Integrative Analysis of the Ethanol Tolerance of Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results
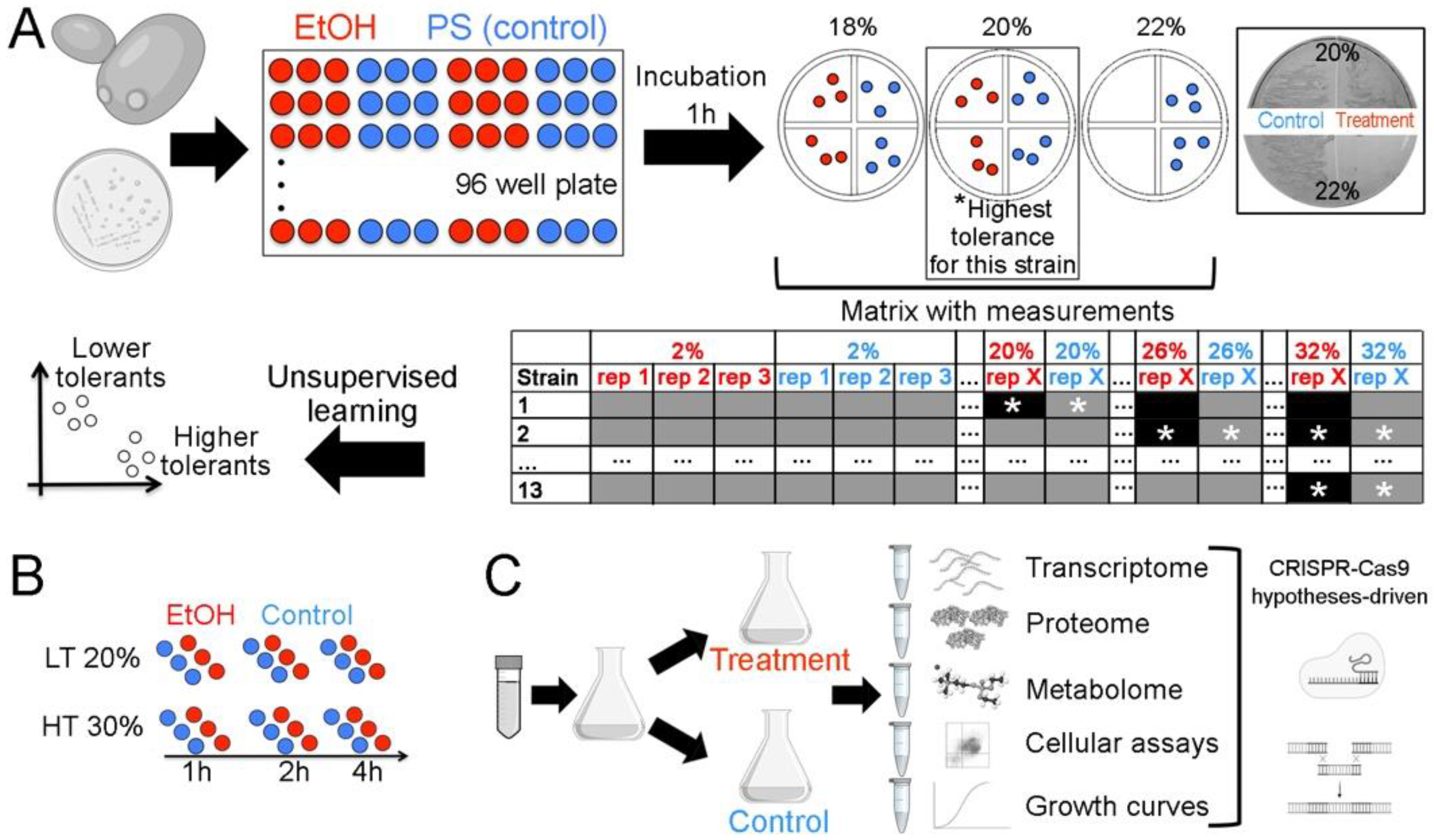
2.1. Rationale and Overview
2.2. Defining the Highest EtOH Tolerance for Each Strain
2.3. Gene, Protein and Metabolite Expression Analyses
2.4. LncRNA Assembly and General Functions of EtOH-Responsive lncRNAs
2.5. Life-Essential Pathways Affected by EtOH
2.6. Effect of EtOH on Degradation/Storage-Related Pathways
2.7. Overall EtOH Stress Data Integration: EtOH Stress Buffering Model
2.8. Peculiarity of the EtOH Stress-Buffering Model of the BMA64-1A Strain
2.9. Effect of EtOH on Lipid Metabolism
3. Discussion
3.1. EtOH Stress-Responsive lncRNAs Are Functionally Diverse and Likely Involved in EtOH Tolerance
3.2. EtOH Causes Extensive Rewiring of Life-Essential Pathways: Longevity, Peroxisome, CTA1 and SUI2 Are Master Key Regulators of EtOH Tolerance Phenotypes
3.3. Membraneless Organelles, Storage, and Degradation Systems Are Related to EtOH Stress: lncRNAs Act on These Systems
3.4. EtOH Stress-Buffering Model
4. Materials and Methods
4.1. Defining the Highest EtOH Tolerance
4.2. Cell Biology Analysis
4.3. Acquisition of Omics Data
4.4. Bioinformatics
4.4.1. Omics Analysis
4.4.2. Analysis of the lncRNAs and Networks
4.4.3. Mutant Generation and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, T.; Khan, A.; Shen, Y.; Chen, L.; Rabinowitz, J.D. Glucose Feeds the Tricarboxylic Acid Cycle via Excreted Ethanol in Fermenting Yeast. Nat. Chem. Biol. 2022, 18, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Auesukaree, C. Molecular Mechanisms of the Yeast Adaptive Response and Tolerance to Stresses Encountered during Ethanol Fermentation. J. Biosci. Bioeng. 2017, 124, 133–142. [Google Scholar] [CrossRef]
- Morard, M.; Macías, L.G.; Adam, A.C.; Lairón-Peris, M.; Pérez-Torrado, R.; Toft, C.; Barrio, E. Aneuploidy and Ethanol Tolerance in Saccharomyces cerevisiae. Front. Genet. 2019, 10, 82. [Google Scholar] [CrossRef]
- Yang, J.; Tavazoie, S. Regulatory and Evolutionary Adaptation of Yeast to Acute Lethal Ethanol Stress. PLoS ONE 2020, 15, e0239528. [Google Scholar] [CrossRef] [PubMed]
- Šoštarić, N.; Arslan, A.; Carvalho, B.; Plech, M.; Voordeckers, K.; Verstrepen, K.J.; van Noort, V. Integrated Multi-Omics Analysis of Mechanisms Underlying Yeast Ethanol Tolerance. J. Proteome Res. 2021, 20, 3840–3852. [Google Scholar] [CrossRef]
