Physiological Investigation and Transcriptome Analysis Reveals the Mechanisms of Setaria italica’s Yield Formation under Heat Stress
Abstract
1. Introduction
2. Results
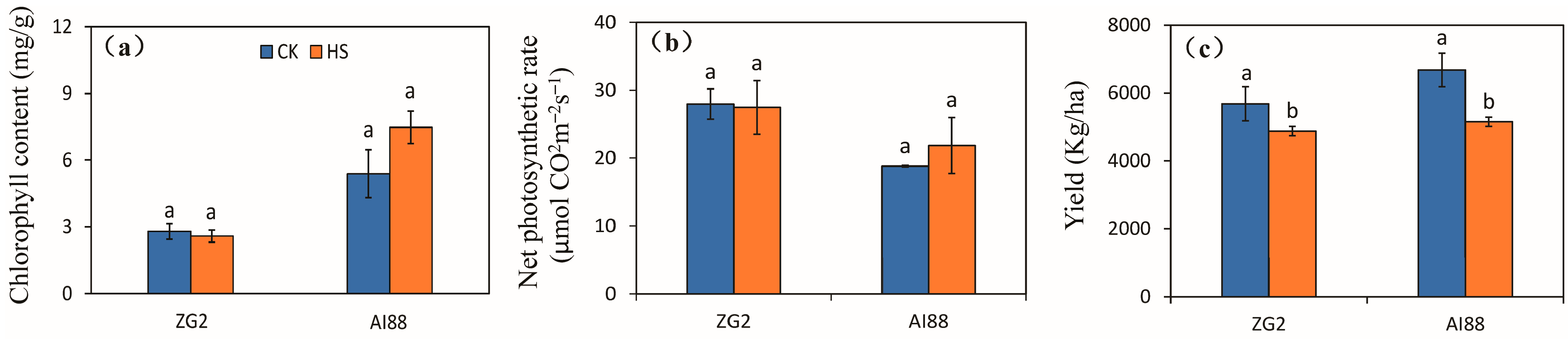
2.1. Yield, Photosynthetic Rate, and Chlorophyll Content of the Two Varieties under Heat Stress
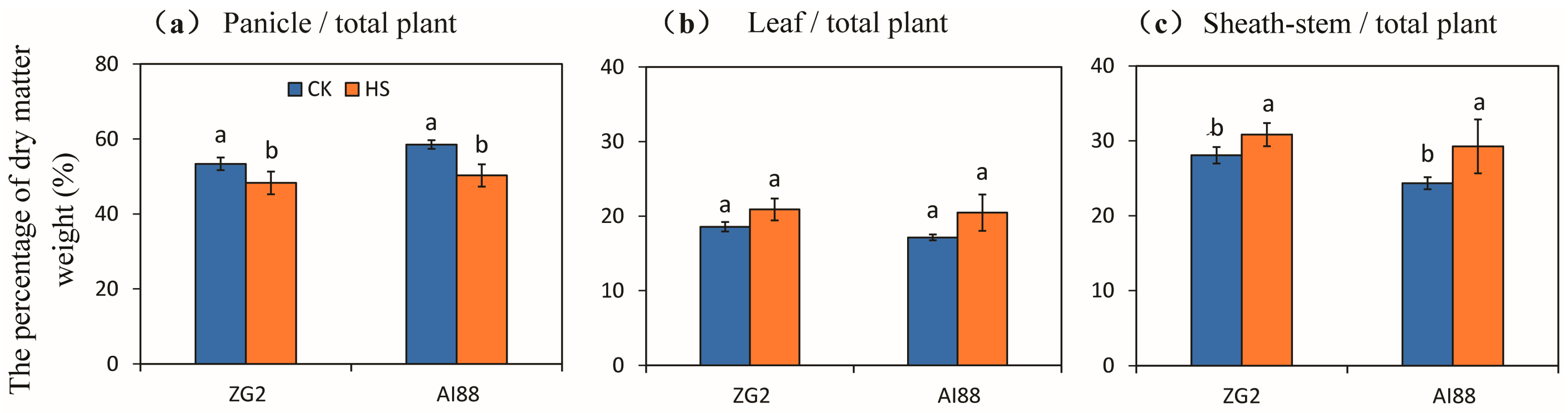
2.2. Accumulation and Allocation of Dry Matter Weight under Heat Stress
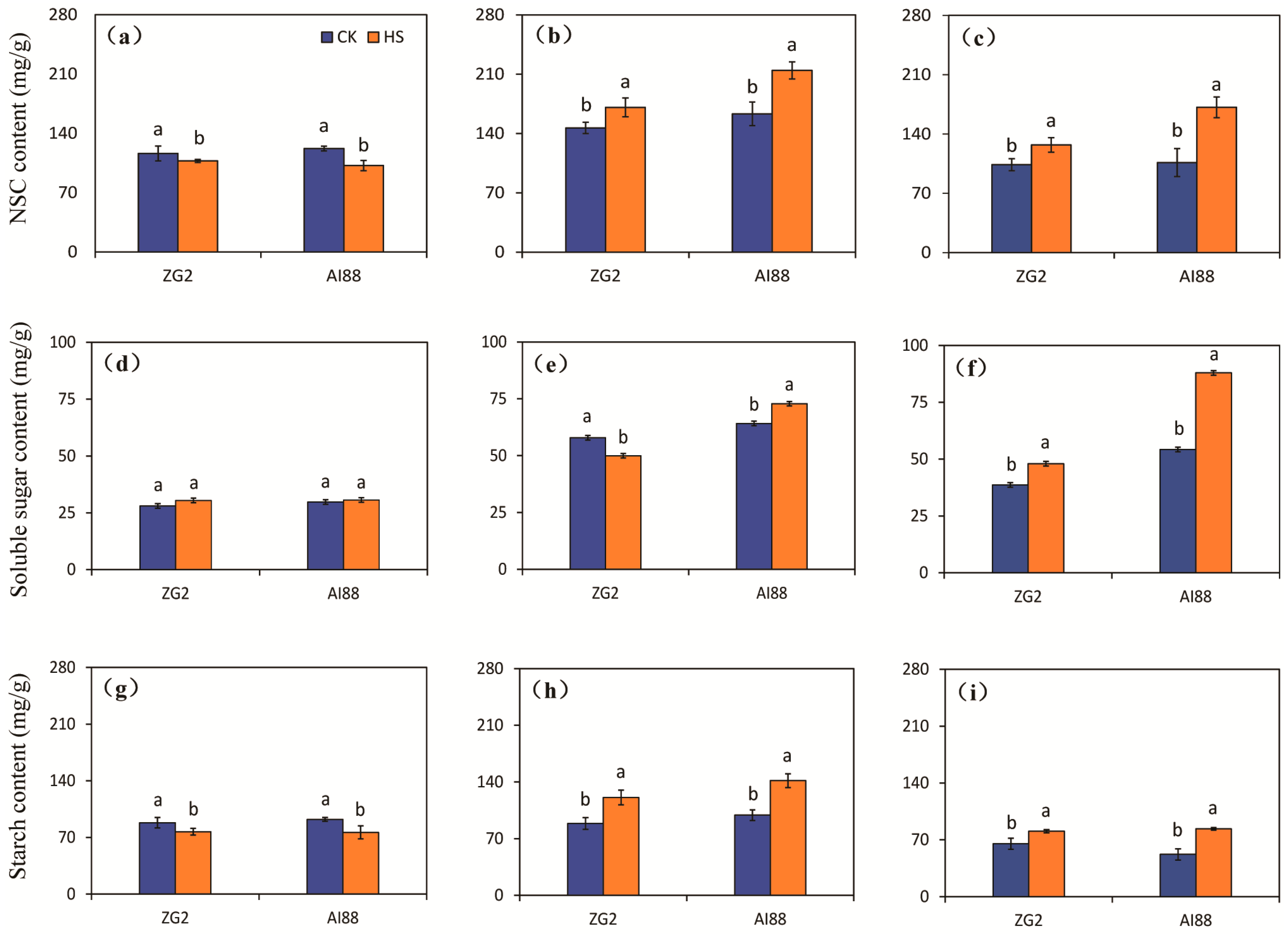
2.3. Content of NSC, Soluble Sugar, and Starch in Grains, Leaves, and Sheath Stems under Heat Stress
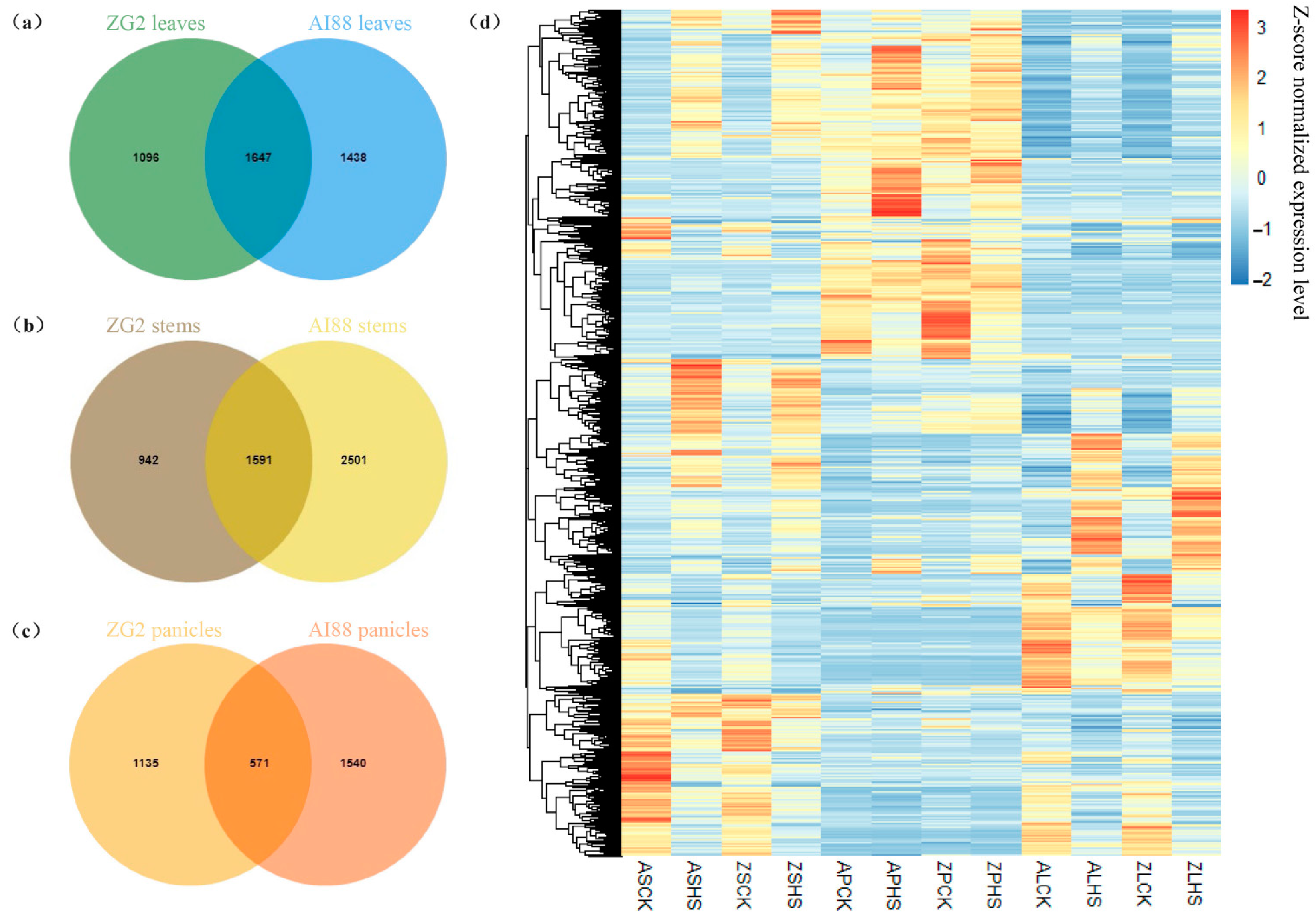
2.4. RNA-Seq Analysis of Heat Stress in Three Tissues
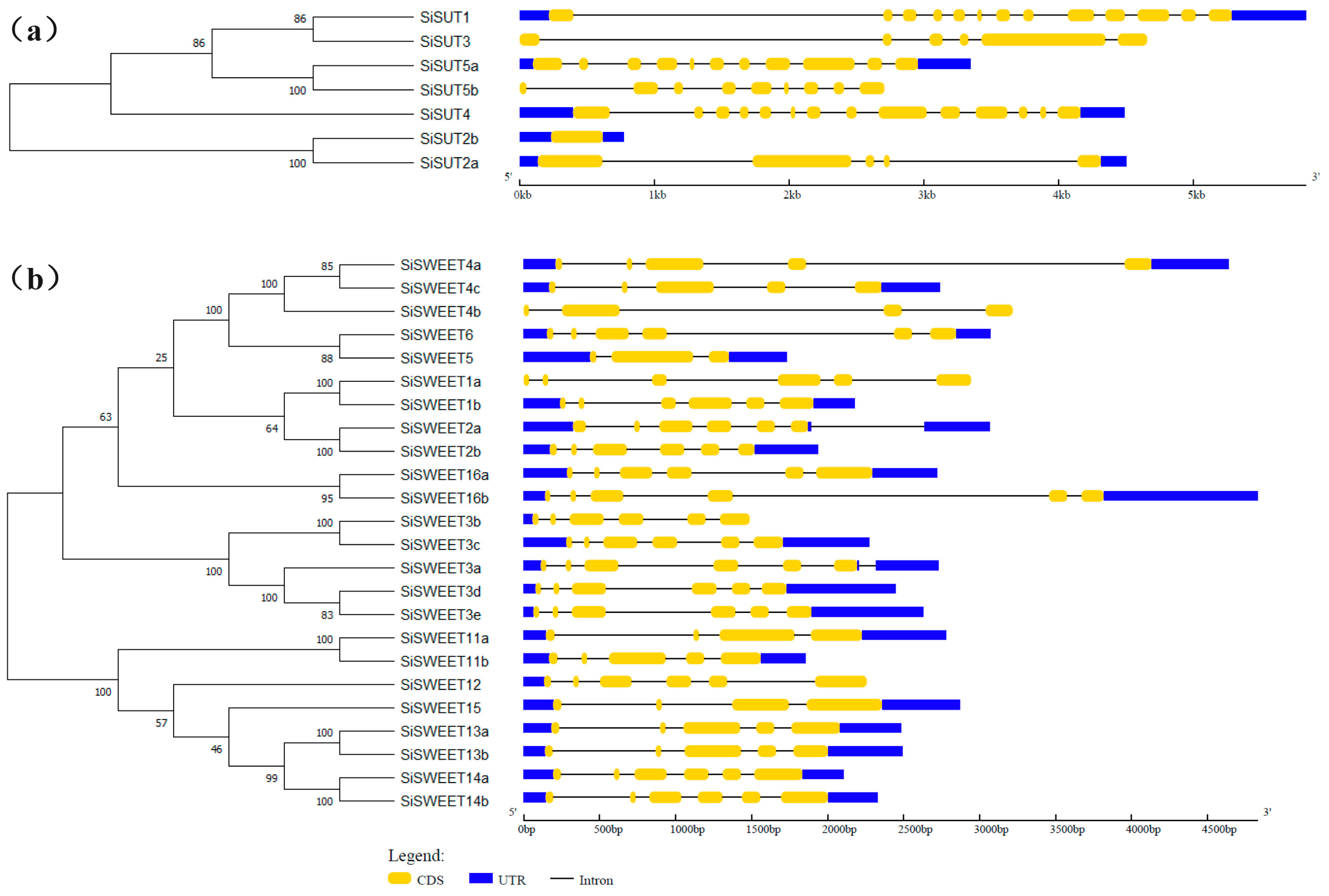
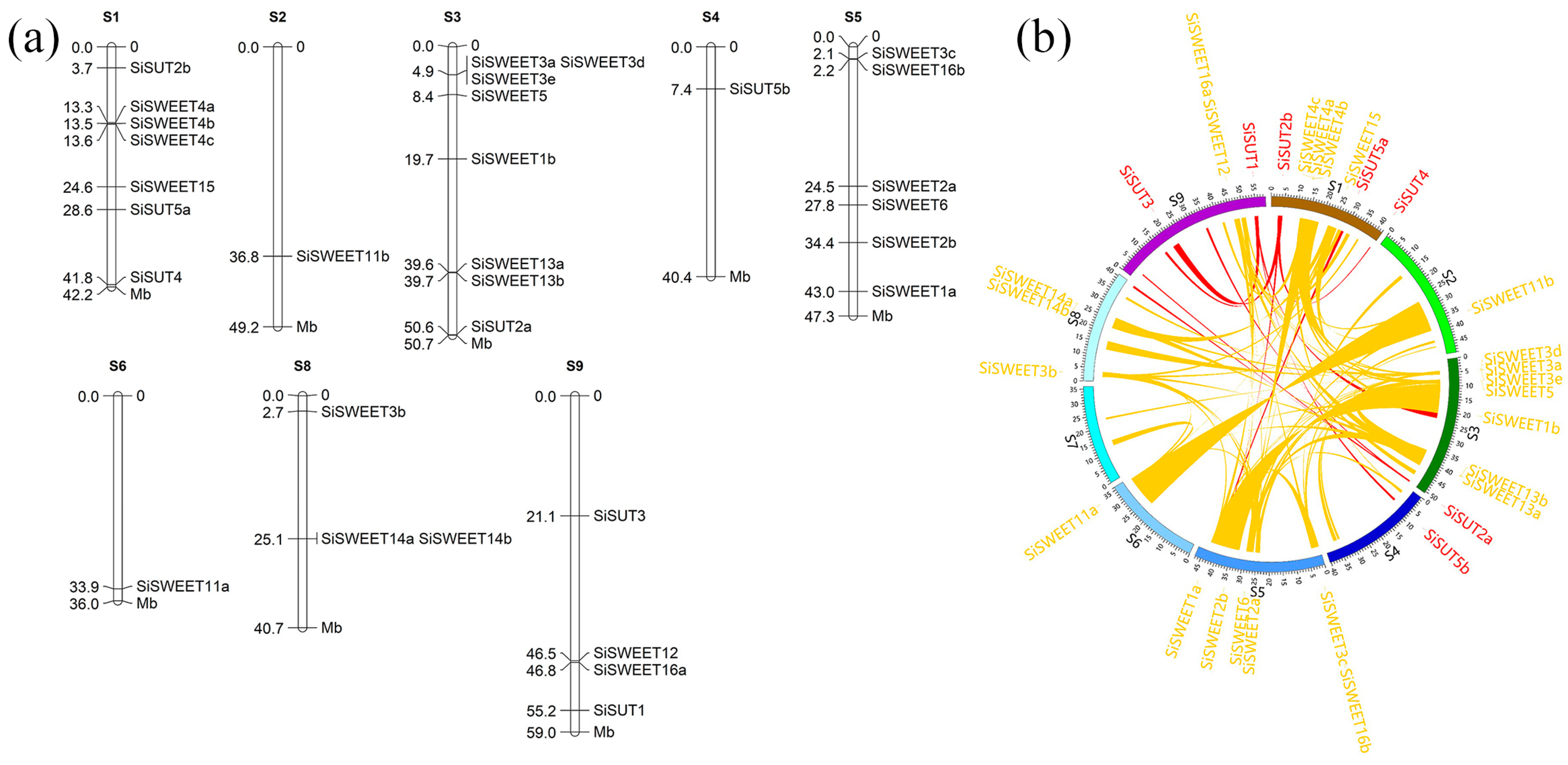
2.5. Identification of Sugar Transport-Related Genes in S. italica Genome
2.6. Expression Pattern of SUTs and SWEETs under Heat Stress
3. Discussion
3.1. Photosynthesis Is Not the Direct Means of Yield Decrease during Heat Stress
3.2. Sugar Transport Is One of the Main Factors That Influence Yield
3.3. Sugar Transporters Played Important Roles in the Sugar Transport under Heat Stress
4. Materials and Methods
4.1. Experimental Setup and Plant Materials
4.2. Measurement of Net Photosynthetic Rate, Chlorophyll Concentration, MDA Content, and SeedSetting Rate
4.3. Analysis of Accumulation and Allocation of Dry Matter Weight
4.4. Assays of Nonstructural Carbohydrate, Soluble Sugar, and Starch Contents
4.5. RNA-Seq Analysis
4.6. Identification of SWEET and SUT Members
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Al Zeidi, M.; Siddique, K.H.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Heeter, K.J.; Harley, G.L.; Abatzoglou, J.T.; Anchukaitis, K.J.; Cook, E.R.; Coulthard, B.L.; Dye, L.A.; Homfeld, I.K. Unprecedented 21st century heat across the Pacific Northwest of North America. NPJ Clim. Atmos. Sci. 2023, 6, 5. [Google Scholar] [CrossRef]
