Taxonomic Revision of the Nearctic Genus Drepanaphis Del Guercio (Hemiptera, Aphididae: Drepanosiphinae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Material and Light Microscopy
2.2. Scanning Electron Microscopy
2.3. Statistical Analysis
2.4. Occurrence Data and Preparation of Maps
3. Results
3.1. Taxonomy
- Genus Drepanaphis Del Guercio, 1909
- Type species Siphonophora acerifoliae Thomas, 1878, by original designation.
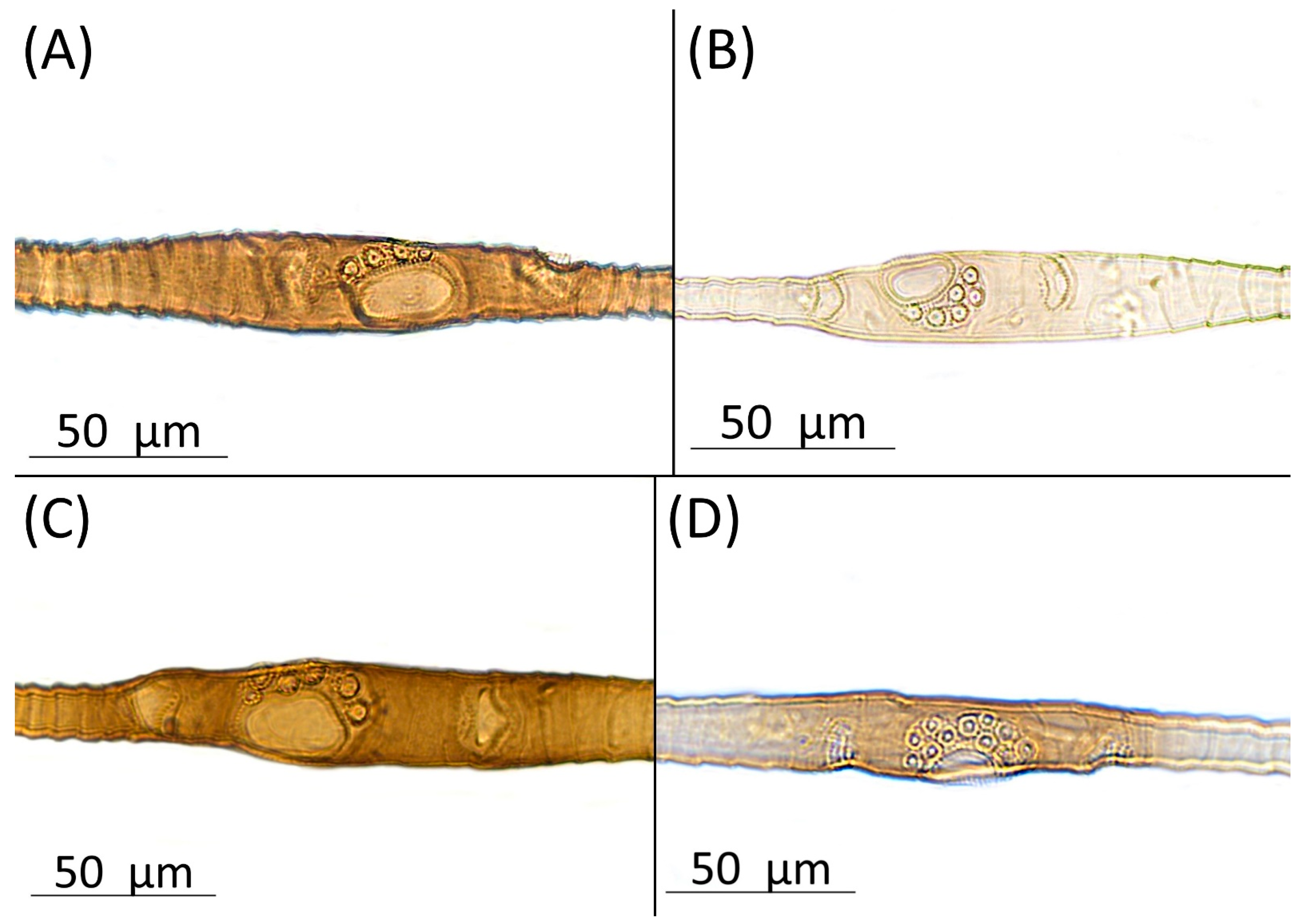
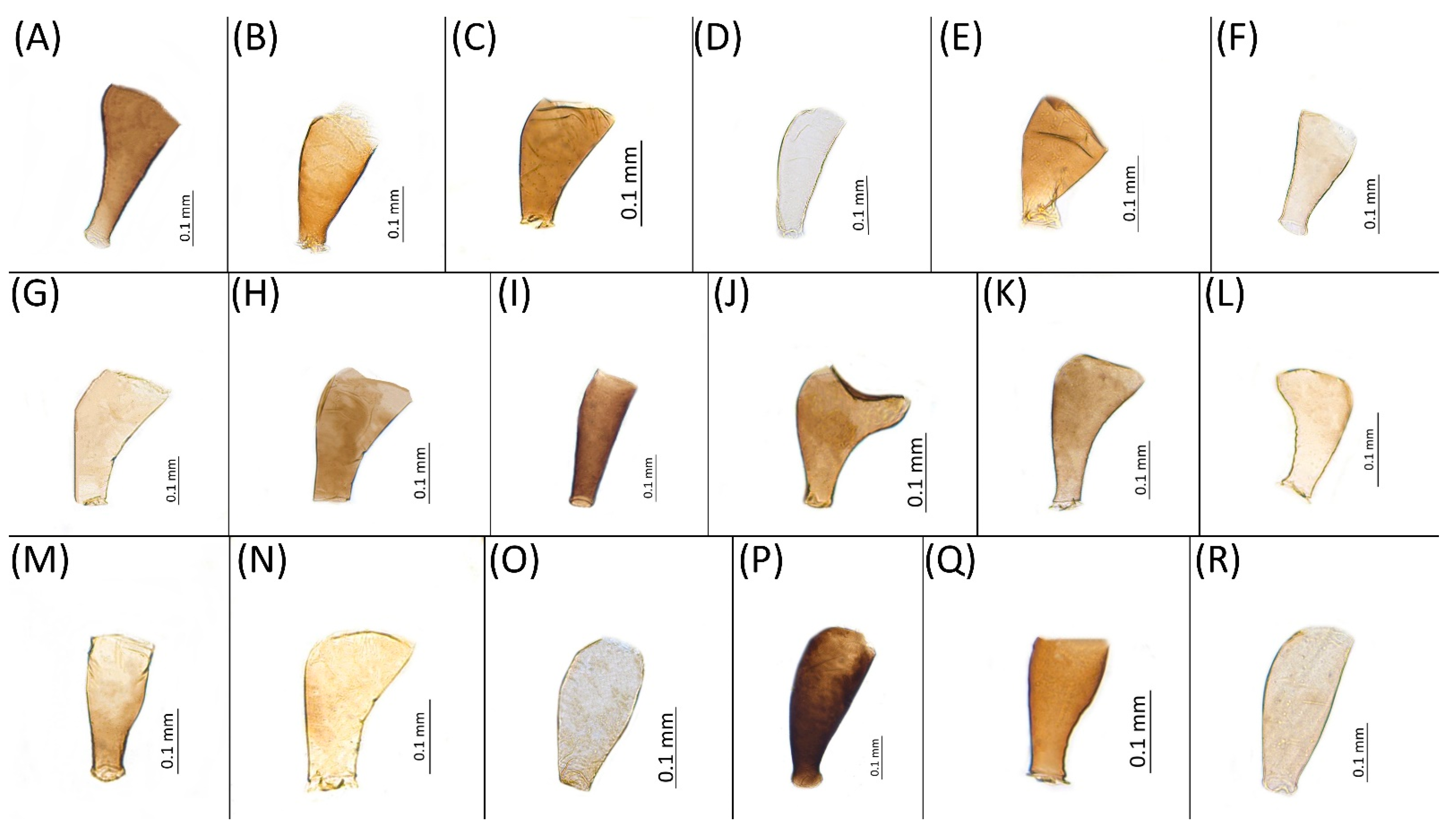
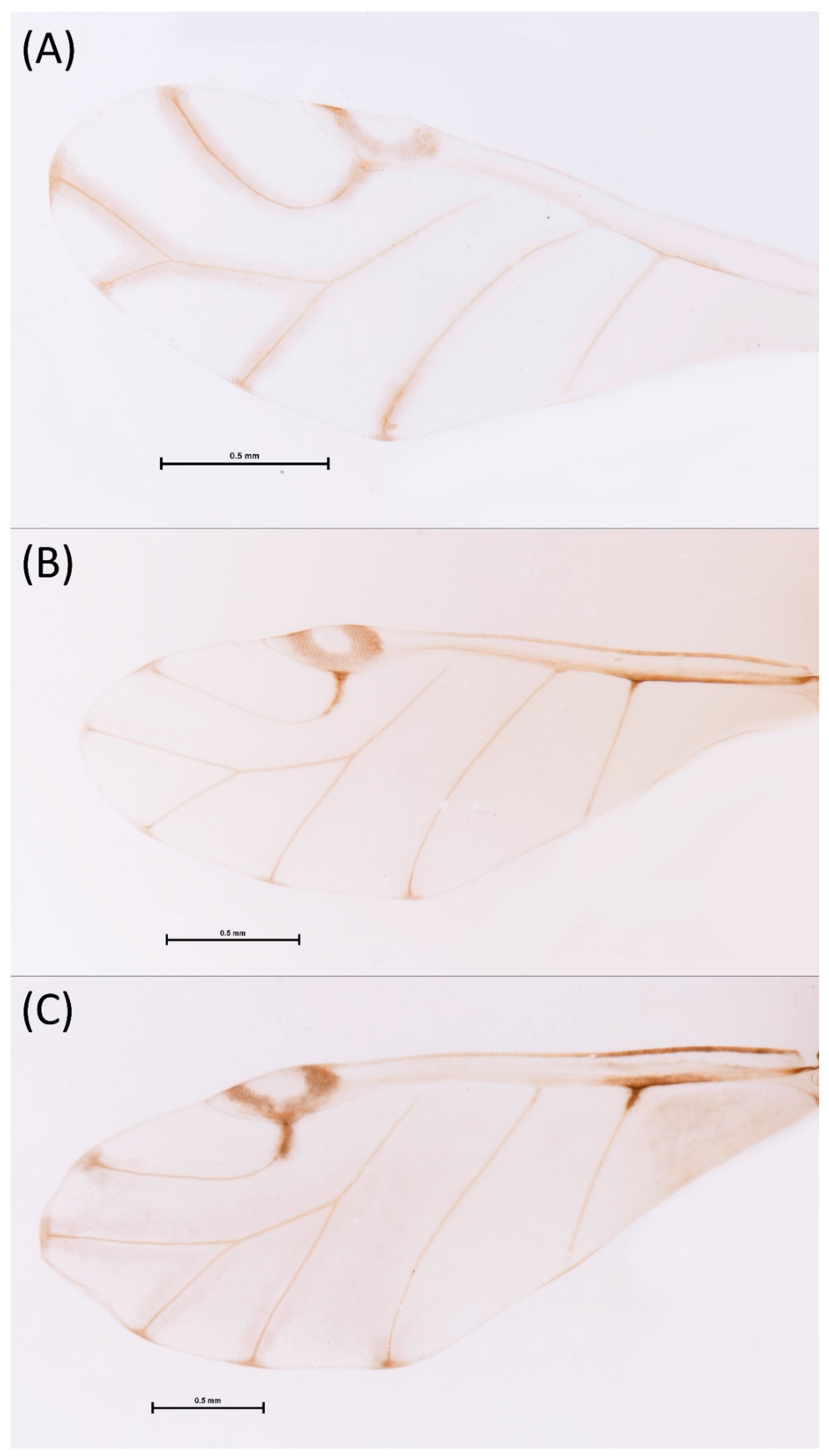
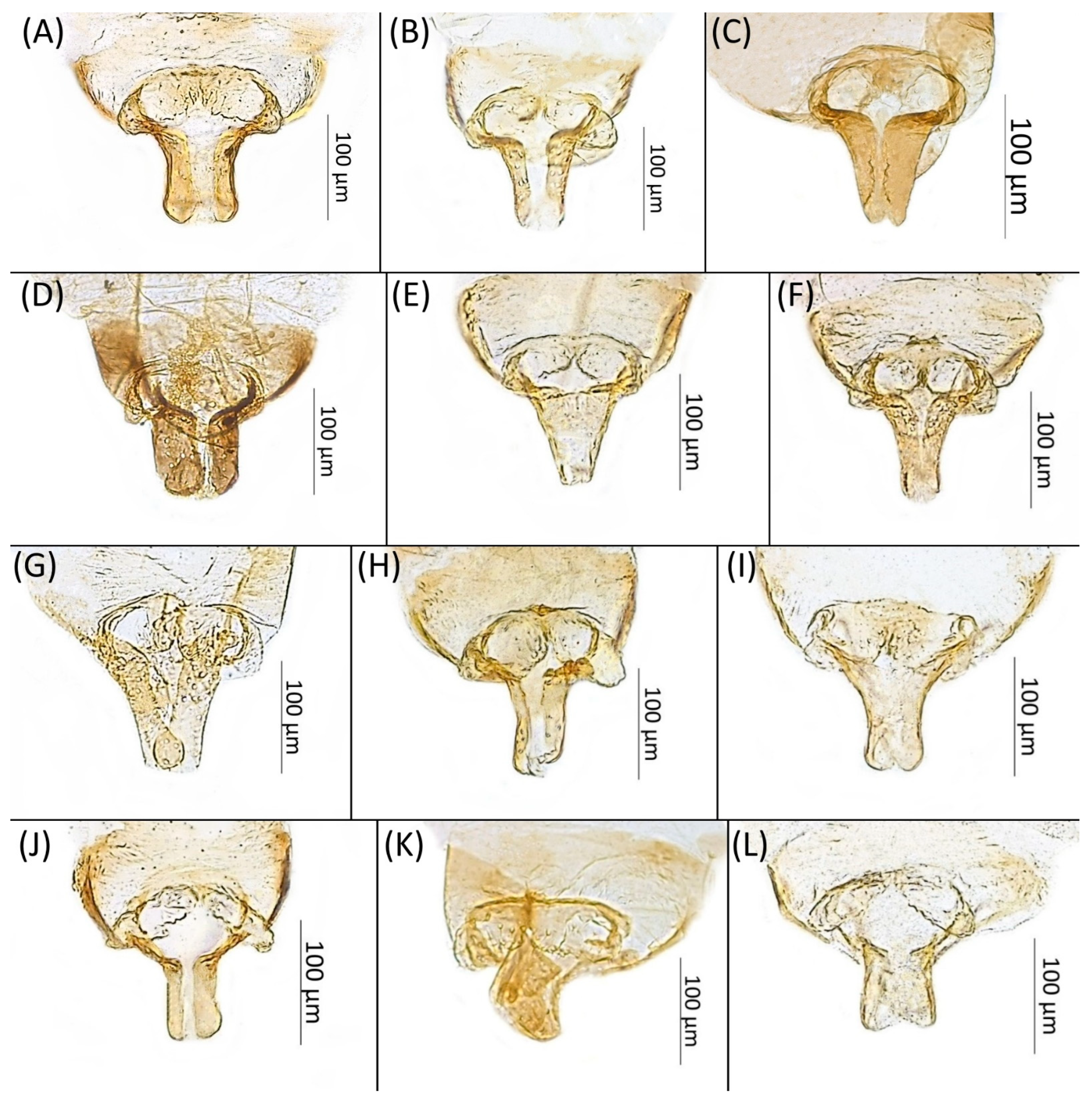
- Diagnosis: Dominant morph is alate viviparous female, characterised by distinct dorsal abdominal tubercles, variably developed on ABD I–IV (Figure 1). Oviparous females apterous, males alate. All morphs with rounded secondary rhinaria on ANT III. Primary and accessory rhinaria on BASE ciliated (Figure 2). Pterostigma distinct, darkly pigmented, with small area inside without pigmentation or palely pigmented, large area inside without pigmentation (Figure 3). Fore femora pale, dark or darker dorsally (Figure 4). Siphunculi tubular or flask-shaped (Figure 5), placed on ABD VI, swollen at base, without subapical reticulation. Almost all species are associated with species of Acer, except for D. monelli, which is found on species of Aesculus. They usually do not form dense colonies and are not attended by ants.

- Shared morphological characters of alate viviparous females of the genus Drepanaphis
- Shared morphological characters of oviparous females of the genus Drepanaphis
- Shared morphological characters of alate males of the genus Drepanaphis
3.2. Keys to Species of the Genus Drepanaphis
3.2.1. Key to the Identification of Alate Viviparous Females of the Genus Drepanaphis
- 1.
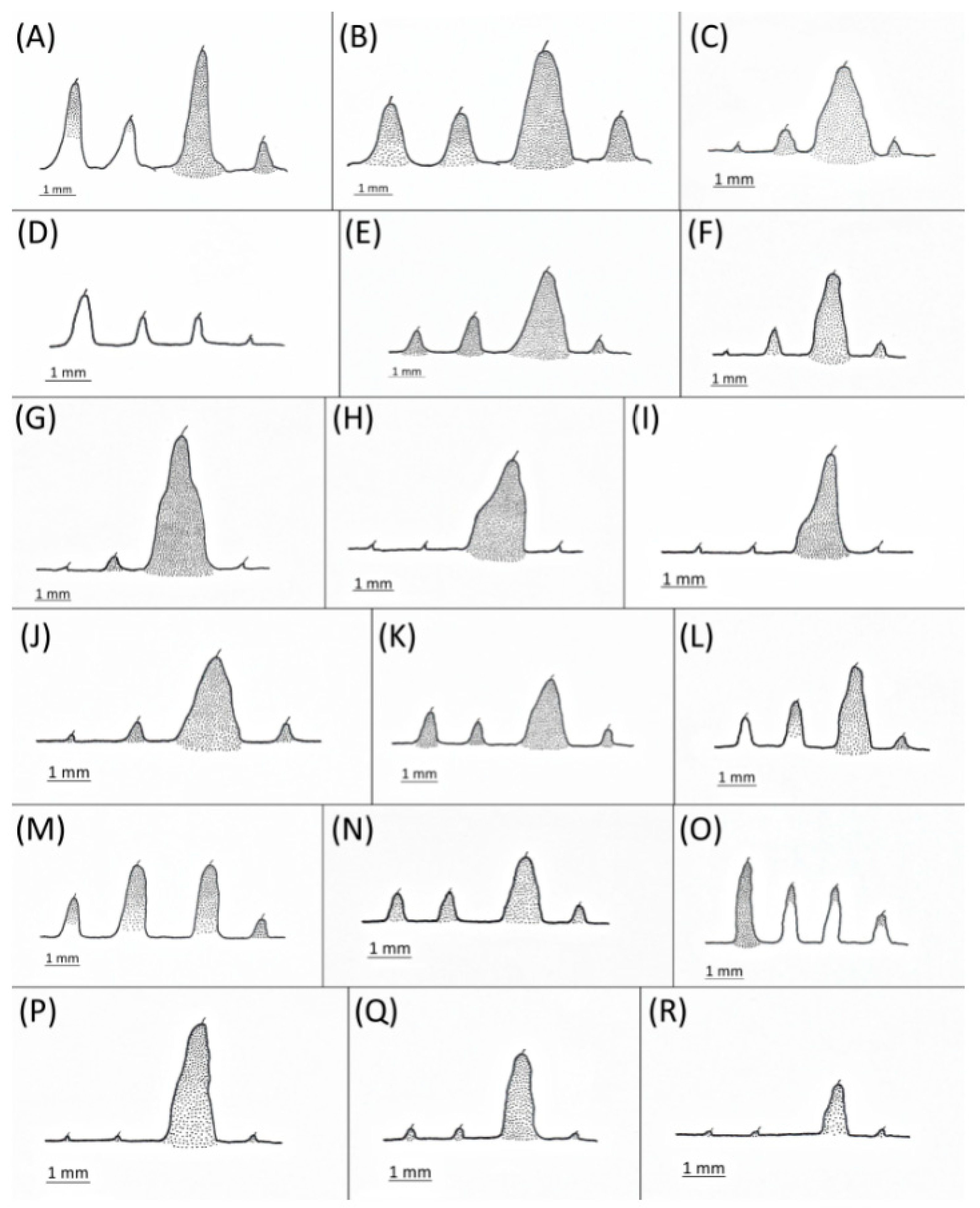
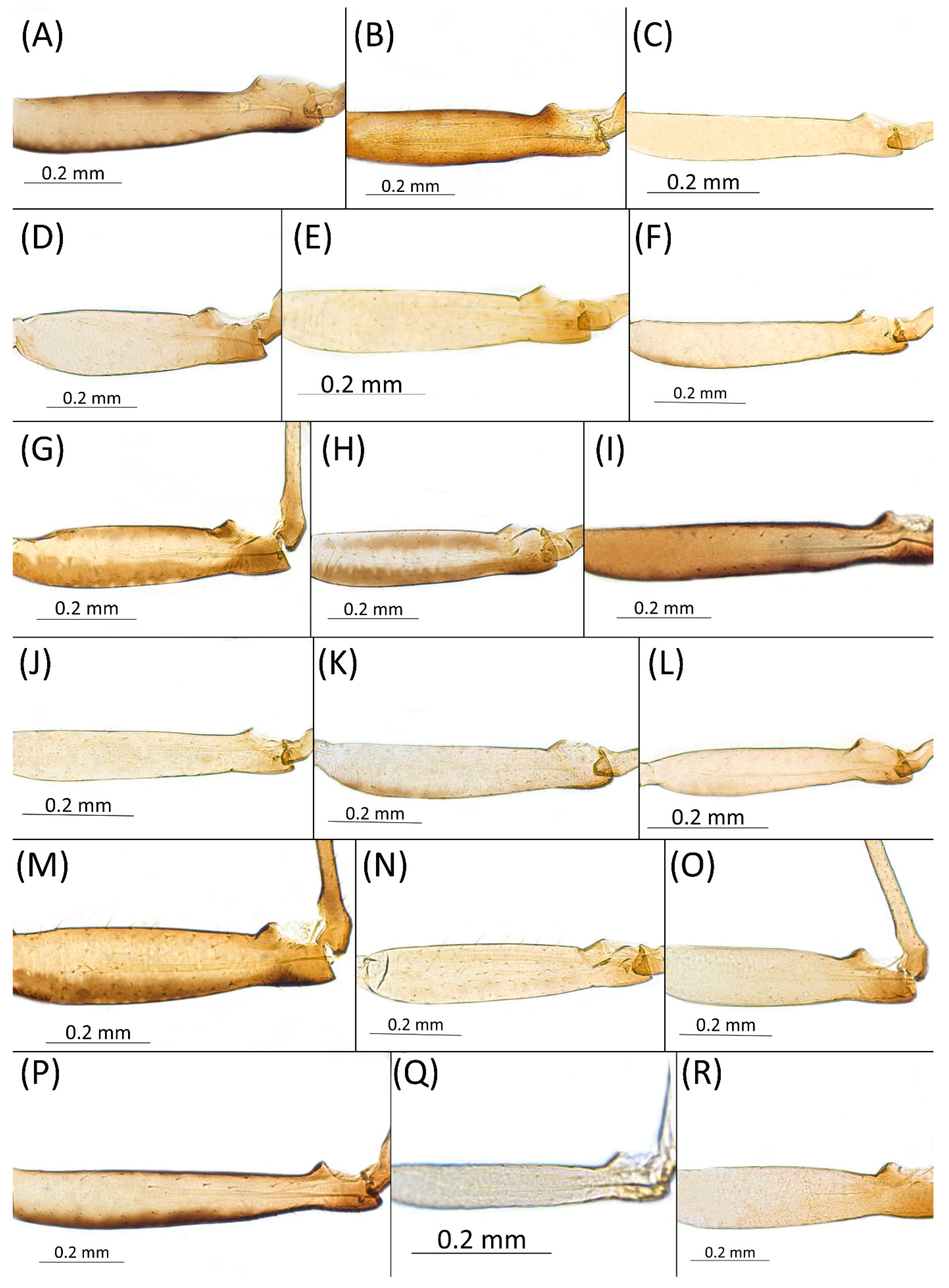
- Femur I pigmented for its full length, especially dorsally (Figure 4A,B,G–I,M,P)……………………………………………………………………………2
- -
- Femur I pale, slightly pigmented basally (Figure 4C–F,J–L,N,O,Q,R)………………………………………………………………………………8
- 2.