- Corte, L.; Roscini, L.; Pierantoni, D.C.; Pellegrino, R.M.; Emiliani, C.; Basaglia, M.; Favaro, L.; Casella, S.; Cardinali, G. Delta-Integration of Single Gene Shapes the Whole Metabolomic Short-Term Response to Ethanol of Recombinant Saccharomyces cerevisiae Strains. Metabolites 2020, 10, 140. [Google Scholar] [CrossRef]
- Goud, B.S.; Kim, J.H.; Ulaganathan, K. Identification of Genes Associated with Stress Tolerance of High Ethanol–Producing Saccharomyces cerevisiae Strain, NCIM3186, by Differential Gene Expression Analysis. BioEnergy Res. 2022, 15, 1459–1471. [Google Scholar] [CrossRef]
- Kasavi, C.; Eraslan, S.; Arga, K.Y.; Oner, E.T.; Kirdar, B. A System Based Network Approach to Ethanol Tolerance in Saccharomyces cerevisiae. BMC Syst. Biol. 2014, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Bergdahl, B.; Machado, D.; Dato, L.; Han, T.-L.; Li, J.; Villas-Boas, S.; Herrgård, M.J.; Förster, J.; Panagiotou, G. Linking Genetic, Metabolic, and Phenotypic Diversity among Saccharomyces cerevisiae Strains Using Multi-Omics Associations. Gigascience 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R.; McHale, A.P. Review: Ethanol Production at Elevated Temperatures and Alcohol Concentrations: Part I-Yeasts in General. World J. Microbiol. Biotechnol. 1998, 14, 809–821. [Google Scholar] [CrossRef]
- Niederer, R.O.; Hass, E.P.; Zappulla, D.C. Long Noncoding RNAs in the Yeast S. cerevisiae. In Long Non Coding RNA Biology; Rao, M., Ed.; Springer: Singapore, 2017; pp. 119–132. [Google Scholar]
- Yamashita, A.; Shichino, Y.; Yamamoto, M. The Long Non-Coding RNA World in Yeasts. Biochim. Biophys. Acta-Gene Regul. Mech. 2016, 1859, 147–154. [Google Scholar] [CrossRef]
- Balarezo-Cisneros, L.N.; Parker, S.; Fraczek, M.G.; Timouma, S.; Wang, P.; O’Keefe, R.T.; Millar, C.B.; Delneri, D.; O\textquoterightKeefe, R.T.; Millar, C.B.; et al. Functional and Transcriptional Profiling of Non-Coding RNAs in Yeast Reveal Context-Dependent Phenotypes and in Trans Effects on the Protein Regulatory Network. PLOS Genet. 2021, 17, e1008761. [Google Scholar] [CrossRef]
- Till, P.; Mach, R.L.; Mach-Aigner, A.R. A Current View on Long Noncoding RNAs in Yeast and Filamentous Fungi. Appl. Microbiol. Biotechnol. 2018, 102, 7319–7331. [Google Scholar] [CrossRef]
- Graf, J.; Kretz, M. From Structure to Function: Route to Understanding LncRNA Mechanism. BioEssays 2020, 42, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The Ethanol Stress Response and Ethanol Tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Elkon, I.M.; McGee, M.A.; Higbee, A.J.; Gasch, A.P. Exploiting Natural Variation in Saccharomyces cerevisiae to Identify Genes for Increased Ethanol Resistance. Genetics 2010, 186, 1197–1205. [Google Scholar] [CrossRef]
- Lourenço, A.B.; Roque, F.C.; Teixeira, M.C.; Ascenso, J.R.; Sá-Correia, I. Quantitative 1H-NMR-Metabolomics Reveals Extensive Metabolic Reprogramming and the Effect of the Aquaglyceroporin FPS1 in Ethanol-Stressed Yeast Cells. PLoS ONE 2013, 8, e55439. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Chambers, P.J.; Stanley, G.A.; Borneman, A.; Fraser, S. Transcriptional Changes Associated with Ethanol Tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 88, 231–239. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Zheng, X.; Wu, Z.; Weng, P. RNA-Seq Transcriptomic Analysis of Green Tea Polyphenols Regulation of Differently Expressed Genes in Saccharomyces cerevisiae under Ethanol Stress. World J. Microbiol. Biotechnol. 2019, 35, 59. [Google Scholar] [CrossRef]
- Li, R.; Miao, Y.; Yuan, S.; Li, Y.; Wu, Z.; Weng, P. Integrated Transcriptomic and Proteomic Analysis of the Ethanol Stress Response in Saccharomyces cerevisiae Sc131. J. Proteomics 2019, 203, 103377. [Google Scholar] [CrossRef]
- Santos, R.M.; Nogueira, F.C.S.; Brasil, A.A.; Carvalho, P.C.; Leprevost, F.V.; Domont, G.B.; Eleutherio, E.C.A. Quantitative Proteomic Analysis of the Saccharomyces cerevisiae Industrial Strains CAT-1 and PE-2. J. Proteomics 2017, 151, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Broman, A.T.; Will, J.; Gasch, A.P. Genetic Architecture of Ethanol-Responsive Transcriptome Variation in Saccharomyces cerevisiae Strains. Genetics 2014, 198, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiong, G.; Yuan, S.; Wu, Z.; Miao, Y.; Weng, P. Investigating the Underlying Mechanism of Saccharomyces cerevisiae in Response to Ethanol Stress Employing RNA-Seq Analysis. World J. Microbiol. Biotechnol. 2017, 33, 206. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, R.; Freed, E.F.; Eckert, C.A.; Gill, R.T. Transcriptional Regulatory Networks Involved in C3–C4 Alcohol Stress Response and Tolerance in Yeast. ACS Synth. Biol. 2021, 10, 19–28. [Google Scholar] [CrossRef]
