Exploring the Anti-Colorectal Cancer Mechanism of Norcantharidin Through TRAF5/NF-κB Pathway Regulation and Folate-Targeted Liposomal Delivery
Abstract
:1. Introduction
2. Results
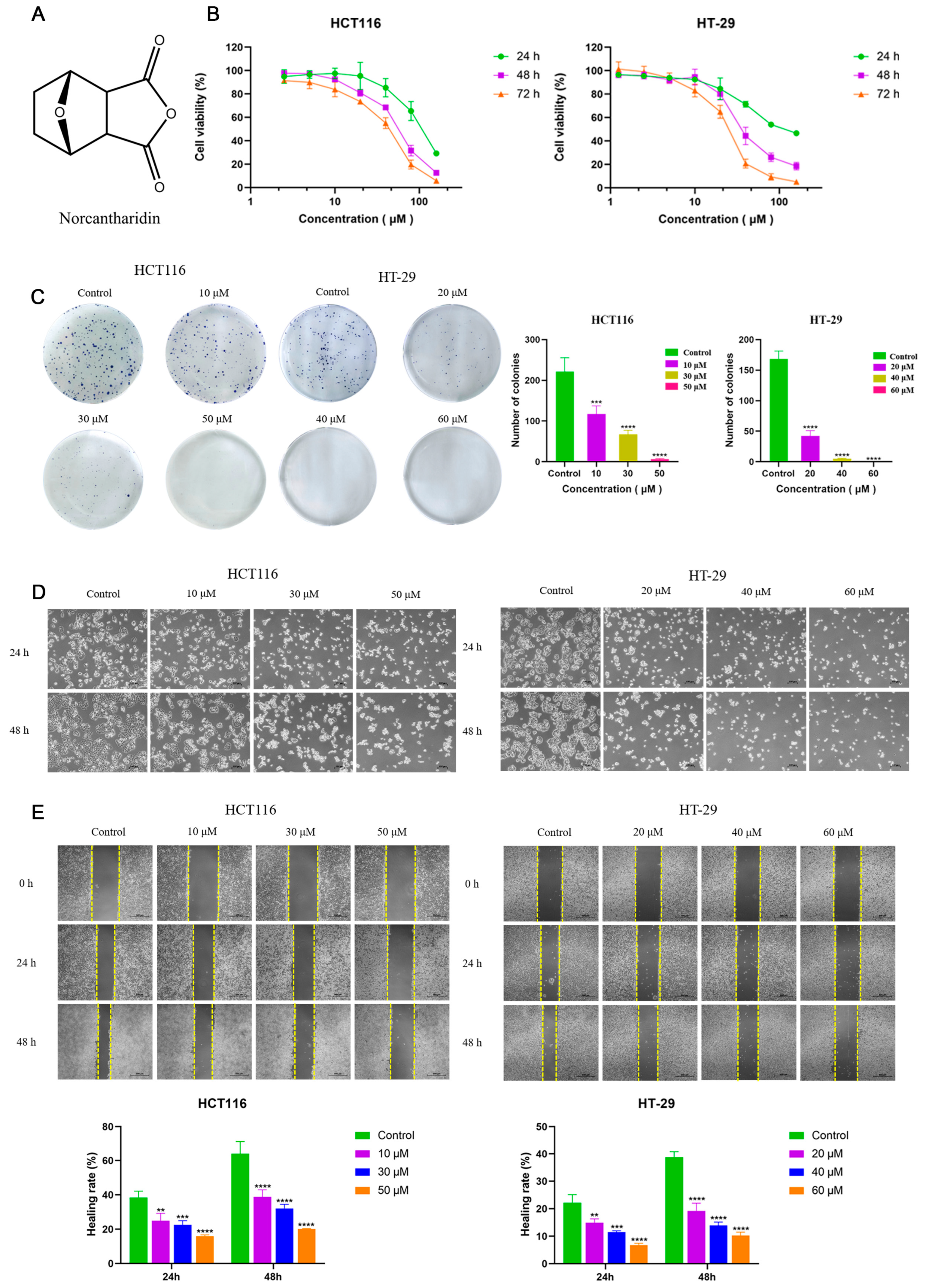
2.1. NCTD Inhibited Cell Proliferation and Migration in CRC Cells
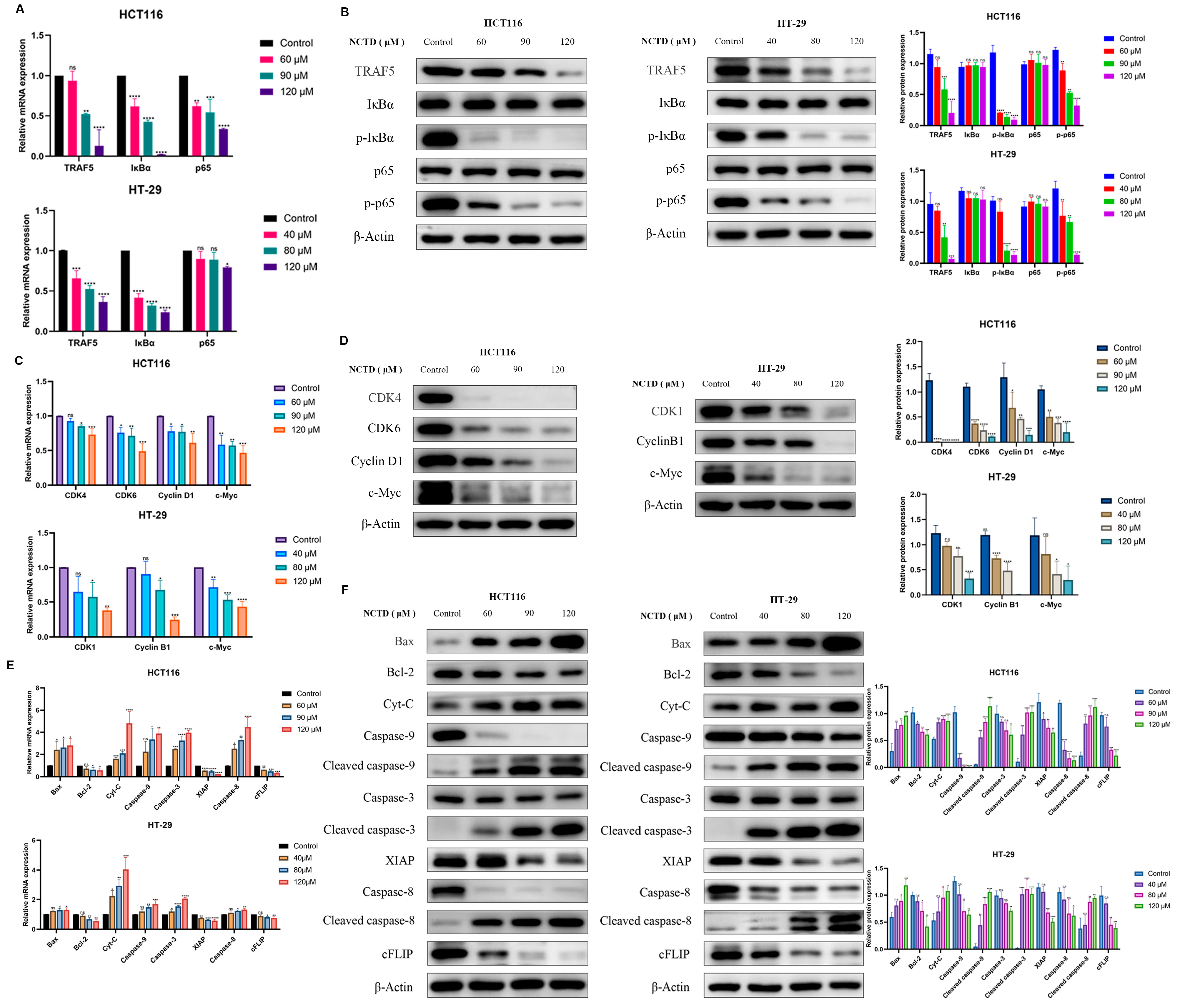
2.2. NCTD Induced Apoptosis and Cell Cycle Arrest
2.3. NCTD Induced Mitochondrial Dysfunction and Enhanced Reactive Oxygen Species (ROS) Levels
2.4. Bioinformatics Analysis of NCTD Intervention HCT116 and HT-29 Cells
2.5. NCTD Inhibits Colon Cancer by Regulating the TRAF5/NF-κB Signaling Pathway
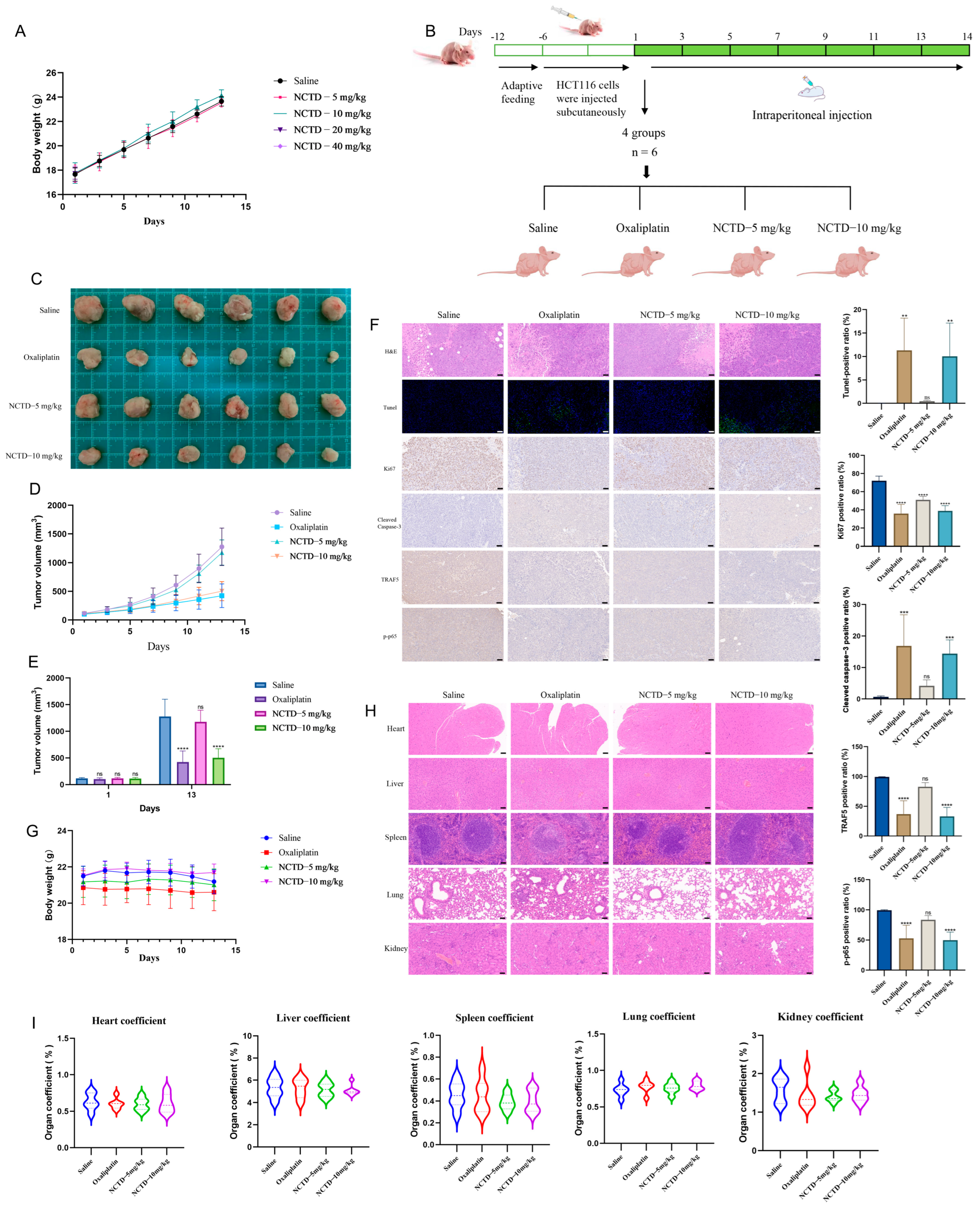
2.6. NCTD Suppressed the Progression of Colon Cancer Transplanted in Nude Mice
2.7. Preparation and Quality Evaluation of NCTD Liposomes Targeting Folate Receptors
2.8. FA-NCTD Liposomes Suppressed the Growth of Colon Cancer Transplanted in Nude Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Culture
4.3. Cell Viability
4.4. Colony Formation
4.5. Cell Morphology
4.6. Wound-Healing Assay
4.7. Cell Apoptosis Assay
4.8. Mitochondrial Membrane Potential
4.9. Cell Cycle Analysis
4.10. Reactive Oxygen Species (ROS)
4.11. Whole-Transcriptome Sequencing
4.12. Bioinformatics Analysis
4.13. Animal Experiments
4.14. Real-Time Quantitative PCR (RT–qPCR) Analysis
4.15. Western Blot
4.16. Hematoxylin–Eosin Staining
4.17. Immunohistochemistry
4.18. Norcantharidin Liposomes Targeting Folate Receptors
4.19. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The Rising Tide of Early-Onset Colorectal Cancer: A Comprehensive Review of Epidemiology, Clinical Features, Biology, Risk Factors, Prevention, and Early Detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Weitz, J.; Koch, M.; Debus, J.; Höhler, T.; Galle, P.R.; Büchler, M.W. Colorectal Cancer. Lancet 2005, 365, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Beretta, G.D.; Kildani, B.; Milesi, L.; Merlin, F.; Mosconi, S.; Pessi, M.A.; Prochilo, T.; Quadri, A.; Gatta, G.; et al. Colon Cancer. Crit. Rev. Oncol. Hematol. 