- Onat, B.; Bakal, H.; Gulluoglu, L.; Arioglu, H. The effects of high temperature at the growing period on yield and yield components of soybean [Glycine max (L.) Merr] varieties. Turk. J. Field Crops 2017, 22, 178–186. [Google Scholar] [CrossRef]
- Xu, Y.F.; Chu, C.C.; Yao, S.G. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Abbass, K.; Qasim, M.Z.; Song, H.M.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular Regulation of Plant Responses to Environmental Temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- Yang, H.; Lu, D.L.; Shen, X.; Cai, X.M.; Lu, W.P. Heat stress at different grain filling stages affects fresh waxy maize grain yield and quality. Cereal Chem. 2015, 92, 258–264. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.P.; Liu, Q.; Liu, J.; Chen, Y.Q.; Sui, P. Yield penalty of maize (Zea mays L.) under heat stress in different growth stages: A review. J. Integr. Agric. 2022, 21, 2465–2476. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Shah, F.; Huang, J.; Cui, K.; Nie, L.; Shah, T.; Chen, C.; Wang, K. Impact of high-temperature stress on rice plant and its traits related to tolerance. J. Agric. Sci. 2011, 149, 545–556. [Google Scholar] [CrossRef]
- Huang, R.Z.; Yu, H.S.; Yang, Y.; Liu, H.Q.; Wu, X.L.; Liu, Z.H.; He, H.; Wu, G.; Wang, W.; Wang, H. Effects of Heat Stress during Seed Filling Stage on Brassica napus Seed Oil Accumulation and Chlorophyll Fluorescence Characteristics. Phyton 2023, 92, 333–348. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhang, C.X.; Zhang, G.H.; Fu, W.M.; Feng, B.H.; Chen, T.T.; Peng, S.; Tao, L.; Fu, G.F. Abscisic acid negatively modulates heat tolerance in rolled leaf rice by increasing leaf temperature and regulating energy homeostasis. Rice 2020, 13, 18. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Li, H.B.; Li, G.Y.; Jin, Q.-Y.; Tao, L.-X.; Fu, G.F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ. Exp. Bot. 2018, 155, 718–733. [Google Scholar] [CrossRef]