- Nitsche, A.; Stadler, P.F. Evolutionary Clues in LncRNAs. Wiley Interdiscip. Rev. RNA 2017, 8, e1376. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.-Z.; Guan, N.-N.; Qu, J.; Huang, Z.-A.; Zhu, Z.-X.; Li, J.-Q. Computational Models for LncRNA Function Prediction and Functional Similarity Calculation. Brief. Funct. Genomics 2019, 18, 58–82. [Google Scholar] [CrossRef]
- Panni, S.; Prakash, A.; Bateman, A.; Orchard, S. The Yeast Noncoding RNA Interaction Network. RNA 2017, 23, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; Yamazaki, T.; Hirose, T. Architectural RNAs (ArcRNAs): A Class of Long Noncoding RNAs That Function as the Scaffold of Nuclear Bodies. Biochim. Biophys. Acta-Gene Regul. Mech. 2016, 1859, 139–146. [Google Scholar] [CrossRef]
- Carlin, D.E.; Demchak, B.; Pratt, D.; Sage, E.; Ideker, T. Network Propagation in the Cytoscape Cyberinfrastructure. PLoS Comput. Biol. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Lázari, L.C.; Wolf, I.R.; Schnepper, A.P.; Valente, G.T. LncRNAs of Saccharomyces cerevisiae Bypass the Cell Cycle Arrest Imposed by Ethanol Stress. PLOS Comput. Biol. 2022, 18, e1010081. [Google Scholar] [CrossRef]
- Santos, J.; Leitão-Correia, F.; Sousa, M.J.; Leão, C. Dietary Restriction and Nutrient Balance in Aging. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gatica, D.; Lahiri, V.; Klionsky, D.J. Cargo Recognition and Degradation by Selective Autophagy. Nat. Cell Biol. 2018, 20, 233–242. [Google Scholar] [CrossRef]
- Wiwie, C.; Kuznetsova, I.; Mostafa, A.; Rauch, A.; Haakonsson, A.; Barrio-Hernandez, I.; Blagoev, B.; Mandrup, S.; Schmidt, H.H.H.W.; Pleschka, S.; et al. Time-Resolved Systems Medicine Reveals Viral Infection-Modulating Host Targets. Syst. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of Autophagy by Spermidine Promotes Longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Ramos-Alonso, L.; Alepuz, P.; Puig, S.; Martínez-Pastor, M.T. Global Translational Repression Induced by Iron Deficiency in Yeast Depends on the Gcn2/EIF2α Pathway. Sci. Rep. 2020, 10, 233. [Google Scholar] [CrossRef]
- van Leeuwen, W.; Rabouille, C. Cellular Stress Leads to the Formation of Membraneless Stress Assemblies in Eukaryotic Cells. Traffic 2019, 20, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.H.; Zhang, B.; Ramachandran, V.; Herman, P.K. Processing Body and Stress Granule Assembly Occur by Independent and Differentially Regulated Pathways in Saccharomyces cerevisiae. Genetics 2013, 193, 109–123. [Google Scholar] [CrossRef]
