Proto-Oncogene FAM50A Can Regulate the Immune Microenvironment and Development of Hepatocellular Carcinoma In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
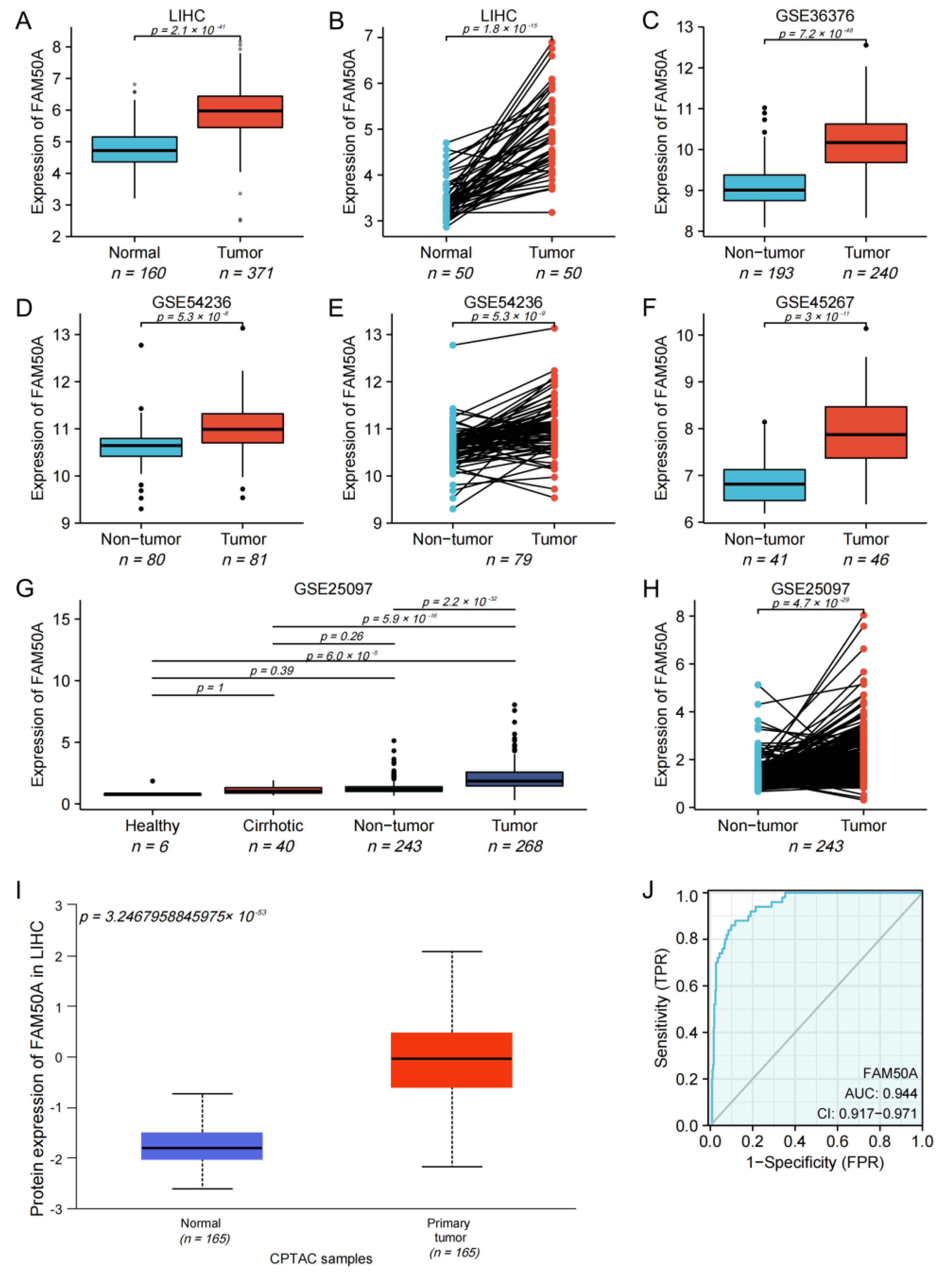
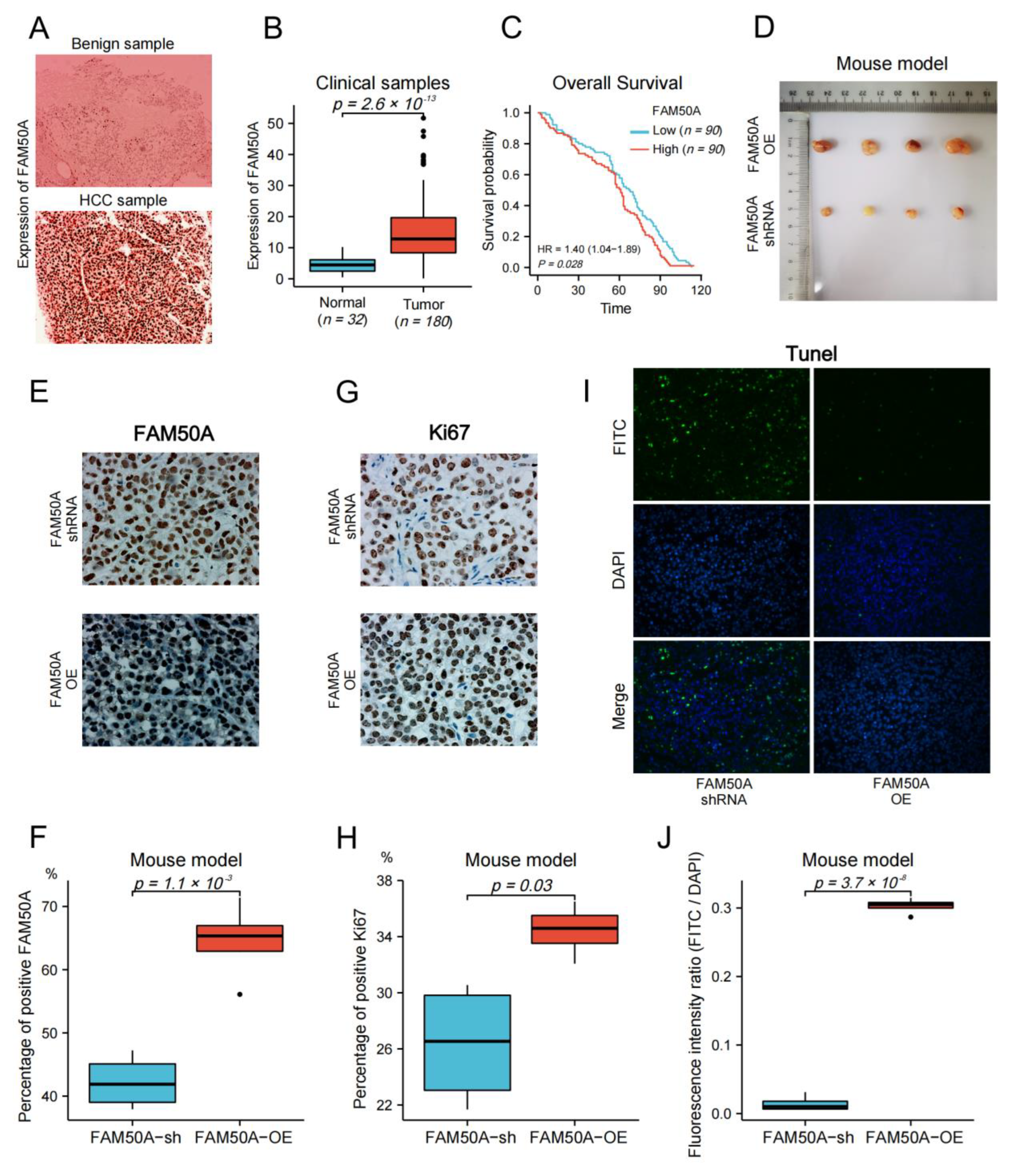
2.1. Expression of FAM50A in HCC
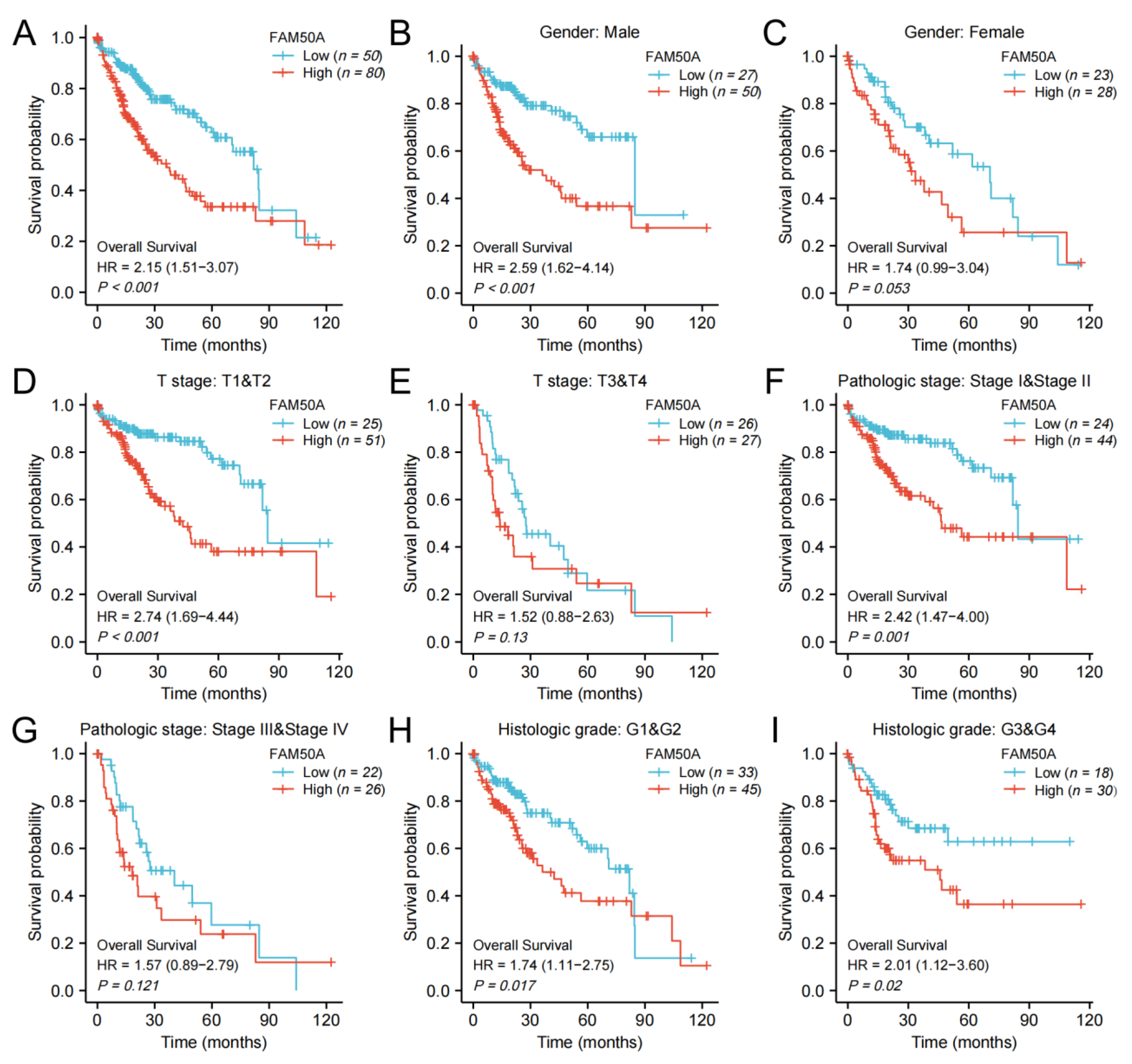
2.2. Prognostic Value of FAM50A in HCC
2.3. Effect of FAM50A on Survival of HCC Patients
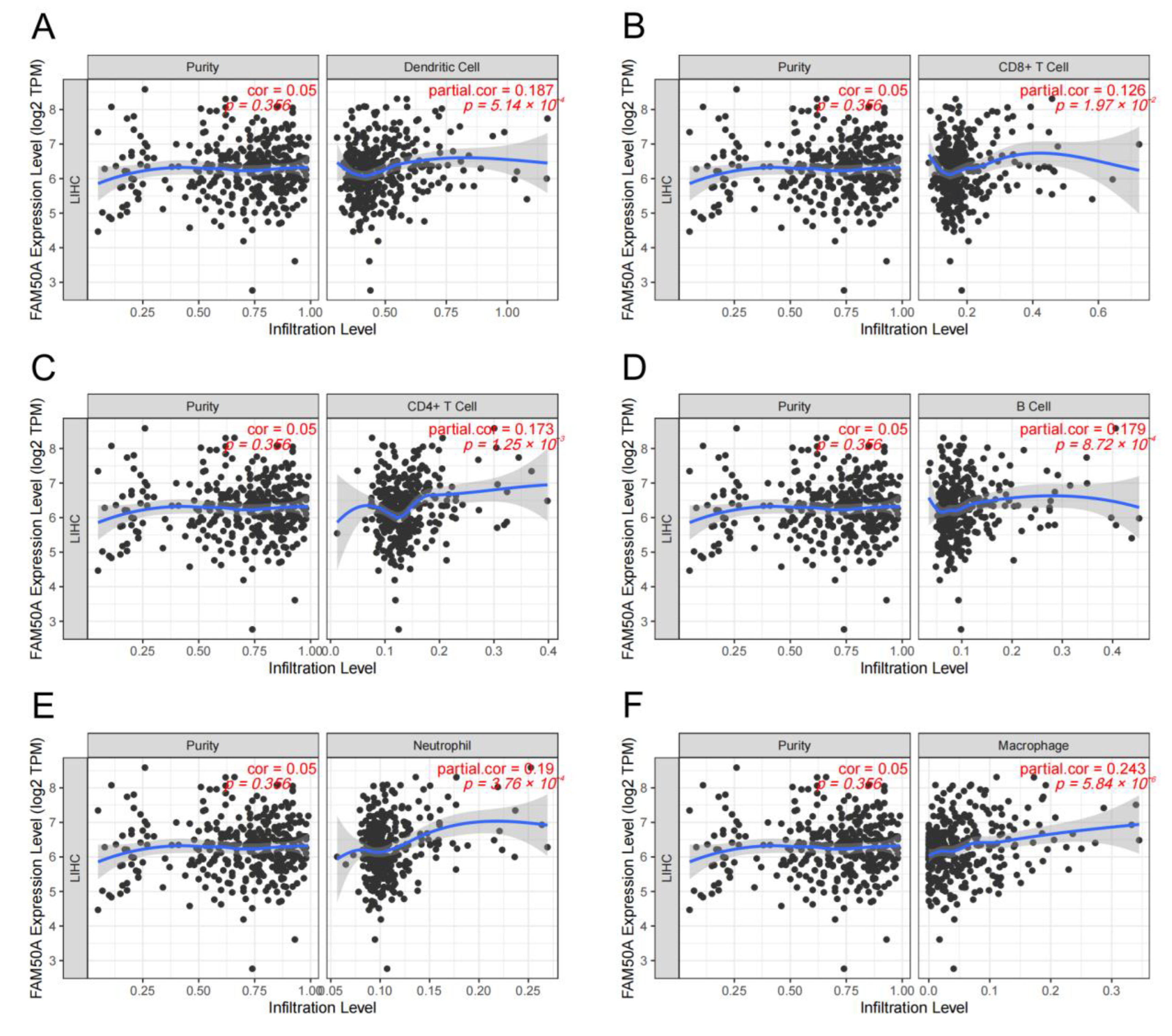
2.4. Relationship of FAM50A and Immune Cell Infiltration in HCC
2.5. Corrections between FAM50A and Stemness of HCC Cells
2.6. Correlation between FAM50A and Immunotherapy
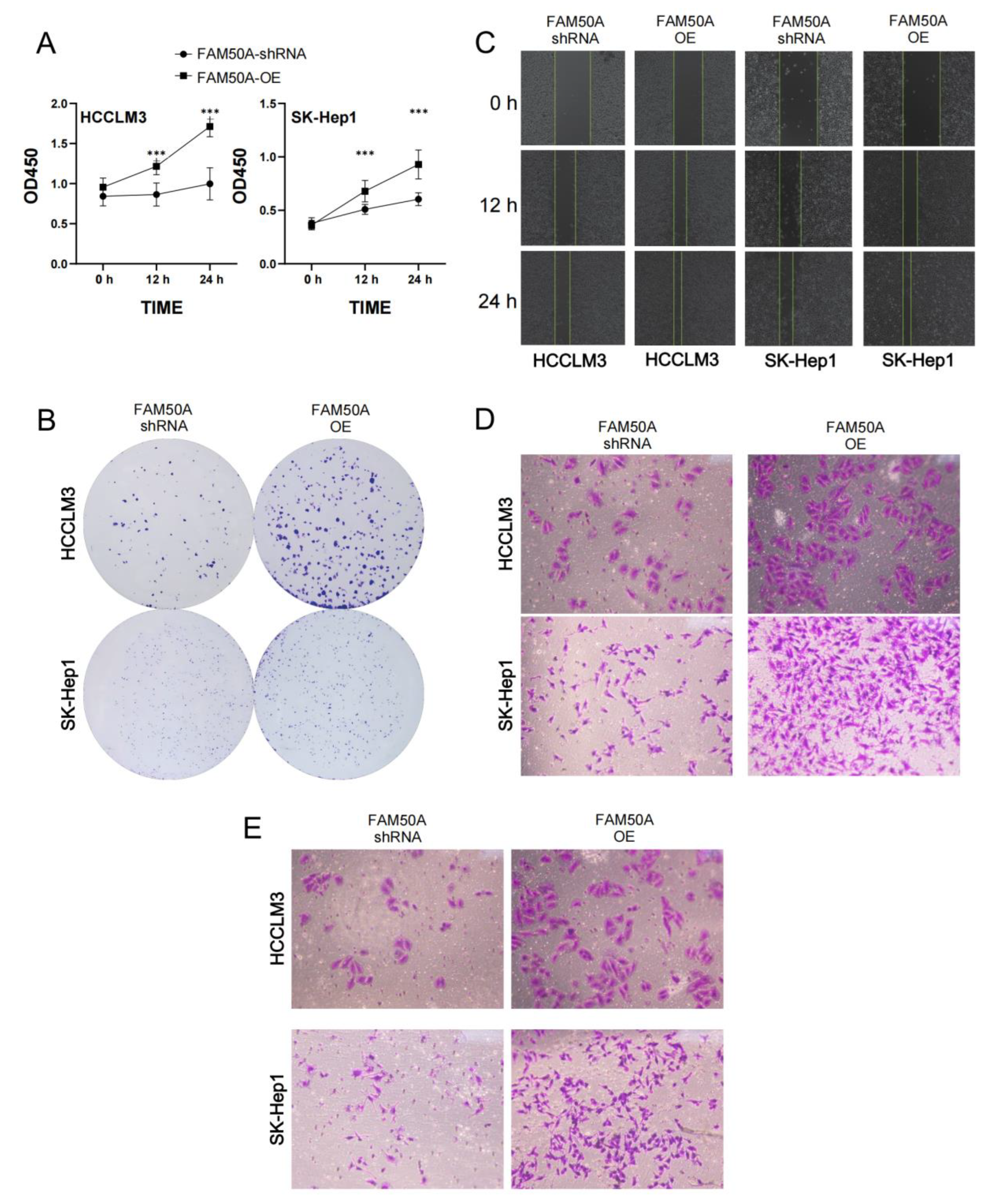
2.7. Effects of FAM50A on HCC In Vitro
2.7.1. Effects of FAM50A on Epithelial–Mesenchymal Transition (EMT)
2.7.2. FAM50A Regulates the Sensitivity of HCC Cells to Lenvatinib
2.7.3. Effect of FAM50A on Cell Cycle and Apoptosis of HCC
2.7.4. Effects of FAM50A on the Malignancy of HCC Cells
2.8. Effect of FAM50A on HCC In Vivo
3. Discussion
4. Materials and Methods
4.1. Data and Tissue Samples Collection
4.2. Cell Culture
4.3. Bioinformatics Analysis by R Software
4.4. Analysis of Immune Infiltration by TIMER
4.5. Plasmids, Lentiviruses, and Regulation of Gene Expression
4.6. RNA Extraction and qRT-PCR
4.7. Western Blot Analysis
4.8. Analysis of Cell Proliferation
4.9. Drug Sensitivity Test
4.10. Analysis of Cell Migration and Invasion
4.11. Flow Cytometric Analysis
4.12. Immunohistochemistry
4.13. TUNEL Staining
4.14. Mouse Model of Subcutaneous Xenograft Tumor