- Li, N.; Bo, C.P.; Zhang, Y.Y.; Wang, L. Phytochrome interacting factors PIF4 and PIF5 promote heat stress induced leaf senescence in Arabidopsis. J. Exp. Bot. 2021, 72, 4577–4589. [Google Scholar] [CrossRef]
- Bandyopadhyay, T.; Singh, R.K.; Ramesh, P.; Maurya, J.; Prasad, M. The Promise of Millets in the Twenty-First Century: Emphasis on Breeding, Nutrition, Food Security and Sustainability. J. Soil. Sci. Plant Nut. 2023, 23, 628–637. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Fahad, S. Linking plants functioning to adaptive responses under heat stress conditions: A mechanistic review. J. Plant Growth Regul. 2022, 41, 2596–2613. [Google Scholar] [CrossRef]
- Nuttall, J.G.; Barlow, K.M.; Delahunty, A.J.; Christy, B.P.; O’Leary, G.J. Acute high temperature response in wheat. Agron. J. 2018, 110, 1296–1308. [Google Scholar] [CrossRef]
- Liu, H.P.; Able, A.J.; Able, J.A. Small RNA, transcriptome and degradome analysis of the transgenerational heat stress response network in durum wheat. Int. J. Mol. Sci. 2021, 22, 5532. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K. Heat stress during flowering in cereals-effects and adaptation strategies. New Phytol. 2020, 226, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Sharma, N.; Adavi, S.B.; Sharma, E.; Altaf, M.A.; Tiwari, R.K.; Kumar, R.; Kumar, A.; Dey, A.; Paul, V.; et al. From source to sink: Mechanistic insight of photoassimilates synthesis and partitioning under high temperature and elevated. Plant Mol. Biol. 2022, 110, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Coast, O.; Sebela, D.; Quiñones, C.; Jagadish, S.V.K. Systematic determination of the reproductive growth stage most sensitive to high night temperature stress in rice (Oryza sativa). Crop Sci. 2020, 60, 391–403. [Google Scholar] [CrossRef]