- Kato, K.; Yamamoto, Y.; Izawa, S. Severe Ethanol Stress Induces Assembly of Stress Granules in Saccharomyces cerevisiae. Yeast 2011, 28, 339–347. [Google Scholar] [CrossRef]
- Wang, C.; Schmich, F.; Srivatsa, S.; Weidner, J.; Beerenwinkel, N.; Spang, A. Context-Dependent Deposition and Regulation of MRNAs in P-Bodies. eLife 2018, 7, 1–25. [Google Scholar] [CrossRef]
- Buchan, J.R.; Muhlrad, D.; Parker, R. P Bodies Promote Stress Granule Assembly in Saccharomyces cerevisiae. J. Cell Biol. 2008, 183, 441–455. [Google Scholar] [CrossRef]
- Eulalio, A.; Behm-Ansmant, I.; Izaurralde, E. P Bodies: At the Crossroads of Post-Transcriptional Pathways. Nat. Rev. Mol. Cell Biol. 2007, 8, 9–22. [Google Scholar] [CrossRef]
- Tudisca, V.; Recouvreux, V.; Moreno, S.; Boy-Marcotte, E.; Jacquet, M.; Portela, P. Differential Localization to Cytoplasm, Nucleus or P-Bodies of Yeast PKA Subunits under Different Growth Conditions. Eur. J. Cell Biol. 2010, 89, 339–348. [Google Scholar] [CrossRef]
- Ferraiuolo, M.A.; Basak, S.; Dostie, J.; Murray, E.L.; Schoenberg, D.R.; Sonenberg, N. A Role for the EIF4E-Binding Protein 4E-T in P-Body Formation and MRNA Decay. J. Cell Biol. 2005, 170, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Kane, P.M. Proton Transport and PH Control in Fungi. In Yeast Membrane Transport. Advances in Experimental Medicine and Biology; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Springer: Cham, Switzerland, 2016; pp. 33–68. [Google Scholar]
- Xie, J.L.; Jarosz, D.F. Mutations, Protein Homeostasis, and Epigenetic Control of Genome Integrity. DNA Repair 2018, 71, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, Y. Control of the DNA Damage Checkpoint by Chk1 and Rad53 Protein Kinases Through Distinct Mechanisms. Science 1999, 286, 1166–1171. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, C.; Yoo, J.Y.; Tagne, J.-B.; Kacherovsky, N.; Lee, T.I.; Young, E.T. Combined Global Localization Analysis and Transcriptome Data Identify Genes That Are Directly Coregulated by Adr1 and Cat8. Mol. Cell. Biol. 2005, 25, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Walther, K.; Schüller, H.-J. Adr1 and Cat8 Synergistically Activate the Glucose-Regulated Alcohol Dehydrogenase Gene ADH2 of the Yeast Saccharomyces cerevisiae. Microbiology 2001, 147, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- KEGG: Kyoto Encyclopedia of Genes and Genomes Alcohol Dehydrogenase Reaction: R00623. Available online: https://www.kegg.jp/entry/R00623 (accessed on 10 October 2022).