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| TIME | Tumor immune microenvironment |
| AFP | Alpha-fetoprotein |
| TME | Tumor microenvironment |
| PBMC | Peripheral Blood Mononuclear Cells |
| CLL | Chronic Lymphoblastic Leukemia |
| TCGA | The Cancer Genome Atlas |
| GEO | Gene Expression Overview |
| UALCAN | University of Alabama at Birmingham Cancer data analysis Portal |
| TIMER | Tumor Immune Estimation Resource |
| ROC | Receiver Operating Characteristic |
| AUROC | Area Under ROC |
| CI | Confidence Interval |
| OS | Overall Survival |
| DSS | Disease-Specific Survival |
| OCLR | One-class logistic regression |
| ICB | Immune Checkpoint Blockade |
| TIDE | Tumor Immune Dysfunction and Exclusion |
| shRNA | short hairpin RNA |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| EMT | Epithelial–Mesenchymal Transition |
| VEGFR | Vascular Endothelial Growth Factor Receptors |
| IC50 | Half-maximal inhibitory concentration |
| FCM | Flow cytometry |
| ECM | Extracellular matrix |
| BMM | Basement Membrane Matrix |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
| FITC | Fluorescein isothiocyanate |
| NAFLD | Nonalcoholic fatty liver disease |
| CPTAC | Clinical Proteomic Tumor Analysis Consortium |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal Bovine Serum |
| FPKM | Fragments Per Kilobase Million |
| TPM | Transcripts Per Million |
| PVDF | Polyvinylidene fluoride |