- Wing veins distinctly bordered (Figure 7A)………………………………………………………………………………3
- -
- Wing veins clear (Figure 7B)…………………………………………………………………………………………………4
- 3.
- Conspicuous four pairs of dorsal abdominal tubercles. ABD I pale at base and darker at tips, ABD II pale, ABD III–IV dark brown; third pairs of tubercles biggest (Figure 1A)…………………………………………………………………………………………………………D. acerifoliae
- -
- Conspicuous one pair of dark brown dorsal abdominal tubercles on tergite III (Figure 1G)……………………………………………………………………………………D. keshenae
- 4.
- Conspicuous four pairs of dorsal abdominal tubercles……………………………………5
- -
- Conspicuous one pair of dorsal abdominal tubercles on tergite III………………………6
- 5.
- First and second pair of dorsal abdominal tubercles equal, third pair biggest, fourth pair smallest (Figure 1B); BASE with 4 accessory rhinaria; ANT III with 9–15 secondary rhinaria…………………………………………………………………………………………D. carolinensis
- -
- 6.
- Fore femora > 0.8 mm long, frontal setae 0.09–0.12 mm long……………………D. spicata
- -
- Fore femora < 0.8 mm long, frontal setae > 0.09 mm long………………………………7
- 7.
- Fore femora dark (Figure 4I), BASE always with four accessory rhinaria, on Aesculus glabra…………………………………………………………………………………D. monelli
- -
- Fore femora darker dorsally (Figure 4H), BASE with four or five accessory rhinaria, on Acer grandidentatum…………………………………………………………………D. knowltoni
- 8.
- Wing veins diffusely bordered (Figure 7C)………………………………………………9
- -
- Wing veins clear (Figure 7B)………………………………………………………………10
- 9.
- BL > 1.85 mm long; body with brown dorsal sclerotisation; SIPH shaded or dark (Figure 5K)………………………………………………………………………………D. parva
- -
- BL < 1.85 mm long; body without brown dorsal sclerotisation; SIPH pale (Figure 5L)……………………………………………………………………………D. robinsoni sp. nov.
- 10.
- Two pairs of frontal setae…………………………………………………………………11
- -
- One pair of frontal setae……………………………………………………………………13
- 11.
- Conspicuous one pair of dorsal abdominal tubercles on tergite III (Figure 1R)……………………………………………………………………………………D. utahensis
- -
- Conspicuous more than one pair of dorsal abdominal tubercles……………………12
- 12.
- Entire body pale; DAT I–III short, first pair larger than second and third (Figure 1D)………………………………………………………………………………………D. granovskyi
- -
- DAT I biggest, darker than others (Figure 1O)………………………………………D. simpsoni
- 13.
- Pterostigma distinct, darkly pigmented, with small area inside without pigmentation; SIPH completely dark; dorsal setae blunt………………………………………………………14
- -
- Pterostigma palely pigmented with large area inside without pigmentation, SIPH shaded or pale, dorsal setae pointed……………………………………………………17
- 14.
- BASE with more than four accessory rhinaria…………………………………………15
- -
- BASE with four accessory rhinaria………………………………………………………16
- 15.
- ANT III with 2–5 secondary rhinaria, frontal setae 0.06–0.08 mm long, dorsal setae 0.03–0.05 mm long…………………………………………………………D. choanotricha
- -
- ANT III with 8–14 secondary rhinaria, frontal setae 0.05–0.06 mm long, dorsal setae 0.01–0.02 mm long……………………………………………………………………D. tissoti
- 16.
- ANT III with 6–9 secondary rhinaria, dorsal setae 0.02–0.04 mm long……D. idahoensis
- -
- ANT III with 12–18 secondary rhinaria, dorsal setae 0.01–0.02 mm long……D. nigricans
- 17.
- Conspicuous four pairs of dorsal abdominal tubercles (Figure 1N)………D. saccharini
- -
- Conspicuous three pairs of dorsal abdominal tubercles, on ABD III biggest, on DAT II and IV very small (Figure 1F)…………………………………………………D. kanzensis
3.2.2. Key to the Identification of Known Oviparous Females of the Genus Drepanaphis
- 1.
- BASE with more than four accessory rhinaria………………………………………………2
- -
- BASE with four accessory rhinaria…………………………………………………………4
- 2.
- ANT segment ratio PT/BASE < 12; >70 pseudosensoria on hind tibiae; legs and antennae very dark, siphunculi tubular………………………………………………D. sabrinae
- -
- ANT segment ratio PT/BASE > 12, siphunculi flask-shaped……………………………3
- 3.
- ANT III > 0.9 mm long; > 30 pseudosensoria; SIPH/BL 0.07–0.09…………………D. tissoti
- -
- ANT III < 0.9 mm long; < 30 pseudosensoria; SIPH/BL 0.12………………D. choanotricha
- 4.
- Two pairs of frontal setae………………………………………………………………………5
- -
- One pair of frontal setae…………………………………………………………………………7
- 5.
- ANT III < 0.05 mm long; dorsal setae evidently forked………………………D. granovskyi
- -
- ANT III > 0.05 mm long…………………………………………………………………………6
- 6.
- URS/ANT III < 0.13 mm long…………………………………………………………D. utahensis
- -
- URS/ANT III > 0.13 mm long…………………………………………………………D. simpsoni
- 7.
- Legs and SIPH dark……………………………………………………………………………8
- -
- Legs and SIPH pale……………………………………………………………………………11
- 8.
- SIPH/BL > 0.09; HT II/ANT VI < 0.1…………………………………………………D. monelli
- -
- SIPH/BL < 0.09; HT II/ANT VI > 0.1……………………………………………………………9
- 9.
- URS/BASE < 0.07……………………………………………………………………D. carolinensis
- -
- URS/BASE > 0.07………………………………………………………………………………10
- 10.
- ANT > 2.6 mm long; SIPH > 0.2 mm long…………………………………………D. acerifoliae
- -
- ANT < 2.6 mm long; SIPH < 0.2 mm long…………………………………………………D. keshenae
- 11.
- ANT segment ratio PT/BASE > 12………………………………………………D. nigricans
- -
- ANT segment ratio PT/BASE < 12…………………………………………………………12
- 12.
- SIPH/BL < 0.08; URS < 0.09………………………………………………………D. kanzensis
- -
- SIPH/BL > 0.08; URS > 0.09…………………………………………………………………13
- 13.
- ANT segment ratio VI/III < 1; 22–23 pseudosensoria………………………D. idahoensis
- -
- ANT segment ratio VI/III > 1; 59–71 pseudosensoria……………………………D. spicata
3.2.3. Key to the Identification of Known Alate Males of the Genus Drepanaphis
- 1.
- Femur I pigmented for its entire length, two pairs of frontal setae, dorsal abdominal tubercles inconspicuous………………………………………………………D. granovskyi
- -
- Femur I pigmented dorsally…………………………………………………………………2
- -
- Femur I pale, slightly pigmented basally…………………………………………………7
- 2.
- Wing veins distinctly bordered, conspicuous one pair of dorsal abdominal tubercles on tergite III……………………………………………………………………………………3
- -
- Wing veins clear……………………………………………………………………………4
- 3.
- Hind tibiae with dark area in apical part; PT/BASE < 0.09……………………D. acerifoliae
- -
- Hind tibiae pale; PT/BASE > 0.09…………………………………………………D. keshenae
- 4.
- Dorsal abdominal tubercles inconspicuous, BL 2.8 mm long…………………D. spicata
- -
- Conspicuous one pair of dorsal abdominal tubercles on tergite III……………………5
- 5.
- URS/ANT III > 0.12………………………………………………………………D. monelli
- -
- URS/ANT III < 0.12…………………………………………………………………………6
- 6.
- ANT III with > 80 rhinaria, hind tibiae < 1.2 mm long; SIPH < 0.25………D. carolinensis
- -
- ANT III with < 80 rhinaria, hind tibiae > 1.2 mm long; SIPH > 0.25…………D. knowltoni
- 7.
- Conspicuous more than one pair of dorsal abdominal tubercles, wing veins diffusely bordered, body with brown dorsal sclerotisation………………………………D. parva
- -
- Wing veins clear…………………………………………………………………………………8
- 8.
- Dorsal abdominal tubercles inconspicuous, two pairs of frontal setae…………………9
- -
- Conspicuous one pair of dorsal abdominal tubercles on tergite III………………………10
- 9.
- ANT segment ratio PT/BASE < 6; SIPH/BL < 0.09………………………………D. simpsoni
- -
- ANT segment ratio PT/BASE > 6; SIPH/BL > 0.09………………………………D. utahensis
- 10.