- Falcone, C.; Mazzoni, C. RNA Stability and Metabolism in Regulated Cell Death, Aging and Diseases. FEMS Yeast Res. 2018, 18, foy050. [Google Scholar] [CrossRef] [PubMed]
- Lakhotia, S.C. Long Non-Coding RNAs Coordinate Cellular Responses to Stress. Wiley Interdiscip. Rev. RNA 2012, 3, 779–796. [Google Scholar] [CrossRef]
- Reynaud, A.; Facca, C.; Sor, F.; Faye, G. Disruption and Functional Analysis of Six ORFs of Chromosome IV: YDL103c (QRI1), YDL105w (QRI2), YDL112w (TRM3), YDL113c, YDL116w (NUP84) and YDL167c (NRP1). Yeast 2001, 18, 273–282. [Google Scholar] [CrossRef]
- Xu, K.; Bezakova, I.; Bunimovich, L.; Yi, S.V. Path Lengths in Protein-Protein Interaction Networks and Biological Complexity. Proteomics 2011, 11, 1857–1867. [Google Scholar] [CrossRef]
- Klein, C.; Marino, A.; Sagot, M.-F.; Vieira Milreu, P.; Brilli, M. Structural and Dynamical Analysis of Biological Networks. Brief. Funct. Genomics 2012, 11, 420–433. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Assenov, Y.; Domingues, F.S.; Albrecht, M. Topological Analysis and Interactive Visualization of Biological Networks and Protein Structures. Nat. Protoc. 2012, 7, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.; Dakik, P.; Medkour, Y.; McAuley, M.; Mitrofanova, D.; Titorenko, V. Some Metabolites Act as Second Messengers in Yeast Chronological Aging. Int. J. Mol. Sci. 2018, 19, 860. [Google Scholar] [CrossRef]
- Mirzaei, H.; Suarez, J.A.; Longo, V.D. Protein and Amino Acid Restriction, Aging and Disease: From Yeast to Humans. Trends Endocrinol. Metab. 2014, 25, 558–566. [Google Scholar] [CrossRef]
- Du, X.; Takagi, H. N-Acetyltransferase Mpr1 Confers Ethanol Tolerance on Saccharomyces cerevisiae by Reducing Reactive Oxygen Species. Appl. Microbiol. Biotechnol. 2007, 75, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Ghose, S.; Nagotu, S. Increased Peroxisome Proliferation Is Associated with Early Yeast Replicative Ageing. Curr. Genet. 2022, 68, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tapia, E.; Nana, R.K.; Querol, A.; Pérez-Torrado, R. Ethanol Cellular Defense Induce Unfolded Protein Response in Yeast. Front. Microbiol. 2016, 7, 189. [Google Scholar] [CrossRef]
- Ramachandran, V.; Shah, K.H.; Herman, P.K. The CAMP-Dependent Protein Kinase Signaling Pathway Is a Key Regulator of P Body Foci Formation. Mol. Cell 2011, 43, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Parker, R. The Discovery and Analysis of P Bodies. Adv. Exp. Med. Biol. 2013, 768, 23–43. [Google Scholar] [CrossRef]
- Roy, R.; Rajyaguru, P.I. Stress Granules and P-Bodies: An Insight into MRNA Translational Control and Decay. Proc. Indian Natl. Sci. Acad. 2018, 97, a032813. [Google Scholar] [CrossRef]
- Youn, J.Y.; Dyakov, B.J.A.; Zhang, J.; Knight, J.D.R.; Vernon, R.M.; Forman-Kay, J.D.; Gingras, A.C. Properties of Stress Granule and P-Body Proteomes. Mol. Cell 2019, 76, 286–294. [Google Scholar] [CrossRef]
- Ivanov, P.; Kedersha, N.; Anderson, P. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol. 2019, 11, a032813. [Google Scholar] [CrossRef]
- Standart, N.; Weil, D. P-Bodies: Cytosolic Droplets for Coordinated MRNA Storage. Trends Genet. 2018, 34, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Grousl, T.; Vojtova, J.; Hasek, J.; Vomastek, T. Yeast Stress Granules at a Glance. Yeast 2022, 39, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, A.; Koppenol, R.; de Almeida, L.P.; Matos, C.A.; Nóbrega, C. Stress Granules, RNA-Binding Proteins and Polyglutamine Diseases: Too Much Aggregation? Cell Death Dis. 2021, 12, 592. [Google Scholar] [CrossRef]
- Pitchiaya, S.; Mourao, M.D.A.; Jalihal, A.P.; Xiao, L.; Jiang, X.; Chinnaiyan, A.M.; Schnell, S.; Walter, N.G. Dynamic Recruitment of Single RNAs to Processing Bodies Depends on RNA Functionality. Mol. Cell 2019, 74, 521–533.e6. [Google Scholar] [CrossRef] [PubMed]
- KEGG: Kyoto Encyclopedia of Genes and Genomes Glycolysis/Gluconeogenesis-Saccharomyces cerevisiae (Budding Yeast): Sce00010. Available online: https://www.kegg.jp/pathway/sce00010 (accessed on 10 October 2022).