| SPF | specific-pathogen-free |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized Controlled Trial of Screening for Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, L.; Gao, X.; Hu, J.; Wang, J.; Dai, Z.; Wang, J.F.; Zhang, Z.; Lu, S.; Huang, X.; et al. Plasma Microrna Panel to Diagnose Hepatitis B Virus-Related Hepatocellular Carcinoma. J. Clin. Oncol. 2011, 29, 4781–4788. [Google Scholar] [CrossRef]
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechene, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. Galad Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735. [Google Scholar] [CrossRef]
- Xu, L.; Kim, Y.; Spolverato, G.; Gani, F.; Pawlik, T.M. Racial Disparities in Treatment and Survival of Patients with Hepatocellular Carcinoma in the United States. Hepatobil. Surg. Nutr. 2016, 5, 43–52. [Google Scholar] [CrossRef]
- Zhang, G.; Li, R.; Deng, Y.; Zhao, L. Conditional Survival of Patients with Hepatocellular Carcinoma: Results From the Surveillance, Epidemiology, and End Results Registry. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 515–523. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase Iii Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Qin, S.; Bi, F.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable Or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase Ii-Iii Trial. J. Clin. Oncol. 2021, 39, 3002–3011. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab Versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab Or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab Versus Chemotherapy for Pd-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. Fda Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable Or Metastatic