- ANT segment ratio PT/BASE > 6; SIPH pale……………………………………D. kanzensis
- -
- ANT segment ratio PT/BASE < 6; SIPH dark.………………………………………D. choanotricha
3.3. Checklist of Species in the Genus Drepanaphis
- Family: Aphididae Latreille, 1802Subfamily: Drepanosiphinae Herrich-Schaeffer, 1857Genus: Drepanaphis Del Guercio, 1909Drepanaphis Del Guercio, 1909: 4: 49= Phymatosiphum Davis, 1909: 2: 196= Drepanphis Takahashi, 1923: 4: 66 (subsequent misspelling)1. Drepanaphis acerifoliae (Thomas, 1878)2. Drepanaphis carolinensis Smith, 19413. Drepanaphis choanotricha Smith & Dillery, 19684. Drepanaphis granovskyi Smith & Knowlton, 19435. Drepanaphis idahoensis Smith & Dillery, 19686. Drepanaphis kanzensis Smith, 19417. Drepanaphis keshenae Granovsky, 19318. Drepanaphis knowltoni Smith & Dillery, 19689. Drepanaphis monelli (Davis, 1909)10. Drepanaphis nigricans Smith, 194111. Drepanaphis parva Smith, 194112. Drepanaphis robinsoni Malik sp. nov.13. Drepanaphis sabrinae Miller, 193714. Drepanaphis saccharini Smith & Dillery, 196815. Drepanaphis simpsoni Smith, 195916. Drepanaphis spicata Smith, 194117. Drepanaphis tissoti Smith, 1941 stat. rev.18. Drepanaphis utahensis Smith & Knowlton, 1943
3.4. Review of Species
3.4.1. Drepanaphis acerifoliae (Thomas, 1878)
- Type species Siphonophora acerifoliae Thomas, 1878 by original designationType species Siphonophora acerifoliae Thomas, 1878 by original designation= Siphonophora acerifoliae Thomas, 1878: 1(2): 4 [17]= Siphonophora acericola Thomas, 1878: 1(2): plate 1 (subsequent misspelling) [17]= Drepanosiphum acerifolii Monell, 1879: 5(1): 27 [40]= Drepanosiphum acerifoliae Davidson, 1909: 2(4): 303 [41]Drepanaphis acerifoliae Del Guercio, 1909: 2 4(4): 50 [16]= Drepanaphis acerifolii Davis, 1910: 3(5): 419 (subsequent misspelling) [42]= Drepanaphis allegheneyensis Miller, 1936: 68: 81 [43]Figure 1A, Figure 2A, Figure 3A, Figure 4A, Figure 5A, Figure 7A, Figure 8A, Figure 9A, Figure 10A, Figure 11A, Figure 11A, Figure 12A, Figure 13A, Figure 14A and Figure 15; Table 1, Table 2 and Table 3Material examined: Paratype. Siphonophora acerifoliae Thomas, 757, Ft. Dodge, Iowa, Dubuque, Iowa, Peoria, Ill. Sept. I, 1887, SI. 7169, Ill. Nat.Hist. Sur.//Aphididae, Drepanaphis acerifoliae Thomas, See slide by Davis, Det. F. C. Hottes, Ill. Nat. Hist. Sur.//PARATYPE, Siphonophora acerifoliae Thomas//INHS Insect Collection 1058753—15 alate viv. fem. [INHS]. Lectotype. Aphididae, Drepanaphis acerifoliae (Thomas), acerifolae, Ft. Dodge + Dubuque, Iowa, also Peoria, Ill., 757, Sept. I, 1897, Det. F.C. Hottes, Ill. Nat. Hist Sur. ’28, SI 7168//Lecto-type, Siphonophora acerifoliae Thomas, Viviparous ♀ ♀//INHS, Insect Collection 457903—five alate viv. fem. [INHS].Additional material examined—Table S6.
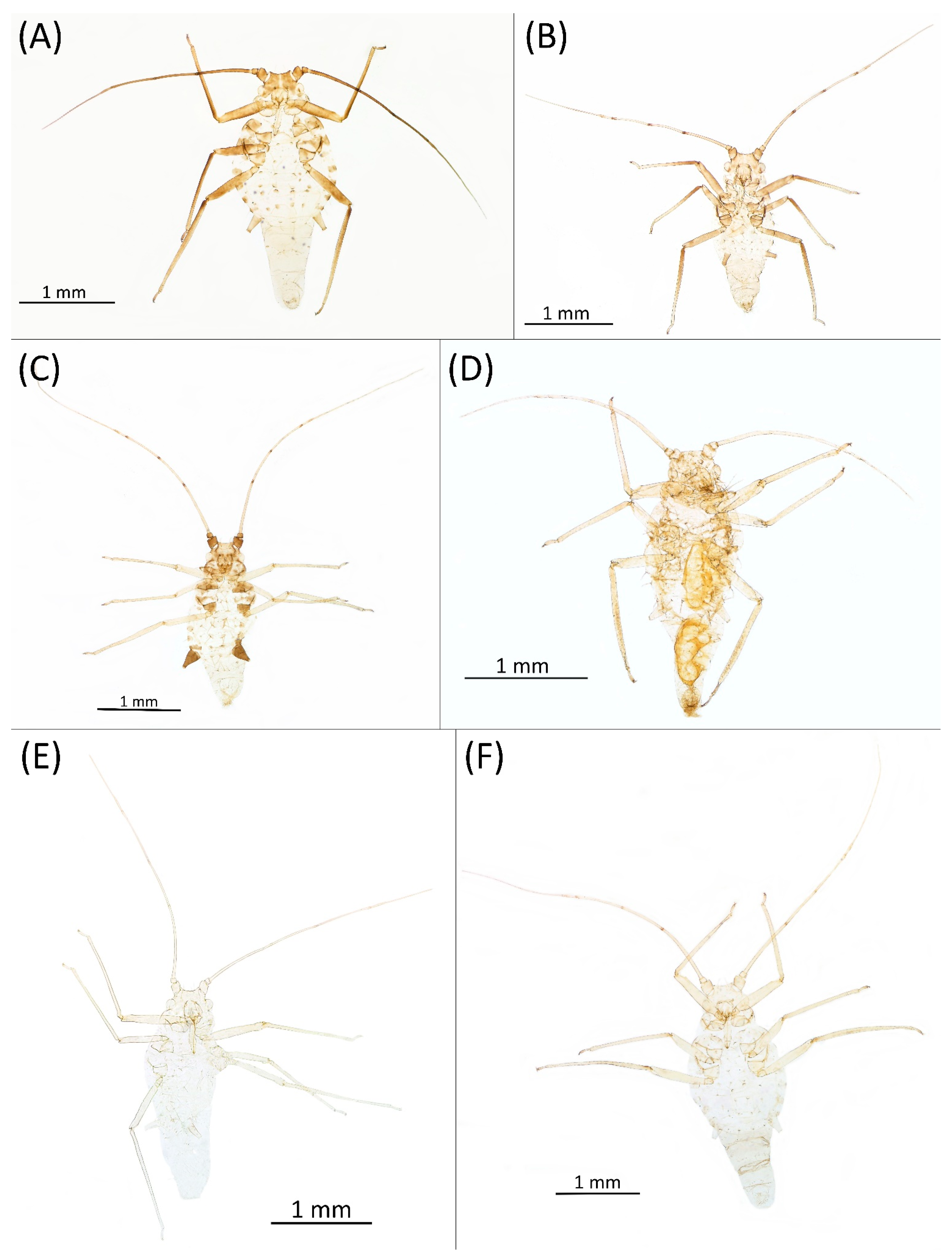
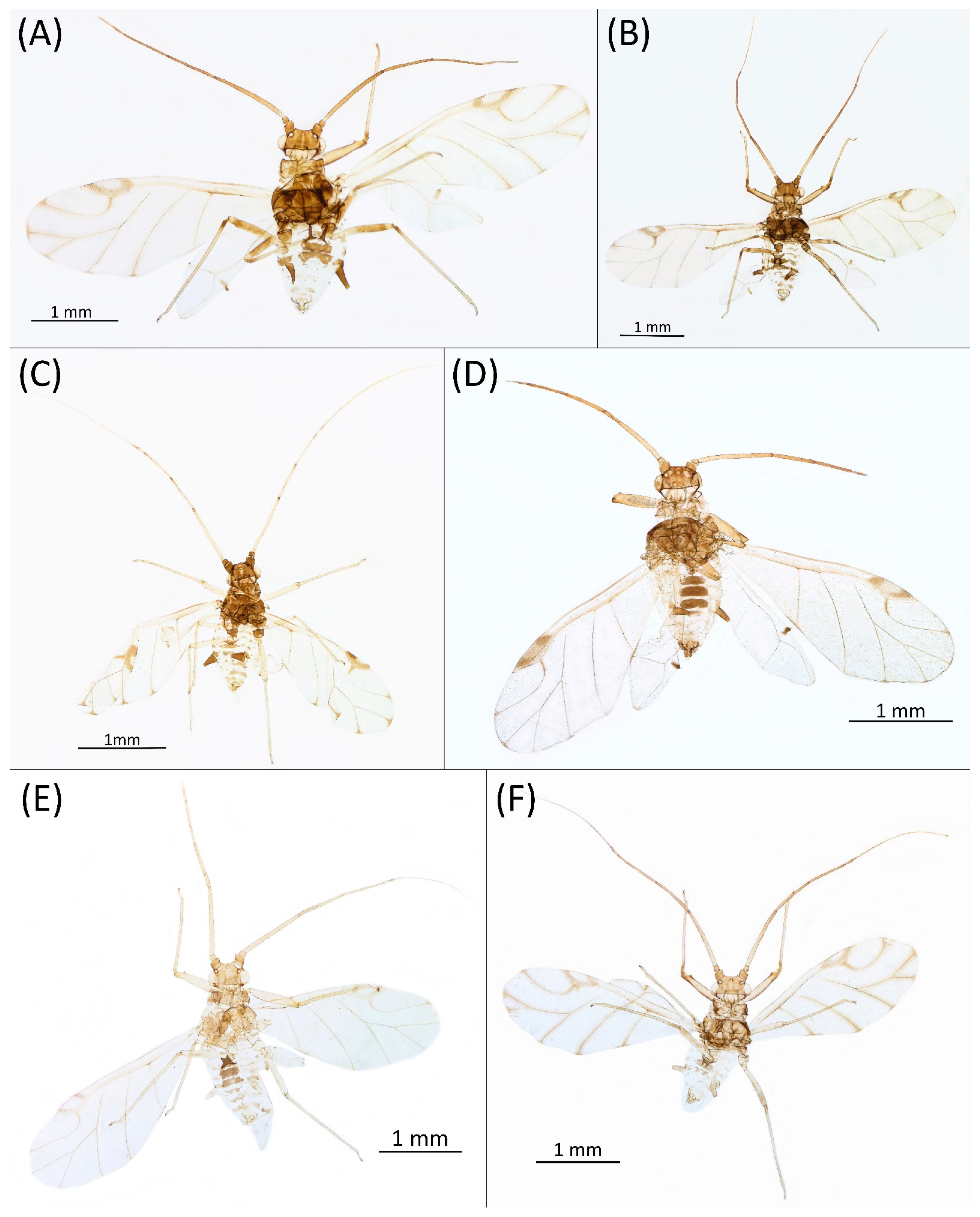
- Alate viviparous female—re-description (n = 26)Colour. In life: Head and thorax brown to dark brown with white wax stripes. Eyes red. Antennae pale with dark apices of ANT III–V. Fore femora darker than middle and hind femora, dark brown dorsally. Middle and hind femora pale brown to brown. Tibiae pale brown. Wing veins dark bordered, pterostigma dark brown. Abdomen covered by white wax dots. Tergites I–V brown, tergites I–II slightly lighter than tergites III–V. Tergites VI–VIII fully covered by wax. Siphunculi dark (Figure 8A).

- Pigmentation of mounted specimens: Head and pronotum brown, rest of thorax dark brown (Figure 9A). ANT I–II brown, ANT III–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3A). Wing veins brown bordered (Figure 7A). Abdomen pale brown, marginal sclerites dark brown. DAT I pale at base, darker at tips; DAT II pale; DAT III–IV dark brown (Figure 1A). Siphunculi brown to dark brown; cauda, subgenital and anal plate pale. Fore femora darker dorsally (Figure 4A). Middle and hind femora brown with dark brown smudge. Tibiae brown with darker distal parts. Tarsi dark brown.