- Gonzalez-Schain, N.; Dreni, L.; Lawas, L.M.F.; Galbiati, M.; Colombo, L.; Heuer, S.; Kater, M.M. Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol. 2016, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.B.; Jiang, Y.; Salah, A.; Cai, M.L.; Peng, W.; Zhang, L.J.; Cao, C.G. Response of source-sink characteristics and rice quality to high natural field temperature during reproductive stage in irrigated rice system. Front. Plant Sci. 2022, 13, 911181. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Hafeez, M.B.; Ullah, A.; Siddique, K.H.; Farooq, M. Grain Development in Wheat under Combined Heat and Drought Stress: Plant Responses and Management. Environ. Exp. Bot. 2021, 188, 104517. [Google Scholar] [CrossRef]
- Farooq, M.; Nadeem, F.; Gogoi, N.; Ullah, A.; Alghamdi, S.S.; Nayyar, H.; Siddique, K.H.M. Heat stress in grain legumes during reproductive and grain-filling phases. Crop Pasture Sci. 2017, 68, 985–1005. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar] [CrossRef]
- Braun, D.M.; Lu, W.; Yongling, R. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef]
- Chen, L.Q. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Lu, M.Z.; Snyder, R.; Grant, J.; Tegeder, M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Crop J. 2020, 101, 217–236. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Hu, M.H.; Xu, W.W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol. Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef]
- Mirosavljevic, M.; Mikic, S.; Spika, A.K.; Zupunski, V.; Zhou, R.; Abdelhakim, L.; Ottosen, C.O. The effect of heat stress on some main spike traits in 12 wheat cultivars at anthesis and mid-grain filling stage. Plant Soil Environ. 2021, 67, 71–76. [Google Scholar] [CrossRef]
- Zhang, C.X.; Fu, G.F.; Yang, X.Q.; Yang, Y.J.; Zhao, X.; Chen, T.T.; Zhang, X.F.; Jin, Q.Y.; Tao, L.X. Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J. Agron. Crop Sci. 2016, 202, 394–408. [Google Scholar] [CrossRef]
- Sosso, D.; Luo, D.P.; Li, Q.B.; Sasse, J.; Yang, J.L.; Gendrot, G.; Suzuki, M.; E Koch, K.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on sweet-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.; Porcheron, B.; Kodjovi, G.C.; Moumen, B.; Vriet, C.; Maurousset, L.; Doidy, J. Genome-wide transcriptional responses to water deficit during seed development in pisum sativum, focusing on sugar transport and metabolism. Physiol. Plantarum. 2023, 175, e14062. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M.; Perumal, R.; Ciampitti, I.A. Impact of high temperature stress on floret fertility and individual grain weight of grain sorghum: Sensitive stages and thresholds for temperature and duration. Front. Plant Sci. 2015, 6, 820. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Yu, J.P.; Qian, Q.; Shang, L.G. Enhancement of Heat and Drought Stress Tolerance in Rice by Genetic Manipulation: A Systematic Review. Rice 2022, 15, 67. [Google Scholar] [CrossRef]
- Jespersen, D.; Zhang, J.; Huang, B.R. Chlorophyll loss associated with heat-induced senescence in bentgrass. Plant Sci. 2016, 249, 1–12. [Google Scholar] [CrossRef]
- Li, G.Y.; Chen, T.T.; Feng, B.H.; Peng, S.B.; Tao, L.X.; Fu, G.F. Respiration, Rather Than Photosynthesis, Determines Rice Yield Loss Under Moderate High-Temperature Conditions. Front. Plant Sci. 2021, 12, 1287–1295. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Xie, H.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Sugar Transport, Metabolism and Signaling in Fruit Development of Litchi chinensis Sonn: A Review. Int. J. Mol. Sci. 2021, 22, 11231. [Google Scholar] [CrossRef]
- Zhang, C.X.; Li, G.Y.; Chen, T.T.; Feng, B.H.; Fu, W.M.; Yan, J.X.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G.F. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 2018, 11, 14–26. [Google Scholar] [CrossRef]