Hepatocellular Carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab Plus a Bevacizumab Biosimilar (Ibi305) Versus Sorafenib in Unresectable Hepatocellular Carcinoma (Orient-32): A Randomised, Open-Label, Phase 2-3 Study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Rimassa, L.; Danesi, R.; Pressiani, T.; Merle, P. Management of Adverse Events Associated with Tyrosine Kinase Inhibitors: Improving Outcomes for Patients with Hepatocellular Carcinoma. Cancer Treat. Rev. 2019, 77, 20–28. [Google Scholar] [CrossRef]
- Xu, S.; Lai, R.; Zhao, Q.; Zhao, P.; Zhao, R.; Guo, Z. Correlation between Immune-Related Adverse Events and Prognosis in Hepatocellular Carcinoma Patients Treated with Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 794099. [Google Scholar] [CrossRef]
- Mou, L.; Tian, X.; Zhou, B.; Zhan, Y.; Chen, J.; Lu, Y.; Deng, J.; Deng, Y.; Wu, Z.; Li, Q.; et al. Improving Outcomes of Tyrosine Kinase Inhibitors in Hepatocellular Carcinoma: New Data and Ongoing Trials. Front. Oncol. 2021, 11, 752725. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhan, Y.; Xu, W.; Liu, X.; Geng, Y.; Liu, L.; Da, J.; Wang, J.; Zhang, X.; Jin, H.; et al. Prognostic and Immunological Role of Fam20C in Pan-Cancer. Biosci. Rep. 2021, 41, BSR20201920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Fu, L.; Li, D.; Dai, X.; Liu, L.; Zhang, J.; Zheng, L.; Cui, M. Identification of Mrnas Related to Endometrium Function Regulated by Lncrna Cd36-005 in Rat Endometrial Stromal Cells. Reprod. Biol. Endocrinol. 2018, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Kostianets, O.; Antoniuk, S.; Filonenko, V.; Kiyamova, R. Immunohistochemical Analysis of Medullary Breast Carcinoma Autoantigens in Different Histological Types of Breast Carcinomas. Diagn. Pathol. 2012, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.A.; Seymour, E.; Saiya-Cork, K.; Parkin, B.; Shedden, K.; Malek, S.N. A Quantitative Analysis of Subclonal and Clonal Gene Mutations Before and After Therapy in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2016, 22, 4525–4535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Shang, J.; He, H.; Yang, Q. Integrative Analysis Reveals the Prognostic Value and Functions of Splicing Factors Implicated in Hepatocellular Carcinoma. Sci. Rep. 2021, 11, 15175. [Google Scholar] [CrossRef]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International Trends in Hepatocellular Carcinoma Incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Dalekos, G.N.; Yurdaydin, C.; Buti, M.; Goulis, J.; Arends, P.; Sypsa, V.; Manolakopoulos, S.; Mangia, G.; Gatselis, N.; et al. Incidence and Predictors of Hepatocellular Carcinoma in Caucasian Chronic Hepatitis B Patients Receiving Entecavir Or Tenofovir. J. Hepatol. 2015, 62, 363–370. [Google Scholar] [CrossRef]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224 (Suppl. 2), S343–S351. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Feld, J.J.; Jacobson, I.M.; Hezode, C.; Asselah, T.; Ruane, P.J.; Gruener, N.; Abergel, A.; Mangia, A.; Lai, C.L.; Chan, H.L.; et al. Sofosbuvir and Velpatasvir for