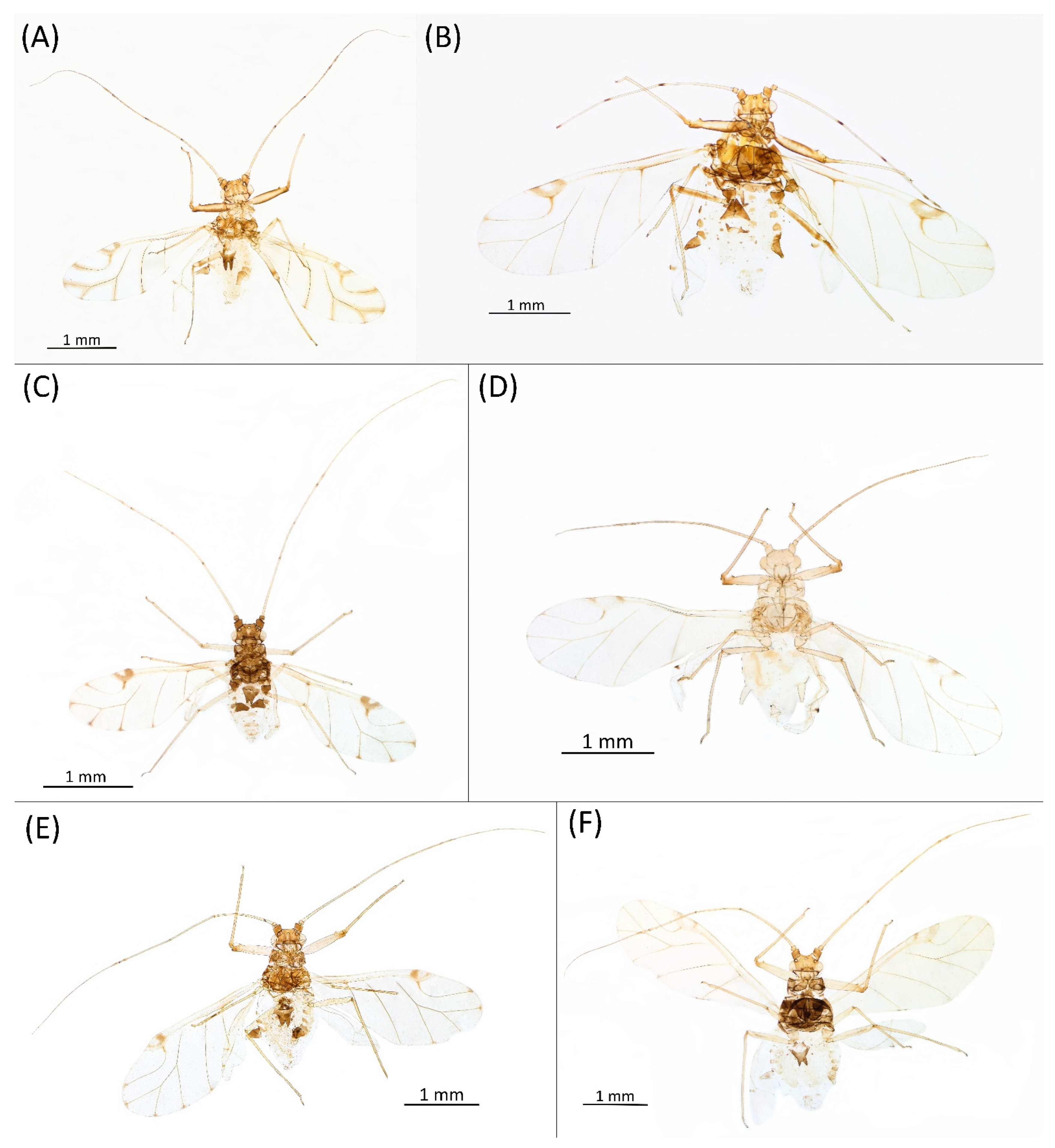
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.04–0.05 mm long with pointed apices, one pair of pointed frontal setae on ventral side 0.09–0.1 mm long. ANT/BL 1.57–2.26; ANT/HW 9.23–13.74; PT/BASE 6.93–12.02. ANT III with 9–14 secondary rhinaria, BASE with 4 accessory rhinaria (Figure 2A), URS with 4–8 accessory setae (Figure 10A). Other ratios: ANT IV/ANT III 0.64–0.85; ANT V/ANT III 0.64–0.83; ANT VI/ANT III 1.12–1.74; URS/ANT III 0.08–0.13; URS/BASE 0.59–0.88; URS/SIPH 0.25–0.52; HT II/ANT III 0.09–0.14; HT II/BASE 0.62–0.9; TIBIA III/BL 0.49–0.73; SIPH/BL 0.1–0.15; SIPH/CAUDA 1.5–2.9. Dorsal abdominal segments with four pairs of distinct tubercles. DAT I 0.14–0.28 mm long, DAT II 0.07–0.14 mm long. DAT III largest, 0.23–0.37 mm long (Figure 11A). DAT IV smallest, 0.04–0.06 mm long. Pointed setae at ends of tubercles, 0.02–0.03 mm long. Dorsal setae with pointed apices, 0.04–0.06 mm long, on small sclerites on ABD I–V. Marginal sclerites with 3–10 setae. Siphunculi flask-shaped (Figure 5A).


- Oviparous female—re-description (n = 6)Colour. In life: Head, thorax and abdomen reddish brown. Last segments of abdomen slightly darker. Marginal sclerites dark brown. Eyes red. Antennae dark brown. Siphunculi dark with lighter area on bases. Femora dark dorsally [10].Pigmentation of mounted specimens: Head brown, pronotum pale brown. ANT brown to dark brown with darker apices of segments. Cauda, subgenital and anal plate pale. Femora, tarsi and siphunculi brown. Tibiae dark brown with darker knee areas and distal parts. Abdominal sclerites brown (Figure 12A).
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, 0.1–0.12 mm long; one pair of latero-dorsal setae, one pair of postero-dorsal setae, 0.05–0.06 mm long with blunt apices on dorsal side; one pair of pointed frontal setae on ventral side, 0.1 mm long. ANT/BL 0.93–1.04. Other ratios: ANT VI/ANT III 1.28–1.83; PT/BASE 5.77–9.0; SIPH/BL 0.07–0.08; FEMUR III/BL 0.2–0.22; TIBIA III/BL 0.37–0.43; HT II/ANT VI 0.11–0.14; URS/ANT III 0.14–0.17; URS/BASE 0.71–0.92; URS/SIPH 0.44–0.5. ANT III without secondary rhinaria. URS with 4–8 accessory setae. Hind tibiae with 50–110 pseudosensoriae distributed along almost their entire lengths. Dorsal setae 0.07–0.14 mm long. Pleural and spinal setae on ABD I–V, placed on small dark sclerites. Marginal sclerites on ABD I–V bigger. Siphunculi tubular.Alate male—re-description (n = 3)Colour. In life: Head and thorax brown to dark brown with white wax stripes. Eyes red. Antennae pale with dark apices of segments. Wing veins dark brown bordered. Abdomen covered by white wax dots. ABD I–II and VI–VII more intensively covered by wax. Siphunculi dark. Femora pale brown. Tibiae pale brown with darker knee areas.Pigmentation of mounted specimens: Head brown, thorax dark brown. Abdomen pale with brown spinal and marginal sclerites. ANT dark brown with darker apices of segments. ANT III slightly lighter at base. Wing veins brown bordered. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Dorsal abdominal tubercles and siphunculi dark brown. Cauda and anal plate pale. Fore femora brown, darker dorsally. Middle and hind femora brown with dark brown smudge. Tibiae brown with darker knee areas and distal parts. Tarsi brown (Figure 13A).
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.07 mm long. ANT/BL 1.58. Other ratios: ANT VI/ANT III 1.4; PT/BASE 8.3; SIPH/BL 0.1–0.13; FEMUR III/BL 0.26–0.3; TIBIA III/BL 0.54–0.61; URS/ANT III 0.1–0.13; URS/SIPH 0.33–0.39. ANT III with 80–100 rhinaria, ANT IV with 38–50 rhinaria, ANT V with 17–21 rhinaria. URS with 6–8 accessory setae. DAT I inconspicuous or very small, 0.05–0.07 mm long. DAT III distinct, 0.15–0.18 mm long with setae 0.03 mm long at end. Dorsal setae 0.03–0.05 mm long, on small sclerites, bigger on ABD II–V. ABD IV–V with two spinal sclerites, each with two setae 0.03–0.04 mm long. Marginal sclerites with 3–10 setae 0.03–0.05 mm long. Siphunculi flask-shaped. Genitalia with basal part of phallus elongated, with broadly rounded apices (Figure 14A).
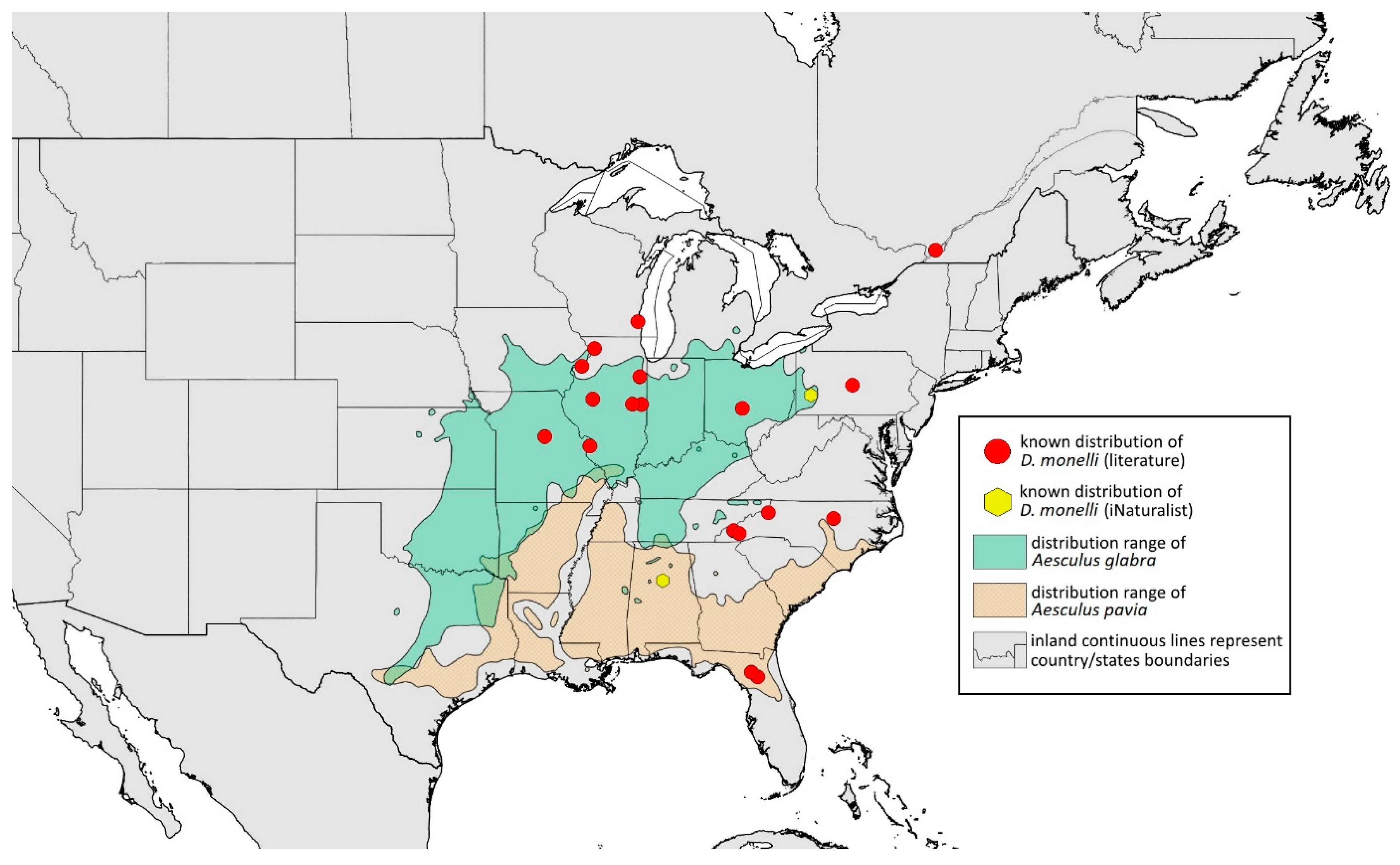
- Remarks: The locus typicus is not designated since the type slides bear three locality names—Fort Dodge and Dubuque in Iowa and Peoria in Illinois (after Hottes and Frison [10], as well as Smith and Dillery [27]).Host plants: Acer rubrum, Acer saccharinum, Acer saccharum, also found on plantings of Acer platanoides (in North America), occasionally on Acer nigrum.Distribution: Canada: British Columbia (North Vancouver^); Manitoba (Winnipeg); New Brunswick (Middle Kouchibouguac‡, Rothesay‡); Ontario (Front of Yonge‡, Hamilton^, Havelock-Belmont-Methuen^, Leamington, London, North Perth‡, Perth South‡, Puslinch^, Smith-Ennismore-Lakefield‡); Quebec (Orsainville in Quebec City, Shawinigan (Lac Wapizagonke)‡). USA: California (Berkeley, Lodi, Palo Alto (vicinity of Stanford University), San Jose); Colorado (Boulder, Denver, Fort Collins, Greeley); Connecticut (Hamden, Hartford, New Haven); Florida (Gainesville, Highlands Hammock State Park, Lake Placid); Idaho (Eagle); Illinois (Albion, Alma*, Alton, Augerville, Bloomfield Precinct*, Cairo, Carbondale, Catlin, Danville, Dixon Springs, Edwardsville, Elizabethtown, Fairmount Township, Golconda, Grayville, Havana, Herod, Kankakee, Le Roy, Macomb, MURShall, Mattoon, Metropolis, Mount Carmel, Mount Carroll, Mount Pulaski*, Newton, Normal, Oregon, Pekin, Peoria, vicinity of Perry*, Rock Island, Quincy, Shawneetown, Starved Rock State Park, Springfield, Tonti, Urbana); Iowa (Dubuque, Fort Dodge); Kansas (Maple Hill*); Maine (Orono); Maryland (Beltsville, Laurel†); Minnesota (Saint James); Missouri (Columbia, Crane, Kansas City, Saint Louis, Steelville); Nebraska (Ashland, Lincoln, Weeping Water); North Carolina (Alamance, Tunnel Bypass Trail near Bryson City, Burlington, Chapel Hill, Cherokee, Franklin, Great Smoky Mountains National Park, Greensboro, Raleigh, Reidsville, Roaring Gap, Roxboro, Wilkesboro); Ohio (Columbus, Ostrander); Oregon (Corvallis); Pennsylvania (Bryn Athyn, Chambersburg, Houserville, Lancaster, Loganville, Miquon, New Bloomfield, Philipsburg, Pittsburgh, State College); South Carolina (Easley, Hardeeville); Tennessee (Cosby Horse Trail near Cosby); Utah (Bountiful, East Canyon Cashe Co., Logan, Payson, Provo, Salt Lake City); Virginia (Chatham); Washington (Yakima); Washington, D.C.; West Virginia (Martinsburg“); Wisconsin (Montello*, Shields in Marquette County). Europe: Hungary (Cegléd, Gazdagrét, Pesterzsébet, Tabán, Törökvész); Italy (Calendasco, Carlazzo, Milan (Bosco in Città, Sempione Parc), Nola); Serbia (Belgrade (Banjica, New Belgrade, Vrčin, Zemun near Danube), Novi Sad (Bistrica, Novo Naselje)); Spain (Astorga, León, Lleida) (Figure 15) ([10,17,21,23,24,27,41,44,45,46,47,48,49,50,51,52,53,54,55,56,57]; Centre for Biodiversity Genomics—Canadian Specimens [‡]; Illinois Natural History Survey Insect Collection [*]; International Barcode of Life project (iBOL) [^]; Natural History Museum of Denmark Entomology Collection [“]; new record in this publication [†]).

- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): Canada: Ontario (LaSalle, Ottawa); Quebec (Dorval Island). USA: Alabama (Fort Payne, Hoover); California (Albany); Florida (Parkland); Georgia (Cashes Valley, Kennesaw, LaFayette); Illinois (New Lenox); Indiana (Zionsville); Iowa (Cedar Rapids); Kentucky (eastern outskirts of Louisville); Massachusetts (Williamstown); North Carolina (vicinity of Barnardsville, Charlotte, Durham); Ohio (Rest Area Southbound Wapakoneta); Pennsylvania (Bethel Park, Buckingham Springs, vicinity of Strickersville, Villanova University); Virginia (Dulles, Far Hills, Forest Lakes, Holly Knoll Cir near Great Falls, Great Falls Park, Herndon, Woodbridge); Wisconsin (Cross Plains). Europe: Spain (Santiago de Compostela).
3.4.2. Drepanaphis carolinensis Smith, 1941
- Drepanaphis carolinensis Smith, 1941: 57(2): 228, 231 [23]Figure 1B, Figure 3B, Figure 4B, Figure 5B, Figure 7B, Figure 8B, Figure 9B, Figure 11B, Figure 12B, Figure 13B, Figure 14B and Figure 16; Table 1, Table 2 and Table 3Material examined: Holotype. Drepanaphis carolinensis Smith, Holotype Type No 55834. D.D.N.N.M.//N.C, Aphids, Host Acer, Raleigh, N.C. 193. Date 4–28-40. C.F. Smith—six alate viv. fem. (USNM) Paratype. Drepanaphis carolinensis Smith//N. C. Aphids, Host Maple, Raleigh, N.C. Date 4-26-40, 193, C. F. Smith//INHS, Insect Collection 1,058,855—four alate viv. fem. Paratype. Drepanaphis carolinensis Smith//N. C. Aphids, Host Acer, Raleigh, N.C. Date 4-30-40, 193, C. F. Smith//INHS, Insect Collection 1,058,858—four alate viv. fem. Paratype. Drepanaphis carolinensis Smith//N. C. Aphids, Host Sugar Maple, Milburnie, NC, Date 21 May 1940, C. F. Smith//INHS, Insect Collection 1,058,859—three alate viv. fem. Paratype. Drepanaphis carolinensis Smith//N. C. Aphids, Host Acer, Raleigh, N.C. Date 4-30-40, 193, C. F. Smith (IECA).Additional material examined—Table S6.Alate viviparous female—re-description (n = 16)Colour. In life: Head, thorax and abdomen reddish brown. Head and pronotum with longitudinal white wax stripes. Abdomen covered by white wax dots, more intensively on ABD I–II and VI–VIII. Eyes red, antennal segments pale with dark apices. Wings clear with small area of dark brown pigmentation at end, radius veins brown. Pterostigma brown. Femora and siphunculi dark. Tibiae pale. DAT dark brown (Figure 8B).Pigmentation of mounted specimens: Head, pronotum, ANT I–II brown. Rest of thorax dark brown (Figure 9B). ANT III–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with small area of dark brown pigmentation at end, radius veins dark brown (Figure 7B). Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3B). Abdomen pale brown, marginal sclerites dark brown. DAT (Figure 1B) and siphunculi dark brown. Cauda, subgenital and anal plate pale. Fore femora brown darker dorsally (Figure 4B). Middle and hind femora brown with darker smudge. Hind femora with darker stripes at margins. Tibiae brown with darker knee areas and distal parts. Tarsi brown.Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.04 mm long with pointed apices; one pair of pointed frontal setae on ventral side 0.07–0.08 mm long. ANT/BL 1.44–2.07; ANT/HW 9.3–13.25; PT/BASE 5.56–8.1. ANT III with 9–15 secondary rhinaria, BASE with 4 accessory rhinaria, URS with 4–8 accessory setae. Other ratios: ANT IV/ANT III 0.64–0.82; ANT V/ANT III 0.62–0.75; ANT VI/ANT III 1.02–1.6; URS/ANT III 0.09–0.12; URS/BASE 0.56–0.71; URS/SIHP 0.38–0.56; HT II/ANT III 0.11–0.14; HT II/BASE 0.6–0.89; TIBIA III/BL 0.5–0.66; SIPH/BL 0.08–0.14; SIPH/CAUDA 1.33–2.63. DAT with four pairs of distinct tubercles. DAT I 0.13–0.16 mm long, slightly larger than DAT II 0.1–0.13 mm long. DAT III largest, 0.23–0.32 mm long (Figure 11B). DAT IV smallest, 0.06–0.1 mm long. Dorsal setae with pointed apices, 0.02–0.04 mm long, on small sclerites on ABD I–V. Marginal sclerites with 3–6 setae. Siphunculi flask-shaped (Figure 5B).Oviparous female—description (n = 8)Colour. In life: Unknown.Pigmentation of mounted specimens: Head and thorax brown, abdomen pale. ANT I–II brown. ANT III–VI pale brown with darker apices on ANT III–V. Siphunculi, subgenital, anal plate and cauda brown. Fore, middle and hind femora dark brown. Tibiae pale brown with darker knee areas and distal parts. Tarsi pale brown. Dorsal sclerites brown (Figure 12B).Morphometric characters: Head setae: two pairs of fronto-orbital setae, 0.07–0.1 mm long; one pair of postero-dorsal setae, 0.05–0.06 mm long; one pair of latero-dorsal setae, 0.03–0.04 mm long with blunt apices on dorsal side; one pair of pointed frontal setae on ventral side, 0.09–0.1 mm long. ANT/BL 0.94–1.42. Other ratios: ANT VI/ANT III 1.5–2.0; PT/BASE 5.14–7.0; SIPH/BL 0.06–0.08; FEMUR III/BL 0.2–0.26; TIBIA III/BL 0.38–0.5; HT II/ANT VI 0.1–0.14; URS/ANT III 0.14–0.17; URS/BASE 0.64–0.71; URS/SIPH 0.5–0.64. ANT III with one or without secondary rhinaria. URS with 4–8 accessory setae. Hind tibiae with 27–54 pseudosensoria distributed along almost their entire length. Dorsal setae 0.04–0.1 mm long. Marginal sclerites on ABD I–V distinct. Siphunculi tubular.Alate male—re-description (n = 3)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I–II, IV–VI brown; ANT III light brown with darker apices. Thorax, DAT and siphunculi dark brown. Abdominal sclerotisation dark brown. Wings clear with small area of dark brown pigmentation at end, radius veins brown. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Cauda and anal plate pale. Fore femora brown darker dorsally. Middle and hind femora brown with darker smudge. Hind femora with darker stripes at margins. Tibiae brown with darker knee areas and distal parts. Tarsi brown (Figure 13B).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae, 0.02–0.03 mm long on dorsal side; one pair of frontal setae on ventral side, 0.05 mm long. ANT/BL 1.53–1.55. Other ratios: ANT VI/ANT III 1.08–1.42; PT/BASE 6.64–7.67; SIPH/BL 0.09–0.1; FEMUR III/BL 0.25–0.3; TIBIA III/BL 0.53–0.63; URS/ANT III 0.09–0.1; URS/SIPH 0.45–0.5. ANT III with 69–102 rhinaria, ANT IV with 43–56 rhinaria, ANT V with 25–38 rhinaria. URS with 4–6 accessory setae. DAT I and II inconspicuous or very small, 0.03–0.04 mm long. DAT III distinct, 0.1–0.15 mm long; DAT IV on spinal sclerites, 0.04–0.05 mm long. Dorsal setae on abdomen with pointed apices, 0.02–0.03 mm long, on small sclerites. Spinal sclerites on ABD V with 4–5 setae, 0.03–0.04 mm long. Marginal sclerites with 3–6 setae. Siphunculi flask-shaped. Genitalia with basal part of phallus short, hook-shaped (Figure 14B).Host plants:Acer saccharum, occasionally on Acer nigrum and Acer rubrum.Distribution: Canada: Ontario (Algonquin Provincial Park^, Bon Echo Provincial Park^, Guelph (Clairfields)^, Ottawa^, Owen Sound^). USA: Florida (Waccasassa River in Levy County); Illinois (Arlington Heights*, Palatine*, Urbana*); Maine (Orono); Massachusetts (Amherst, Taunton); Minnesota (Walsh); Michigan (Albion); New Jersey (Rahway†); North Carolina (Chapel Hill, Greensboro, Moravian Falls, Raleigh—locus typicus); Ohio (Columbus); Pennsylvania (Harrisburg, State College); Tennessee (Gatlinburg^, Great Smoky Mountains National Park); Washington, D.C.; Wisconsin (Saint Croix Falls) (Figure 16) ([23,24,27,58]; Illinois Natural History Survey Insect Collection [*]; International Barcode of Life project (iBOL) [^]; new record in this publication [†]).