- Liu, P.; Yin, B.Z.; Gu, L.; Zhang, S.; Ren, J.; Wang, Y.; Duan, W.; Zhen, W. Heat stress affects tassel development and reduces the kernel number of summer maize. Front. Plant Sci. 2023, 14, 1186921. [Google Scholar] [CrossRef]
- Anwar, K.; Joshi, R.; Morales, A.; Das, G.; Yin, X.; Anten, N.P.R.; Raghuvanshi, S.; Bahuguna, R.N.; Singh, M.P.; Singh, R.K.; et al. Genetic diversity reveals synergistic interaction between yield components could improve the sink size and yield in rice. Food Energy Secur. 2022, 1, e334. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.T.; Ding, M.Q.; Lu, W.P.; Lu, D.L. Weakened carbon and nitrogen metabolisms under post-silking heat stress reduce the yield and dry matter accumulation in waxy maize. J. Integr. Agric. 2020, 19, 78–88. [Google Scholar] [CrossRef]
- Luan, R.; Liu, J.; Tao, L.; Fu, G.; Zhang, C. Comparative transcriptome analysis reveals osbgs and osgsls influence sugar transport through callose metabolism under heat stress in rice. Int. J. Mol. Sci. 2023, 24, 3175. [Google Scholar] [CrossRef]
- Zhou, R.; Kjær, K.H.; Rosenqvist, E.; Yu, X.; Wu, Z.; Ottosen, C.O. Physiological response to heat stress during seedling and anthesis stage in tomato genotypes differing in heat tolerance. J. Agron. Crop Sci. 2017, 203, 68–80. [Google Scholar] [CrossRef]
- Kaushal, N.; Awasthi, R.; Gupta, K.; Gaur, P.; Siddique, K.H.M.; Nayyar, H. Heatstress-induced reproductive failures in chickpea (Cicer arietinum) are associated with impaired sucrose metabolism in leaves and anthers. Funct. Plant Biol. 2013, 40, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Signaling Role of Sucrose Metabolism in Development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef]
- Andargie, M.; Li, J.X. Expression of the Arabidopsis SWEET genes during rice false smut infection in the transgenic Arabidopsis thaliana containing increased levels of ATP and sucrose. J. Plant Biochem. Biotechnol. 2019, 28, 509–520. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Hu, Y.B.; Chen, L.Q.; Sosso, D.; Ducat, D.C.; Hou, B.H.; Frommer, W.B. Functional role of oligomerization for bacterial and plant sweet sugar transporter family. Proc. Natl. Acad. Sci. USA 2013, 110, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.X.; Jiang, Y.J.; Lu, Y.Z.; Zhao, P.; Wu, B.J.; Li, H.X.; Wang, Y.; Xu, S.B.; Sun, Q.X.; Liu, Z.S. Genome-wide identification and transcriptome profiling reveal great expansion of SWEET gene family and their wide-spread responses to abiotic stress in wheat (Triticum aestivum L.). J. Integr. Agric. 2020, 19, 1704–1720. [Google Scholar] [CrossRef]
- Kim, P.; Xue, C.Y.; Song, H.D.; Gao, Y.; Feng, L.; Li, Y.H.; Xuan, Y.H. Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol. J. 2021, 19, 409–411. [Google Scholar] [CrossRef]
- Hutin, M.; Sabot, F.; Ghesquière, A.; Koebnik, R.; Szurek, B. A knowledge-based molecular screen uncovers a broad-spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J. 2015, 84, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Streubel, J.; Pesce, C.; Hutin, M.; Koebnik, R.; Boch, J.; Szurek, B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. Oryzae. New Phytol. 2013, 200, 808–819. [Google Scholar] [CrossRef]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, Y.; Vu, N.T.Q.; Shen, S.; Xia, K.; Zhang, M. Crispr/cas9-mediated mutation of ossweet14 in rice cv. Zhonghua11 confers resistance to xanthomonas oryzae pv. Oryzae without yield penalty. BMC Plant Biol. 2020, 20, 313. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. Tophat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, 4–36. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pachter, L. Differential gene and transcript expression analysis of rna-seq experiments with tophat and cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Arndt, W.; Miller, B.L.; Wheeler, T.J.; Schreiber, F.; Bateman, A.; Eddy, S.R. Hmmer web server: 2015 update. Nucleic. Acids. Res. 2015, 43, 30–38. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST plus: Architecture and applications. BMC Bioinform. 2009, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Marchler Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.N.; Chitsaz, F.; Geer, L.Y.; Bryant, S.H. Cdd: Ncbi’s conserved domain database. Acc. Chem. Res. 2015, 43, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. Mega11 molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Paterson, A.H. Mcscanx: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, 49–63. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Yang, M.; Liu, J.; Huang, H.; Luan, R.; Yue, H.; Zhang, C. Physiological Investigation and Transcriptome Analysis Reveals the Mechanisms of Setaria italica’s Yield Formation under Heat Stress. Int. J. Mol. Sci. 2024, 25, 3171. https://doi.org/10.3390/ijms25063171
Hu M, Yang M, Liu J, Huang H, Luan R, Yue H, Zhang C. Physiological Investigation and Transcriptome Analysis Reveals the Mechanisms of Setaria italica’s Yield Formation under Heat Stress. International Journal of Molecular Sciences. 2024; 25(6):3171. https://doi.org/10.3390/ijms25063171
Chicago/Turabian StyleHu, Manicao, Meng Yang, Jingyang Liu, Haozhe Huang, Ruiwei Luan, Hongliang Yue, and Caixia Zhang. 2024. "Physiological Investigation and Transcriptome Analysis Reveals the Mechanisms of Setaria italica’s Yield Formation under Heat Stress" International Journal of Molecular Sciences 25, no. 6: 3171. https://doi.org/10.3390/ijms25063171
APA StyleHu, M., Yang, M., Liu, J., Huang, H., Luan, R., Yue, H., & Zhang, C. (2024). Physiological Investigation and Transcriptome Analysis Reveals the Mechanisms of Setaria italica’s Yield Formation under Heat Stress. International Journal of Molecular Sciences, 25(6), 3171. https://doi.org/10.3390/ijms25063171