Hcv Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 2015, 373, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of Nafld and Nash: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global Epidemiology of Nafld-Related Hcc: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Jinjuvadia, R.; Patel, S.; Liangpunsakul, S. The Association between Metabolic Syndrome and Hepatocellular Carcinoma: Systemic Review and Meta-Analysis. J. Clin. Gastroenterol. 2014, 48, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef]
- Petrick, J.L.; Kelly, S.P.; Altekruse, S.F.; McGlynn, K.A.; Rosenberg, P.S. Future of Hepatocellular Carcinoma Incidence in the United States Forecast through 2030. J. Clin. Oncol. 2016, 34, 1787–1794. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Lan, J.; Cui, Y.; Rao, X.; Zhao, J.; Xing, T.; Ju, G.; Song, G.; Lou, J.; et al. Crispr Screens Uncover Protective Effect of Pstk as a Regulator of Chemotherapy-Induced Ferroptosis in Hepatocellular Carcinoma. Mol. Cancer 2022, 21, 11. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, H.; Xie, T.; Yang, X.; Pang, Y.; Ye, S. Elevated Expression of Pdzd11 is Associated with Poor Prognosis and Immune Infiltrates in Hepatocellular Carcinoma. Front. Genet. 2021, 12, 669928. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated Tcga Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416. [Google Scholar] [CrossRef]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kaminska, B.; Huelsken, J.; Omberg, L.; Gevaert, O.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354. [Google Scholar] [CrossRef]