- Miinalainen, I.J.; Chen, Z.-J.; Torkko, J.M.; Pirilä, P.L.; Sormunen, R.T.; Bergmann, U.; Qin, Y.-M.; Hiltunen, J.K. Characterization of 2-Enoyl Thioester Reductase from Mammals. An Ortholog of YBR026p/MRF1’p of the Yeast Mitochondrial Fatty Acid Synthesis Type II. J. Biol. Chem. 2003, 278, 20154–20161. [Google Scholar] [CrossRef] [PubMed]
- KEGG: Kyoto Encyclopedia of Genes and Genomes Fatty Acid Elongation-Saccharomyces cerevisiae (Budding Yeast): Sce00062. Available online: https://www.genome.jp/pathway/sce00062 (accessed on 10 October 2022).
- Shani, N.; Valle, D. A Saccharomyces cerevisiae Homolog of the Human Adrenoleukodystrophy Transporter Is a Heterodimer of Two Half ATP-Binding Cassette Transporters. Proc. Natl. Acad. Sci. USA 1996, 93, 11901–11906. [Google Scholar] [CrossRef] [PubMed]
- Priegnitz, A.; Brzhevskaya, O.N.; Wojtczak, L. Tight Binding of Oxaloacetate to Succinate Dehydrogenase. Biochem. Biophys. Res. Commun. 1973, 51, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Nijland, J.G.; Shin, H.Y.; Dore, E.; Rudinatha, D.; de Waal, P.P.; Driessen, A.J.M. D-Glucose Overflow Metabolism in an Evolutionary Engineered High-Performance D-Xylose Consuming Saccharomyces cerevisiae Strain. FEMS Yeast Res. 2021, 21, foaa062. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, S.; Chen, Y.; Cao, Q.; Wei, C.; Cheng, X.; Zhang, Y. Optimal Assembly Strategies of Transcriptome Related to Ploidies of Eukaryotic Organisms. BMC Genomics 2015, 16, 65. [Google Scholar] [CrossRef]






| Strain or Group | EtOH Tol. (%) | Phenotype | Genotype |
|---|---|---|---|
| BMA64-1A 1,2 | 30 | HT | MATa; his3-11_15; leu2-3_112; ura3-1; trp1Δ2; ade2-1; can1-100 |
| BY4742 | 26 | HT | MATα; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0 |
| X2180-1A | 24 | HT | MATa SUC2 mel gal2 CUP1 |
| BY4741 | 22 | LT | MATa; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0 |
| SEY6210 | 20 | LT | MATα suc2-Δ9 ura3-52 leu2-3112 his3-Δ200 trp1-Δ901 lys2-801 |
| S288C 2 | 20 | LT | MATα SUC2 mal mel gal2CUP1 |
| Strain | LncRNAs | Putative Main Functions |
|---|---|---|
| BMA64-1A | transcr_6448, transcr_20548 * | Branched-chain alcohol tolerance metabolism, regulation of the response to EtOH, and stress granules |
| BY4742 | transcr_10883 **, transcr_10027 *, transcr_9158 *, transcr_7869 *, transcr_63478 | Degradation, metabolic pathways, cell signaling, division, cell wall, transport, transcription, replication, ribosome biogenesis, and storage/degradation pathways |
| X2180-1A | transcr_3746, transcr_6988, transcr_8290 | Degradation, membrane-dependent process, cell wall, cell surveillance, longevity, growth, and transcription |
| BY4741 | transcr_3338, transcr_2916 | Membrane-dependent processes |