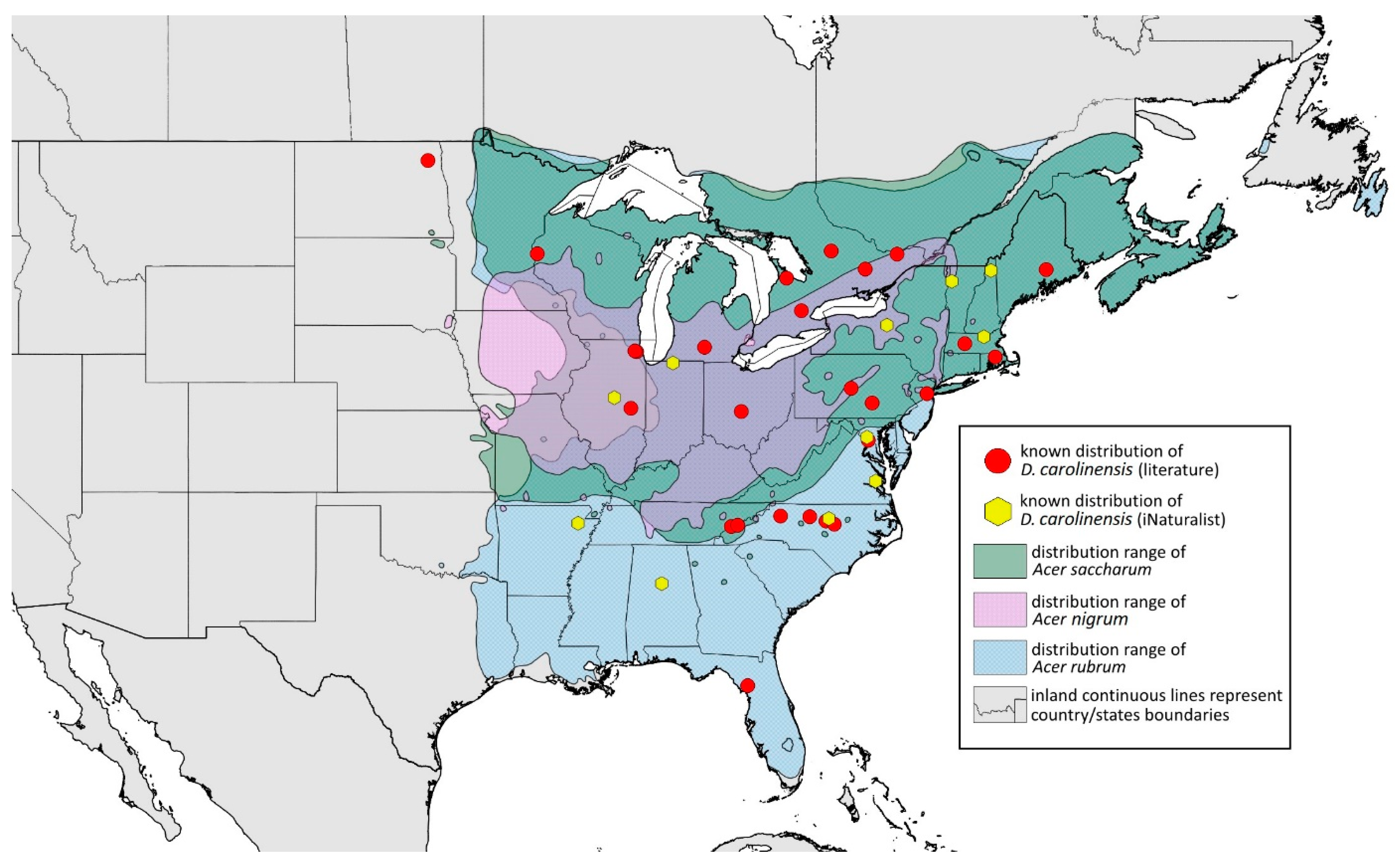
- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): USA: Alabama (Birmingham Botanical Gardens); Arkansas (Jonesboro); Illinois (Bloomington); Indiana (South Bend); Maryland (North Bethesda); Massachusetts (Groton); New Hampshire (Dixville); New York (Onondaga); North Carolina (Durham (Trinity Park)); Vermont (Essex Junction); Virginia (Williamsburg (York River State Park)).
3.4.3. Drepanaphis choanotricha Smith & Dillery, 1968
- Drepanaphis choanotricha Smith & Dillery, 1968: 61(1): 186, 190 [27]Figure 1C, Figure 2B, Figure 3C, Figure 4C, Figure 5C, Figure 9C, Figure 11C, Figure 12C, Figure 13C, Figure 14C and Figure 17; Table 1, Table 2 and Table 3Material examined: Holotype. Drepanaphis choanotricha Smith & Dillery Det. Smith & Dillery, 60-1060 Southern sugar maple, Paratype (blue)//Umstead PK. Raleigh, N. C. 9•11•60, Holotype Red, CFS—two alate viv. fem. [USNM] Paratype. Drepanaphis choanotricha Smith & Dillery Det. Smith & Dillery, 60-1060 Southern Sugar maple//Umstead PK. Raleigh, N. C. 9•11•60, Paratype//INHS, Insect Collection 1058862—four alate viv. fem. Paratype. Drepanaphis choanotricha Smith & Dillery, paratype, BM 1984-340, Det: Smith & Dillery//N.U.S.A, Pl. Southern Sugar maple, Loc. Umstead Pk, Raleigh, N.C., Date II.IX.1960, Leg. C. F. Smith, 60.1060//NHMUK 014314711—three alate viv. fem. Paratype. Drepanaphis choanotricha Smith & Dillery Det. Smith & Dillery, 60-1060 Southern Sugar maple//Umstead PK. Raleigh, N. C. 9•11•60, Paratype//08107//Museum Paris MNHN 25145—three alate viv. fem.Additional material examined—Table S6.Alate viviparous female–re-description (n = 13)Colour. In life: Black with pale legs. Head bearing three longitudinal and one anterior transverse white wax stripes. Pronotum with two or three medial longitudinal stripes. Mesonotum with one pair of latero-anterior and one pair of medio-posterior wax dots. Metanotum with one pair of lateral wax dots. Abdomen with rows of white wax dots, denser at posterior end [27].Pigmentation of mounted specimens: Head, ANT I, II, thorax dorsal abdominal tubercles and siphunculi dark brown (Figure 1C and Figure 9C). ANT III–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear, with dark brown pigmentation of end of veins, radius veins brown. Pterostigma distinct, darkly pigmented, oval with small area inside without pigmentation (Figure 3C). Abdomen pale, dorsal sclerotisation brown. Cauda, subgenital and anal plate pale. Fore femora pale brown (Figure 4C).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.05 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.06–0.08 mm long. ANT/BL 1.79–3.35; ANT/HW 11.73–19.99; PT/BASE 9.04–16.31. ANT III with 2–5 secondary rhinaria, BASE with 5–6 accessory rhinaria (Figure 2B). URS with 6–10 accessory setae. Other ratios: ANT IV/ANT III 0.61–0.99; ANT V/ANT III 0.67–0.83; ANT VI/ANT III 1.81–3.48; URS/ANT III 0.12–0.16; URS/BASE 0.69–0.86; URS/SIPH 0.5–0.76; HT II/ANT III 0.11–0.15; HT II/BASE 0.58–0.79; TIBIA III/BL 0.57–0.74; SIPH/BL 0.1–0.15; SIPH/CAUDA 2.06–4.02. DAT II–IV clearly visible, DAT I inconspicuous. DAT II 0.04–0.06 mm long, DAT III 0.13–0.24 mm long (Figure 11C). DAT IV smallest, 0.01–0.03 mm long. Setae at ends of tubercles 0.03–0.04 mm long with blunt apices. Marginal sclerites with 2–4 blunt setae 0.02–0.04 mm long. Distinct spinal setae 0.04–0.05 mm long with blunt apices on small sclerites. Siphunculi flask-shaped (Figure 5C).Oviparous female—description (n = 2)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax and coxa brown. ANT I, II and siphunculi dark brown. ANT III–VI pale brown with darker apices on ANT III–V. Abdomen pale. Dorsal sclerotisation brown. Fore, middle and hind femora; tarsi subgenital; anal plate; and cauda pale brown (Figure 12C).Morphometric characters: Head setae: two pairs of fronto-orbital setae, 0.1–0.12 mm long; one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.06–0.08 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.1 mm long. ANT/BL 1.67–1.93. Other ratios: ANT VI/ANT III 2.34–2.7; PT/BASE 12.25–13.3; SIPH/BL 0.12; FEMUR III/BL 0.27–0.29; TIBIA III/BL 0.48–0.51; HT II/ANT VI 0.05–0.06; URS/ANT III 0.13–0.16; URS/BASE 0.75–0.83; URS/SIPH 0.38–0.48. ANT III without secondary rhinaria. URS with 6–7 accessory setae. Hind tibiae with 23–29 pseudosensoria, more abundant in distal parts of tibiae. Dorsal setae 0.1–0.13 mm long. ABD I–VI with setae on small dark sclerites. ABD I–V with marginal sclerites. Siphunculi flask-shaped.Alate male—re-description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I, II, thorax, coxa, dorsal abdominal tubercles and siphunculi dark brown. ANT III–VI pale brown with darker apices on ANT III–V. Wings clear with distinct area of dark brown pigmentation at end, radius veins brown. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Abdomen pale, dorsal sclerotisation brown. Cauda and anal plate brown. Fore, middle, hind femora and tarsi pale brown (Figure 13C).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.04 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.07–0.08 mm long. ANT/BL 2.34–2.36. Other ratios: ANT VI/ANT III 2.35–2.51; PT/BASE 12.9–13.4; SIPH/BL 0.12; FEMUR III/BL 0.28–0.31; TIBIA III/BL 0.58–0.59; URS/ANT III 0.13; URS/SIPH 0.53. ANT III with 43–44 rhinaria, ANT IV with 21–23 rhinaria, ANT V with 16–18 rhinaria. BASE with seven very small accessory rhinaria. URS with six accessory setae. DAT III 0.05 mm long with pointed setae 0.02 mm long at end. Dorsal setae 0.02–0.04 mm long with pointed apices. Spinal sclerites on ABD IV with two setae, ABD V with one seta. Marginal sclerites with 3–6 setae, spinal setae on small sclerites. Siphunculi flask-shaped. Genitalia with basal part of phallus elongated, robust, with pilled inner edges (Figure 14C).Host plant: Acer saccharum.