- Jiang, P.; Gu, S.; Pan, D.; Fu, J.; Sahu, A.; Hu, X.; Li, Z.; Traugh, N.; Bu, X.; Li, B.; et al. Signatures of T Cell Dysfunction and Exclusion Predict Cancer Immunotherapy Response. Nat. Med. 2018, 24, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. Timer: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-Five Years of Quantitative Pcr for Gene Expression Analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | HR (95% CI) Univariate Analysis | p Value | HR (95% CI) Multivariate Analysis | p Value |
|---|---|---|---|---|
| Gender (Male vs. Female) | 0.793 (0.557–1.130) | 0.2 | ||
| Age (>60 vs. <=60) | 1.205 (0.850–1.708) | 0.295 | ||
| Pathologic stage (Stage vs. I~II) | 2.504 (1.727–3.631) | <0.001 | 1.469 (0.200–10.784) | 0.705 |
| Histologic grade (G3~4 vs. 1~2) | 1.091 (0.761–1.564) | 0.636 | ||
| T stage (T3~4 vs. 1~2) | 2.598 (1.826–3.697) | <0.001 | 1.643 (0.223–12.104) | 0.626 |
| N stage (N1 vs. N0) | 2.029 (0.497–8.281) | 0.324 | ||
| M stage (M1 vs. M0) | 4.077 (1.281–12.973) | 0.017 | 1.168 (0.277–4.926) | 0.832 |
| Adjacent hepatic tissue inflammation (Mild~Severe vs. None) | 1.194 (0.734–1.942) | 0.475 | ||
| Residual tumor (R1~2 vs. R0) | 1.604 (0.812–3.169) | 0.174 | ||
| Child–Pugh grade (B~C vs. A) | 1.643 (0.811–3.330) | 0.168 | ||
| Vascular invasion (Yes vs. No) | 1.344 (0.887–2.035) | 0.163 | ||
| Tumor status (With tumor vs. Tumor-free) | 2.317 (1.590–3.376) | <0.001 | 2.153 (1.348–3.437) | 0.001 |
| AFP (ng/mL) (>400 vs. ≤400) | 1.075 (0.658–1.759) | 0.772 | ||