2010, 74, 106–133. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, H.; Li, C.; Cai, P.; Qi, F. The Positive Role of Traditional Chinese Medicine as an Adjunctive Therapy for Cancer. Biosci. Trends 2021, 15, 283–298. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The Effects of Cantharidin and Cantharidin Derivates on Tumour Cells. Anticancer Agents Med. Chem. 2009, 9, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-M.; Chen, Y.-J.; Bao, Y.-X. Pharmacological Mechanisms of Norcantharidin against Hepatocellular Carcinoma. Am. J. Cancer Res. 2023, 13, 5024–5038. [Google Scholar]
- Han, Z.; Li, B.; Wang, J.; Zhang, X.; Li, Z.; Dai, L.; Cao, M.; Jiang, J. Norcantharidin Inhibits SK-N-SH Neuroblastoma Cell Growth by Induction of Autophagy and Apoptosis. Technol. Cancer Res. Treat. 2017, 16, 33–44. [Google Scholar] [CrossRef]
- Xu, L.; Su, B.; Mo, L.; Zhao, C.; Zhao, Z.; Li, H.; Hu, Z.; Li, J. Norcantharidin Induces Immunogenic Cell Death of Bladder Cancer Cells through Promoting Autophagy in Acidic Culture. Int. J. Mol. Sci. 2022, 23, 3944. [Google Scholar] [CrossRef]
- Peng, C.; Liu, X.; Liu, E.; Xu, K.; Niu, W.; Chen, R.; Wang, J.; Zhang, Z.; Lin, P.; Wang, J.; et al. Norcantharidin Induces HT-29 Colon Cancer Cell Apoptosis through the Alphavbeta6-Extracellular Signal-Related Kinase Signaling Pathway. Cancer Sci. 2009, 100, 2302–2308. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Chen, M.-F.; Tsai, C.-H.; Hu, D.-N.; Chang, F.-R.; Wu, Y.-C. Involvement of Caspase and MAPK Activities in Norcantharidin-Induced Colorectal Cancer Cell Apoptosis. Toxicol. Vitr. 2010, 24, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhu, Y.; Tang, X.; Tao, W. The Anti-Proliferative Effects of Norcantharidin on Human HepG2 Cells in Cell Culture. Mol. Biol. Rep. 2011, 38, 163–169. [Google Scholar] [CrossRef]