| SEY6210 | transcr_8157, transcr_3536 *, transcr_9136 ** | Membrane-dependent processes, diauxic shift, cell cycle, storage/degradation pathways |
| S288C | transcr_18666, transcr_18820, transcr_21244, transcr_19266, transcr_6225 | Degradation, trehalose metabolism, and ribosomal biogenesis |
| Analyzed Genes and Metabolites | Relevant Action in the EtOH Stress-Buffering Model | Source |
|---|---|---|
| ADR1, CAT8, GUT1, GUT2, INO4, NQM1 and RSF1 | Essential for growth on nonfermentable carbon sources and diauxic shift-responsive genes | * |
| ALD4, ACS1, FAA1, FAA2, FOX2, HFD1, PXA1, PXA2, POX1, and POT1 | Essential to metabolize acetyl-CoA in the cytosol and peroxisomes | * |
| CAT2, YAT1, and YAT2 | Carnitine acetyltransferase genes | SGD database |
| CIT2, FUM1, GDH2, GDH3, KGD1, KGD2, LAT1, LSC1, LSC2, MDH1, MDH2, MDH3, MLS1, PCK1, PDA1, PDC1, and PYC1 | TCA cycle-related genes | *; SGD database; KEGG sce00010 |
| ADH3, ADH5, and SFA1 | Alcohol dehydrogenase may catabolize EtOH to create acetaldehyde/acetate | *; KEGG sce00010; YeastPathways EC Number 1.1.1.1; [1] |
| ETR1 | Acetyl-CoA catabolism in the fatty acid elongation metabolism | * |
| Oxaloacetate, fumarate, and malate | TCA cycle-related metabolites | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, I.R.; Marques, L.F.; de Almeida, L.F.; Lázari, L.C.; de Moraes, L.N.; Cardoso, L.H.; Alves, C.C.d.O.; Nakajima, R.T.; Schnepper, A.P.; Golim, M.d.A.; et al. Integrative Analysis of the Ethanol Tolerance of Saccharomyces cerevisiae. Int. J. Mol. Sci. 2023, 24, 5646. https://doi.org/10.3390/ijms24065646
Wolf IR, Marques LF, de Almeida LF, Lázari LC, de Moraes LN, Cardoso LH, Alves CCdO, Nakajima RT, Schnepper AP, Golim MdA, et al. Integrative Analysis of the Ethanol Tolerance of Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2023; 24(6):5646. https://doi.org/10.3390/ijms24065646
Chicago/Turabian StyleWolf, Ivan Rodrigo, Lucas Farinazzo Marques, Lauana Fogaça de Almeida, Lucas Cardoso Lázari, Leonardo Nazário de Moraes, Luiz Henrique Cardoso, Camila Cristina de Oliveira Alves, Rafael Takahiro Nakajima, Amanda Piveta Schnepper, Marjorie de Assis Golim, and et al. 2023. "Integrative Analysis of the Ethanol Tolerance of Saccharomyces cerevisiae" International Journal of Molecular Sciences 24, no. 6: 5646. https://doi.org/10.3390/ijms24065646
APA StyleWolf, I. R., Marques, L. F., de Almeida, L. F., Lázari, L. C., de Moraes, L. N., Cardoso, L. H., Alves, C. C. d. O., Nakajima, R. T., Schnepper, A. P., Golim, M. d. A., Cataldi, T. R., Nijland, J. G., Pinto, C. M., Fioretto, M. N., Almeida, R. O., Driessen, A. J. M., Simōes, R. P., Labate, M. V., Grotto, R. M. T., ... Valente, G. T. (2023). Integrative Analysis of the Ethanol Tolerance of Saccharomyces cerevisiae. International Journal of Molecular Sciences, 24(6), 5646. https://doi.org/10.3390/ijms24065646