| Albumin (g/dl) (≥3.5 vs. <3.5) | 0.897 (0.549–1.464) | 0.662 | ||
| Prothrombin time (>4 vs. ≤4) | 1.335 (0.881–2.023) | 0.174 | ||
| Fibrosis ishak score (3/4~5/6 vs. 0~1/2) | 0.740 (0.445–1.232) | 0.247 | ||
| FAM50A (High vs. Low) | 2.153 (1.508–3.072) | <0.001 | 2.272 (1.424–3.627) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Li, L.; Tao, S.; Chen, M.; Fei, L.; Yang, Q.; Huang, C.; Chen, L. Proto-Oncogene FAM50A Can Regulate the Immune Microenvironment and Development of Hepatocellular Carcinoma In Vitro and In Vivo. Int. J. Mol. Sci. 2023, 24, 3217. https://doi.org/10.3390/ijms24043217
Xie X, Li L, Tao S, Chen M, Fei L, Yang Q, Huang C, Chen L. Proto-Oncogene FAM50A Can Regulate the Immune Microenvironment and Development of Hepatocellular Carcinoma In Vitro and In Vivo. International Journal of Molecular Sciences. 2023; 24(4):3217. https://doi.org/10.3390/ijms24043217
Chicago/Turabian StyleXie, Xudong, Li Li, Shuai Tao, Mingsheng Chen, Ling Fei, Qunling Yang, Chenlu Huang, and Liang Chen. 2023. "Proto-Oncogene FAM50A Can Regulate the Immune Microenvironment and Development of Hepatocellular Carcinoma In Vitro and In Vivo" International Journal of Molecular Sciences 24, no. 4: 3217. https://doi.org/10.3390/ijms24043217
APA StyleXie, X., Li, L., Tao, S., Chen, M., Fei, L., Yang, Q., Huang, C., & Chen, L. (2023). Proto-Oncogene FAM50A Can Regulate the Immune Microenvironment and Development of Hepatocellular Carcinoma In Vitro and In Vivo. International Journal of Molecular Sciences, 24(4), 3217. https://doi.org/10.3390/ijms24043217