- Peng, C.; Li, Z.; Niu, Z.; Niu, W.; Xu, Z.; Gao, H.; Niu, W.; Wang, J.; He, Z.; Gao, C.; et al. Norcantharidin Suppresses Colon Cancer Cell Epithelial-Mesenchymal Transition by Inhibiting the Avβ6-ERK-Ets1 Signaling Pathway. Sci. Rep. 2016, 6, 20500. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Hou, F.; Liu, M.; Zhou, L.; Li, D.; Liu, J.; Fan, Z.; Li, Q. Norcantharidin Anti-Angiogenesis Activity Possibly through an Endothelial Cell Pathway in Human Colorectal Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 499–503. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Stratton, B.; Engel, A.; Deng, W. Liposome Technologies towards Colorectal Cancer Therapeutics. Acta Biomater. 2021, 127, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the Folate Receptor α in Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.-X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.K.L.; et al. A Novel Faecal Lachnoclostridium Marker for the Non-Invasive Diagnosis of Colorectal Adenoma and Cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted Nanoparticles for Colorectal Cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in Colorectal Cancer: Rationale, Challenges and Potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Gu, X.-D.; Xu, L.-L.; Zhao, H.; Gu, J.-Z.; Xie, X.-H. Cantharidin Suppressed Breast Cancer MDA-MB-231 Cell Growth and Migration by Inhibiting MAPK Signaling Pathway. Braz. J. Med. Biol. Res. 2017, 50, e5920. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-D.; Liu, L.; Wu, M.-Y.; Jiang, M.; Shou, L.-M.; Wang, W.-J.; Wu, J.; Zhang, Y.; Gong, F.-R.; Chen, K.; et al. The Combination of Cantharidin and Antiangiogenic Therapeutics Presents Additive Antitumor Effects against Pancreatic Cancer. Oncogenesis 2018, 7, 94. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.; Zhou, X.; Xu, W.; Wang, G.; Tang, X.; Luo, L.; Tu, J.; Zhu, Y.; Hu, W.; et al. Cantharidin Induces G2/M Phase Arrest and Apoptosis in Human Gastric Cancer SGC-7901 and BGC-823 Cells. Oncol. Lett. 2014, 8, 2721–2726. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, X.; Gong, Z.; Su, Q.; Yu, R.; Wang, B.; Yang, T.; Dai, B.; Zhan, Y.; Zhang, D.; et al. Cantharidin Treatment Inhibits Hepatocellular Carcinoma Development by Regulating the JAK2/STAT3 and PI3K/Akt Pathways in an EphB4-Dependent Manner. Pharmacol. Res. 2020, 158, 104868. [Google Scholar] [CrossRef] [PubMed]
- Huan, S.K.-H.; Wang, K.-T.; Yeh, S.-D.; Lee, C.-J.; Lin, L.-C.; Liu, D.-Z.; Wang, C.-C. Scutellaria Baicalensis Alleviates Cantharidin-Induced Rat Hemorrhagic Cystitis through Inhibition of Cyclooxygenase-2 Overexpression. Molecules 2012, 17, 6277–6289. [Google Scholar] [CrossRef]
- Qualls, H.J.; Holbrook, T.C.; Gilliam, L.L.; Njaa, B.L.; Panciera, R.J.; Pope, C.N.; Payton, M.E. Evaluation of Efficacy of Mineral Oil, Charcoal, and Smectite in a Rat Model of Equine Cantharidin Toxicosis. J. Vet. Intern. Med. 2013, 27, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Prasad, S.B. Bioactive Component, Cantharidin from Mylabris Cichorii and Its Antitumor Activity against Ehrlich Ascites Carcinoma. Cell Biol. Toxicol. 2012, 28, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.W.-G.; Tseng, Y.-S.; Lin, C.-C.; Hsi, Y.-T.; Lo, Y.-S.; Chuang, Y.-C.; Lin, S.-H.; Yu, C.-Y.; Hsieh, M.-J.; Chen, M.-K. Norcantharidin Induce Apoptosis in Human Nasopharyngeal Carcinoma through Caspase and Mitochondrial Pathway. Environ. Toxicol. 2018, 33, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Z.; Fu, J.-Y.; Zhao, Z.-M.; Chen, C.-Q. Inhibitory Effect of Norcantharidin on the Growth of Human Gallbladder Carcinoma GBC-SD Cells in Vitro. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 72–80. [Google Scholar]
- Wan, X.-Y.; Zhai, X.-F.; Jiang, Y.-P.; Han, T.; Zhang, Q.-Y.; Xin, H.-L. Antimetastatic Effects of Norcantharidin on Hepatocellular Carcinoma Cells by Up-Regulating FAM46C Expression. Am. J. Transl. Res. 2017, 9, 155–166. [Google Scholar]
- Tixeira, R.; Caruso, S.; Paone, S.; Baxter, A.A.; Atkin-Smith, G.K.; Hulett, M.D.; Poon, I.K.H. Defining the Morphologic Features and Products of Cell Disassembly during Apoptosis. Apoptosis 2017, 22, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial Membrane Potential Regulates Matrix Configuration and Cytochrome c Release during Apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Marzo, I.; Brenner, C.; Zamzami, N.; Susin, S.A.; Beutner, G.; Brdiczka, D.; Rémy, R.; Xie, Z.H.; Reed, J.C.; Kroemer, G. The Permeability Transition Pore Complex: A Target for Apoptosis Regulation by Caspases and Bcl-2-Related Proteins. J. Exp. Med. 1998, 187, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Zhang, G.; Li, J.; Wu, X.; Ma, X.; Shan, G.; Mei, Y. Long Noncoding RNA EMS Connects C-Myc to Cell Cycle Control and Tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 14620–14629. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, F.; Jin, W.; Yan, B.; Chen, X.; He, Y.; Yang, W.; Du, W.; Zhang, Q.; Guo, Y.; et al. Theabrownin Inhibits Cell Cycle Progression and Tumor Growth of Lung Carcinoma through C-Myc-Related Mechanism. Front. Pharmacol. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Benaud, C.M.; Dickson, R.B. Adhesion-Regulated G1 Cell Cycle Arrest in Epithelial Cells Requires the Downregulation of c-Myc. Oncogene 2001, 20, 4554–4567. [Google Scholar] [CrossRef]
- Jackman, M.; Marcozzi, C.; Barbiero, M.; Pardo, M.; Yu, L.; Tyson, A.L.; Choudhary, J.S.; Pines, J. Cyclin B1-Cdk1 Facilitates MAD1 Release from the Nuclear Pore to Ensure a Robust Spindle Checkpoint. J. Cell Biol. 2020, 219, e201907082. [Google Scholar]
- Hikami, S.; Shiozaki, A.; Kitagawa-Juge, M.; Ichikawa, D.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; Otsuji, E. The Role of cIAP1 and XIAP in Apoptosis Induced by Tumor Necrosis Factor Alpha in Esophageal Squamous Cell Carcinoma Cells. Dig. Dis. Sci. 2017, 62, 652–659. [Google Scholar] [CrossRef]
- Riedl, S.J.; Renatus, M.; Schwarzenbacher, R.; Zhou, Q.; Sun, C.; Fesik, S.W.; Liddington, R.C.; Salvesen, G.S. Structural Basis for the Inhibition of Caspase-3 by XIAP. Cell 2001, 104, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, Z.; Wang, X.; Zhou, F.; Ma, X.; Wei, W.; Tian, D.; Yu, H. The Emerging Role of Epigenetics and Gut Microbiota in Vogt-Koyanagi-Harada Syndrome. Gene 2022, 818, 146222. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, H.; Zhao, L.; Zhang, Y.; Wan, Q.; Shen, Y.; Bu, X.; Wan, M.; Shen, C. Up-Regulation of OLR1 Expression by TBC1D3 through Activation of TNFα/NF-κB Pathway Promotes the Migration of Human Breast Cancer Cells. Cancer Lett. 2017, 408, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, F.; Zhou, Y.Q.; Lu, W.; Hu, F.L.; Fan, X. Silencing of TRAF5 Enhances Necroptosis in Hepatocellular Carcinoma by Inhibiting LTBR-Mediated NF-κB Signaling. PeerJ 2023, 11, e15551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, B.-H.; Wu, Q.-H.; Li, Q.-L.; Yang, K.-D. LncRNA HCG18 Upregulates TRAF4/TRAF5 to Facilitate Proliferation, Migration and EMT of Epithelial Ovarian Cancer by Targeting miR-29a/b. Mol. Med. 2022, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Fang, L.; Li, Y.; Du, J.; Zhou, B.; Wang, X.; Zhou, H.; Gao, L.; Wang, K.; Zhang, J. miR-873 Inhibits Colorectal Cancer Cell Proliferation by Targeting TRAF5 and TAB1. Oncol. Rep. 2018, 39, 1090–1098. [Google Scholar] [CrossRef]
- Wang, H.; Ahn, K.S.; Alharbi, S.A.; Shair, O.H.; Arfuso, F.; Sethi, G.; Chinnathambi, A.; Tang, F.R. Celastrol Alleviates Gamma Irradiation-Induced Damage by Modulating Diverse Inflammatory Mediators. Int. J. Mol. Sci. 2020, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-κB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Li, X.; Zhang, J.; Zheng, L.; Zhang, W.; Lin, C.; Lin, W.; Li, X. CDCA3 Promotes Cell Proliferation by Activating the NF-κB/Cyclin D1 Signaling Pathway in Colorectal Cancer. Biochem. Biophys. Res. Commun. 2018, 500, 196–203. [Google Scholar] [CrossRef]
- Chen, M.; Ye, K.; Zhang, B.; Xin, Q.; Li, P.; Kong, A.-N.; Wen, X.; Yang, J. Paris Saponin II Inhibits Colorectal Carcinogenesis by Regulating Mitochondrial Fission and NF-κB Pathway. Pharmacol. Res. 2019, 139, 273–285. [Google Scholar] [CrossRef]
- Li, Z.; Xuan, Z.; Chen, J.; Song, W.; Zhang, S.; Jin, C.; Zhou, M.; Zheng, S.; Song, P. Inhibiting the NF-κB Pathway Enhances the Antitumor Effect of Cabazitaxel by Downregulating Bcl-2 in Pancreatic Cancer. Int. J. Oncol. 2020, 57, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-P.; Song, J.-L.; Yi, X.-P.; Li, Y.-X. Double Inhibition of NF-κB and XIAP via RNAi Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine. Oncol. Rep. 2013, 29, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.L.; Corn, R.A.; Stanic, A.K.; Goenka, S.; Aronica, M.; Stanley, S.; Ballard, D.W.; Joyce, S.; Boothby, M. Antiapoptotic Function of NF-kappaB in T Lymphocytes Is Influenced by Their Differentiation Status: Roles of Fas, c-FLIP, and Bcl-xL. Cell Death Differ. 2003, 10, 1032–1044. [Google Scholar] [CrossRef]
- van der Heijden, M.; Zimberlin, C.D.; Nicholson, A.M.; Colak, S.; Kemp, R.; Meijer, S.L.; Medema, J.P.; Greten, F.R.; Jansen, M.; Winton, D.J.; et al. Bcl-2 Is a Critical Mediator of Intestinal Transformation. Nat. Commun. 2016, 7, 10916. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, J.; Gao, Y.; Han, G.; Ding, W.; Huang, X. MicroRNA-4262 Activates the NF-κB and Enhances the Proliferation of Hepatocellular Carcinoma Cells. Int. J. Biol. Macromol. 2016, 86, 43–49. [Google Scholar] [CrossRef]




| Primers | Primer Sequence (5′-3′) |
|---|---|
| TRAF5 | Forward CCGAGCCCCACAATGGCTTA Reverse CCGCTCCACAAACTGGTACT |
| Caspase-3 | Forward CGTGTCATAAAATACCAGTGGA Reverse AAATTCTGTTGCCACCTTTCG |
| p65 | Forward CCTGTCCTTTCTCATCCCATCTTTG Reverse GCTGCCAGAGTTTCGGTTCAC |
| IκBα | Forward GAGACTTTCGAGGAAATACCCC Reverse GTAGCCATGGATAGAGGCTAAG |
| CDK1 | Forward TGCCGCTCTCCACCATCCG Reverse GCACACATCAAACAACCTGACCAC |