3.4.4. Drepanaphis granovskyi Smith & Knowlton, 1943
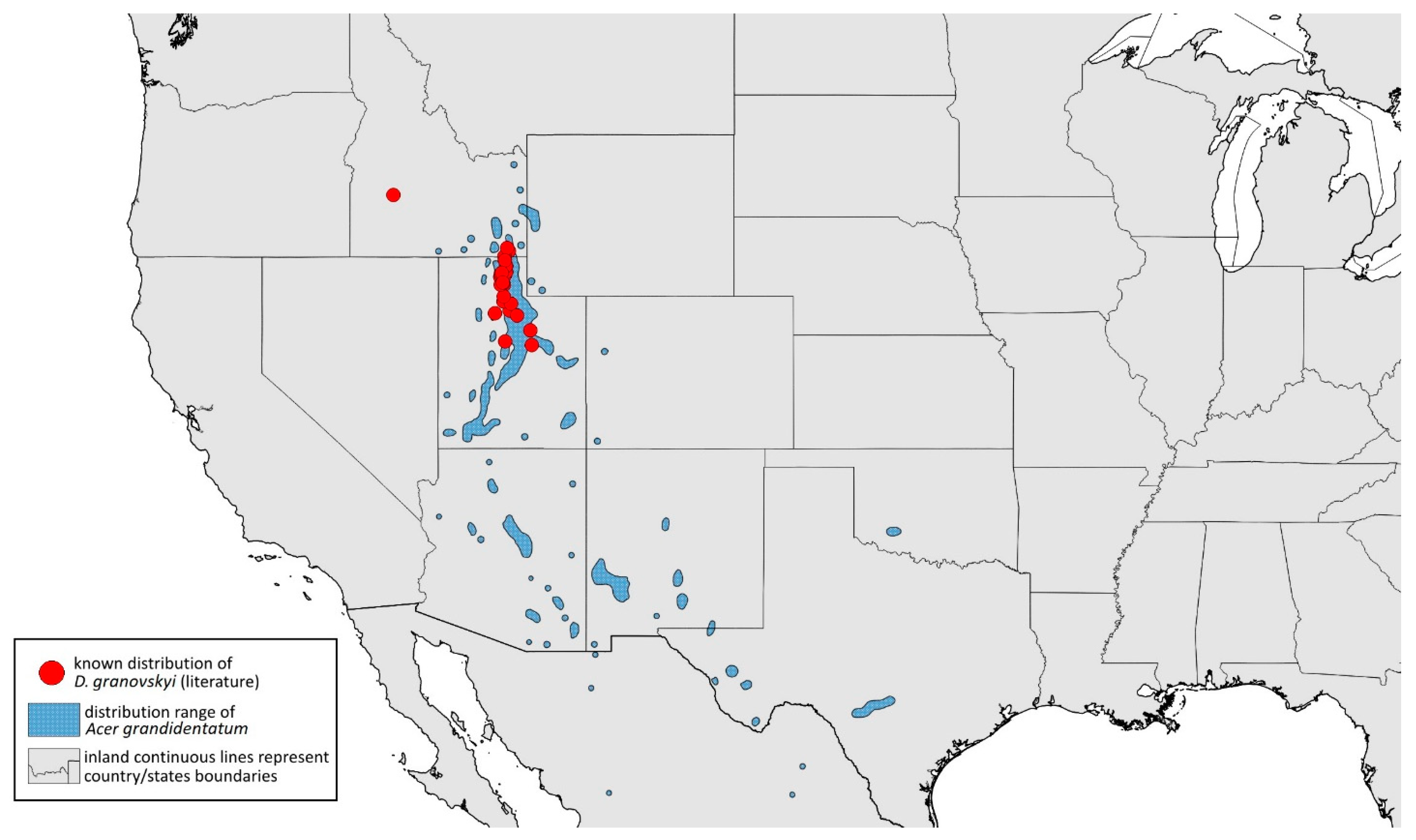
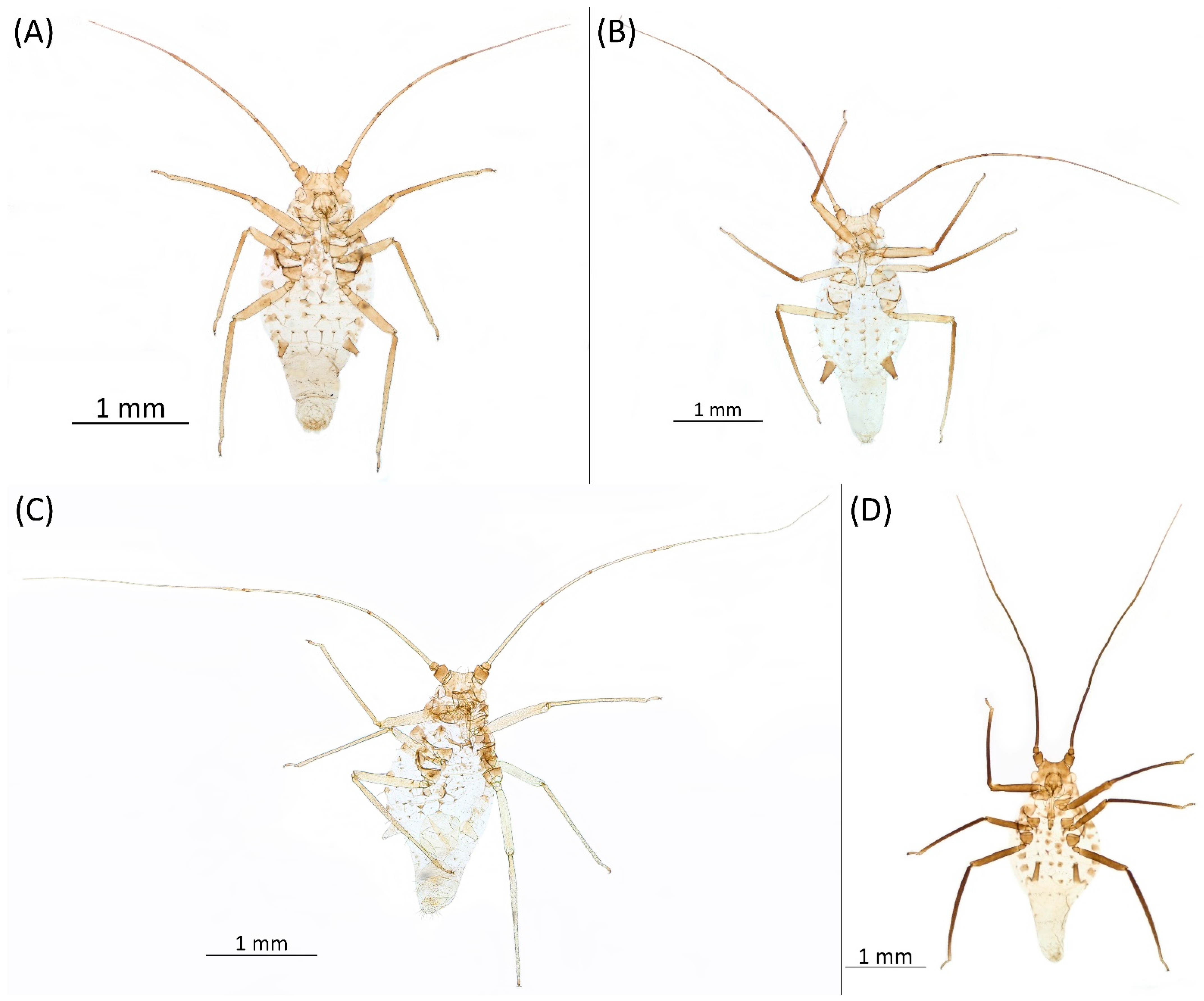
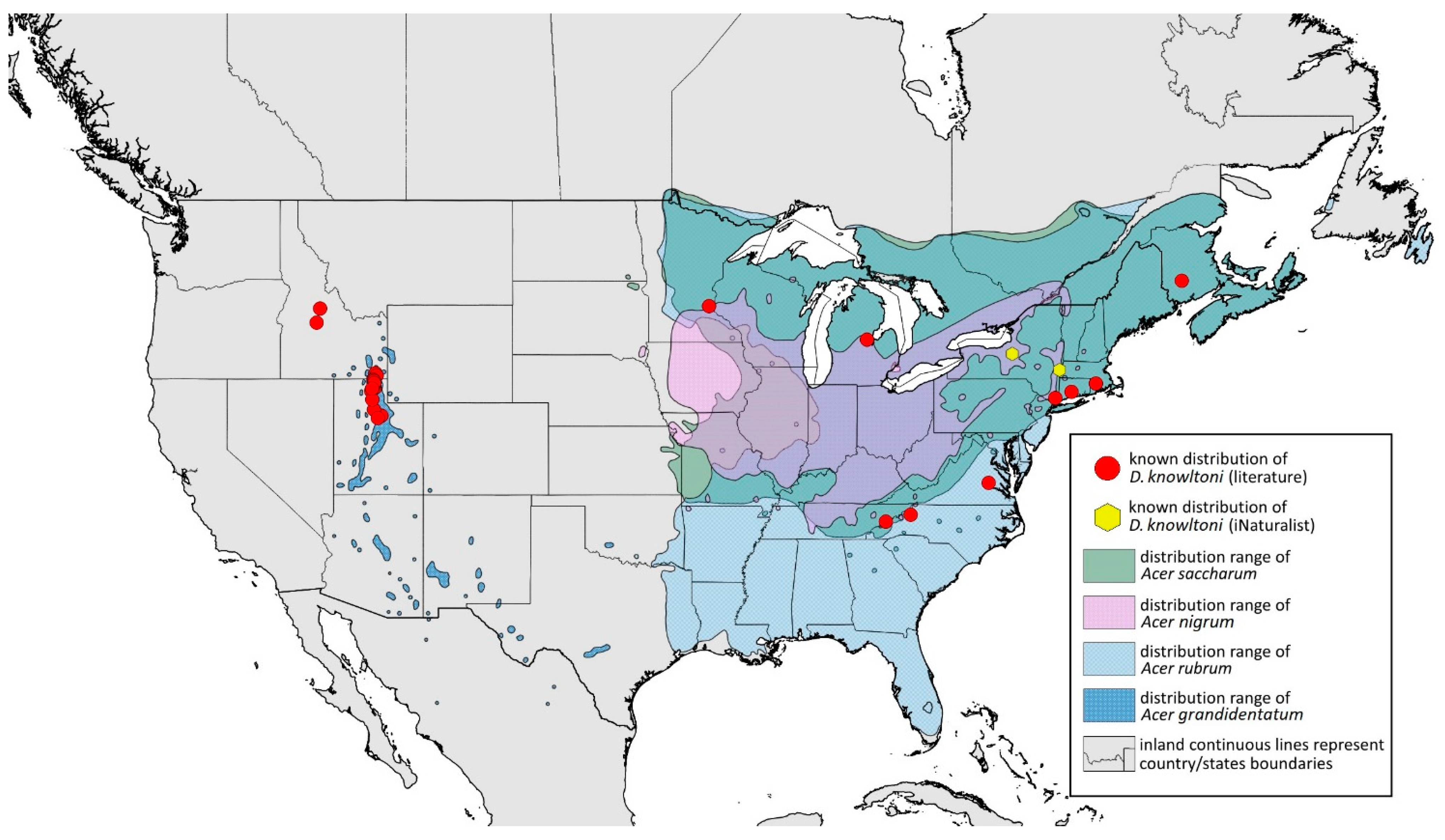
- Drepanaphis granovskyi Smith & Knowlton, 1943: 59(2): 172, 173 [24]Figure 1D, Figure 3D, Figure 4D, Figure 5D, Figure 9D, Figure 11D, Figure 12D, Figure 13D, Figure 14D and Figure 18; Table 1, Table 3 and Table 4Material examined: Type. Drepanaphis granovskyi S-K//Mt. Acer grandidentatum, Liberty, Ut., Aug. 13, 1942, GF. Knowlton—three alate viv. fem. (USNM) Paratype. