossRef]
- Topaloglu, A.K.; Ashley, G.A.; Tong, B.; Shabbeer, J.; Astrin, K.H.; Eng, C.M.; Desnick, R.J. Twenty novel mutations in the alpha-galactosidase A gene causing Fabry disease. Mol. Med. 1999, 5, 806–811. [Google Scholar] [CrossRef]
- Dobrovolny, R.; Dvorakova, L.; Ledvinova, J.; Magage, S.; Bultas, J.; Lubanda, J.C.; Elleder, M.; Karetova, D.; Pavlikova, M.; Hrebicek, M. Relationship between X-inactivation and clinical involvement in Fabry heterozygotes. Eleven novel mutations in the α-galactosidase a gene in the Czech and Slovak population. J. Mol. Med. 2005, 83, 647–654. [Google Scholar] [CrossRef]
- Shabbeer, J.; Yasuda, M.; Benson, S.D.; Desnick, R.J. Fabry disease: Identification of 50 novel α-galactosidase A mutations causing the classic phenotype and three-dimensional structural analysis of 29 missense mutations. Hum. Genomics 2006, 2, 297–309. [Google Scholar] [CrossRef]
- Shabbeer, J.; Robinson, M.; Desnick, R.J. Detection of α-galactosidase A mutations causing fabry disease by denaturing high performance liquid chromatography. Hum. Mutat. 2005, 25, 299–305. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.; Lee, E.J.; Yoon, J.S. Therapeutic effect of curcumin, a plant polyphenol extracted from Curcuma longae, in fibroblasts from patients with graves’ orbitopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4129–4140. [Google Scholar] [CrossRef]
- Rodriguez, L.R.; Bui, S.N.; Beuschel, R.T.; Ellis, E.; Liberti, E.M.; Chhina, M.K.; Cannon, B.; Lemma, M.; Nathan, S.D.; Grant, G.M. Curcumin induced oxidative stress attenuation by N-acetylcysteine co-treatment: A fibroblast and epithelial cell in-vitro study in idiopathic pulmonary fibrosis. Mol. Med. 2019, 25, 27. [Google Scholar] [CrossRef]
- Saidi, A.; Kasabova, M.; Vanderlynden, L.; Wartenberg, M.; Kara-Ali, G.H.; Marc, D.; Lecaille, F.; Lalmanach, G. Curcumin inhibits the TGF-β1-dependent differentiation of lung fibroblasts via PPARγ-driven upregulation of cathepsins B and L. Sci. Rep. 2019, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Morelle, W.; Potelle, S.; Witters, P.; Wong, S.; Climer, L.; Lupashin, V.; Matthijs, G.; Gadomski, T.; Jaeken, J.; Cassiman, D.; et al. Galactose supplementation in patients with TMEM165-CDG rescues the glycosylation defects. J. Clin. Endocrinol. Metab. 2017, 102, 1375–1386. [Google Scholar] [CrossRef]
- Park, J.H.; Hogrebe, M.; Grüneberg, M.; Duchesne, I.; Von Der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Morava, E. Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol. Genet. Metab. 2014, 112, 275–279. [Google Scholar] [CrossRef]
- Witters, P.; Cassiman, D.; Morava, E. Nutritional therapies in congenital disorders of glycosylation (CDG). Nutrients 2017, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Witters, P.; Tahata, S.; Barone, R.; Õunap, K.; Salvarinova, R.; Grønborg, S.; Hoganson, G.; Scaglia, F.; Lewis, A.M.; Mori, M.; et al. Clinical and biochemical improvement with galactose supplementation in SLC35A2-CDG. Genet. Med. 2020, 22, 1102–1107. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Monticelli, M.; Liguori, L.; Allocca, M.; Bosso, A.; Andreotti, G.; Lukas, J.; Monti, M.C.; Morretta, E.; Cubellis, M.V.; Hay Mele, B. Drug Repositioning for Fabry Disease: Acetylsalicylic Acid Potentiates the Stabilization of Lysosomal Alpha-Galactosidase by Pharmacological Chaperones. Int. J. Mol. Sci. 2022, 23, 5105. [Google Scholar] [CrossRef]
- Jehn, U.; Bayraktar, S.; Pollmann, S.; Van Marck, V.; Weide, T.; Pavenstädt, H.; Brand, E.; Lenders, M. α-Galactosidase a Deficiency in Fabry Disease Leads to Extensive Dysregulated Cellular Signaling Pathways in Human Podocytes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Pereira, E.M.; do Monte, S.J.H.; do Nascimento, F.F.; de Castro, J.A.F.; Sousa, J.L.M.; Filho, H.C.S.A.L.C.; da Silva, R.N.; Labilloy, A.; Monte Neto, J.T.; da Silva, A.S. Lysosome-associated protein 1 (LAMP-1) and Lysosome-associated protein 2 (LAMP-2) in a larger family carrier of Fabry disease. Gene 2014, 536, 118–122. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. The cardiovascular phenotype in fabry disease: New findings in the research field. Int. J. Mol. Sci. 2021, 22, 1331. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Xu, J.H.; Huang, X.W.; Zhang, K.Z.; Wen, C.X.; Chen, Y.Z. Down-regulation of p210bcr/abl by curcumin involves disrupting molecular chaperone functions of Hsp90. Acta Pharmacol. Sin. 2006, 27, 694–699. [Google Scholar] [CrossRef]
- Giommarelli, C.; Zuco, V.; Favini, E.; Pisano, C.; Dal Piaz, F.; De Tommasi, N.; Zunino, F. The enhancement of antiproliferative and proapoptotic activity of HDAC inhibitors by curcumin is mediated by Hsp90 inhibition. Cell. Mol. Life Sci. 2010, 67, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Gong, L.; Wang, Z.; Han, F.; Liu, H.; Lu, X.; Liu, L. Curcumin inhibits human cytomegalovirus by downregulating heat shock protein 90. Mol. Med. Rep. 2015, 12, 4789–4793. [Google Scholar] [CrossRef]
- Sang, Q.; Liu, X.; Wang, L.; Qi, L.; Sun, W.; Wang, W.; Sun, Y.; Zhang, H. Curcumin protects an SH-SY5Y cell model of Parkinson’s disease against toxic injury by regulating HSP90. Cell. Physiol. Biochem. 2018, 51, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Canfrán-Duque, A.; Pastor, Ó.; Quintana-Portillo, R.; Lerma, M.; de la Peña, G.; Martín-Hidalgo, A.; Fernández-Hernando, C.; Lasunción, M.A.; Busto, R. Curcumin promotes exosomes/microvesicles secretion that attenuates lysosomal cholesterol traffic impairment. Mol. Nutr. Food Res. 2014, 58, 687–697. [Google Scholar] [CrossRef]
- Smid, B.E.; Van der Tol, L.; Biegstraaten, M.; Linthorst, G.E.; Hollak, C.E.M.; Poorthuis, B.J.H.M. Plasma globotriaosylsphingosine in relation to phenotypes of fabry disease. J. Med. Genet. 2015, 52, 262–268. [Google Scholar] [CrossRef]
- Arends, M.; Wanner, C.; Hughes, D.; Mehta, A.; Oder, D.; Watkinson, O.T.; Elliott, P.M.; Linthorst, G.E.; Wijburg, F.A.; Biegstraaten, M.; et al. Characterization of classical and nonclassical fabry disease: A multicenter study. J. Am. Soc. Nephrol. 2017, 28, 1631–1641. [Google Scholar] [CrossRef]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta-Mol. Cell Res. 2009, 1793, 684–696. [Google Scholar] [CrossRef]
- Li, X.; Ren, X.; Zhang, Y.; Ding, L.; Huo, M.; Li, Q. Fabry disease: Mechanism and therapeutics strategies. Front. Pharmacol. 2022, 13, 1025740. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.B.; Yang, J.; Wang, J.R.; Liu, J.X.; Li, C.L. Curcumin alleviates isoproterenol-induced cardiac hypertrophy and fibrosis through inhibition of autophagy and activation of mTOR. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7500–7508. [Google Scholar] [CrossRef]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Yao, Q.; Ke, Z.Q.; Guo, S.; Yang, X.S.; Zhang, F.X.; Liu, X.F.; Chen, X.; Chen, H.G.; Ke, H.Y.; Liu, C. Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J. Mol. Cell. Cardiol. 2018, 124, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Di Tu, Q.; Jin, J.; Hu, X.; Ren, Y.; Zhao, L.; He, Q. Curcumin Improves the Renal Autophagy in Rat Experimental Membranous Nephropathy via Regulating the PI3K/AKT/mTOR and Nrf2/HO-1 Signaling Pathways. Biomed Res. Int. 2020, 2020, 7069052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Li, B.; Zhang, B.; Du, Y.; Liu, Y.; He, Y.; Chen, X. Curcumin attenuates renal interstitial fibrosis of obstructive nephropathy by suppressing epithelial-mesenchymal transition through inhibition of the TLR4/NF-кB and PI3K/AKT signalling pathways. Pharm. Biol. 2020, 58, 828–837. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin Ameliorates Kidney Function and Oxidative Stress in Experimental Chronic Kidney Disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, L.; Li, W.; Liang, Q.; Li, Z. Curcumin induces apoptosis and autophagy inhuman renal cell carcinoma cells via Akt/mTOR suppression. Bioengineered 2021, 12, 5017–5027. [Google Scholar] [CrossRef]
- Mirza, M.; Volz, C.; Karlstetter, M.; Langiu, M.; Somogyi, A.; Ruonala, M.O.; Tamm, E.R.; Jägle, H.; Langmann, T. Progressive Retinal Degeneration and Glial Activation in the CLN6nclf Mouse Model of Neuronal Ceroid Lipofuscinosis: A Beneficial Effect of DHA and Curcumin Supplementation. PLoS ONE 2013, 8, e0075963. [Google Scholar] [CrossRef]
- Williams, I.M.; Wallom, K.L.; Smith, D.A.; Al Eisa, N.; Smith, C.; Platt, F.M. Improved neuroprotection using miglustat, curcumin and ibuprofen as a triple combination therapy in Niemann-Pick disease type C1 mice. Neurobiol. Dis. 2014, 67, 9–17. [Google Scholar] [CrossRef]
- Andreotti, G.; Citro, V.; De Crescenzo, A.; Orlando, P.; Cammisa, M.; Correra, A.; Cubellis, M.V. Therapy of Fabry disease with pharmacological chaperones: From in silico predictions to in vitro tests. Orphanet J. Rare Dis. 2011, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http//www.R-project.org (accessed on 1 October 2022).
- Kassambara, A. Rstatix:Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 1 October 2022).
- Kassambara, A. Ggpubr: “Ggplot2” Based Publication Ready Plots. R Package Version 0.2. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 1 October 2022).
- Felis, A.; Whitlow, M.; Kraus, A.; Warnock, D.G.; Wallace, E. Current and Investigational Therapeutics for Fabry Disease. Kidney Int. Reports 2020, 5, 407–413. [Google Scholar] [CrossRef]
- Tatti, M.; Motta, M.; Scarpa, S.; Di Bartolomeo, S.D.; Cianfanelli, V.; Tartaglia, M.; Salvioli, R. BCM-95 and (2-hydroxypropyl)-β-cyclodextrin reverse autophagy dysfunction and deplete stored lipids in Sap C-deficient fibroblasts. Hum. Mol. Genet. 2015, 24, 4198–4211. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2018, 175, 168–180. [Google Scholar] [CrossRef]
- Nosengo, N. Can you teach old drugs new tricks? Nature 2016, 534, 314–316. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA-J. Am. Med. Assoc. 2020, 323, 844–853. [Google Scholar] [CrossRef]
- Brasil, S.; Pascoal, C.; Francisco, R.; Marques-da-Silva, D.; Andreotti, G.; Videira, P.A.; Morava, E.; Jaeken, J.; Dos Reis Ferreira, V. CDG therapies: From bench to bedside. Int. J. Mol. Sci. 2018, 19, 1304. [Google Scholar] [CrossRef] [PubMed]
- Hay Mele, B.; Citro, V.; Andreotti, G.; Cubellis, M.V. Drug repositioning can accelerate discovery of pharmacological chaperones. Orphanet J. Rare Dis. 2015, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Pantoom, S.; Hules, L.; Schöll, C.; Petrosyan, A.; Monticelli, M.; Pospech, J.; Cubellis, M.V.; Hermann, A.; Lukas, J. Mechanistic Insight into the Mode of Action of Acid β-Glucosidase Enhancer Ambroxol. Int. J. Mol. Sci. 2022, 23, 3536. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, C.; Witt, M.; Rolfs, A.; Antipova, V.; Wree, A. Gender-specific effects of two treatment strategies in a mouse model of niemann-pick disease type c1. Int. J. Mol. Sci. 2021, 22, 2539. [Google Scholar] [CrossRef] [PubMed]
- Jamalpoor, A.; Gelder, C.A.; Yousef Yengej, F.A.; Zaal, E.A.; Berlingerio, S.P.; Veys, K.R.; Pou Casellas, C.; Voskuil, K.; Essa, K.; Ammerlaan, C.M.; et al. Cysteamine–bicalutamide combination therapy corrects proximal tubule phenotype in cystinosis. EMBO Mol. Med. 2021, 13, e13067. [Google Scholar] [CrossRef]
- Guiraud, S.; Davies, K.E. Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr. Opin. Pharmacol. 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Tambuyzer, E.; Vandendriessche, B.; Austin, C.P.; Brooks, P.J.; Larsson, K.; Miller Needleman, K.I.; Valentine, J.; Davies, K.; Groft, S.C.; Preti, R.; et al. Therapies for rare diseases: Therapeutic modalities, progress and challenges ahead. Nat. Rev. Drug Discov. 2020, 19, 93–111. [Google Scholar] [CrossRef]
- Ridley, K.; Condren, M. Elexacaftor-tezacaftor-ivacaftor: The first triple-combination cystic fibrosis transmembrane conductance regulator modulating therapy. J. Pediatr. Pharmacol. Ther. 2020, 25, 192–197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).