| CDK4 | Forward TGAAATTGGTGTCGGTGCCTATGG Reverse CTCCTCCACCTCCTCCTCCATTG |
| CDK6 | Forward TGCCGCTCTCCACCATCCG Reverse GCACACATCAAACAACCTGACCAC |
| Cyclin B1 | Forward GCCAGTGCCAGAGCCAGAAC Reverse CATTGGGCTTGGAGAGGCAGTATC |
| Cyclin D1 | Forward GCCCTCGGTGTCCTACTTCAAATG Reverse TCCTCCTCGCACTTCTGTTCCTC |
| c-Myc | Forward CCTGGTGCTCCATGAGGAGAC Reverse CAGACTCTGACCTTTTGCCAGG |
| GAPDH | Forward TGGAGTCCACTGGCGTCTTCAC Reverse TTGCTGATGATCTTGAGGCTGTTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Chen, X.; Qiao, C.; Yang, S.; Zhai, Y.; Zhang, J.; Chai, K.; Wang, H.; Zhou, J.; Guo, M.; et al. Exploring the Anti-Colorectal Cancer Mechanism of Norcantharidin Through TRAF5/NF-κB Pathway Regulation and Folate-Targeted Liposomal Delivery. Int. J. Mol. Sci. 2025, 26, 1450. https://doi.org/10.3390/ijms26041450
Zhang F, Chen X, Qiao C, Yang S, Zhai Y, Zhang J, Chai K, Wang H, Zhou J, Guo M, et al. Exploring the Anti-Colorectal Cancer Mechanism of Norcantharidin Through TRAF5/NF-κB Pathway Regulation and Folate-Targeted Liposomal Delivery. International Journal of Molecular Sciences. 2025; 26(4):1450. https://doi.org/10.3390/ijms26041450
Chicago/Turabian StyleZhang, Fanqin, Xiaodong Chen, Chuanqi Qiao, Siyun Yang, Yiyan Zhai, Jingyuan Zhang, Keyan Chai, Haojia Wang, Jiying Zhou, Meiling Guo, and et al. 2025. "Exploring the Anti-Colorectal Cancer Mechanism of Norcantharidin Through TRAF5/NF-κB Pathway Regulation and Folate-Targeted Liposomal Delivery" International Journal of Molecular Sciences 26, no. 4: 1450. https://doi.org/10.3390/ijms26041450
APA StyleZhang, F., Chen, X., Qiao, C., Yang, S., Zhai, Y., Zhang, J., Chai, K., Wang, H., Zhou, J., Guo, M., Lu, P., & Wu, J. (2025). Exploring the Anti-Colorectal Cancer Mechanism of Norcantharidin Through TRAF5/NF-κB Pathway Regulation and Folate-Targeted Liposomal Delivery. International Journal of Molecular Sciences, 26(4), 1450. https://doi.org/10.3390/ijms26041450






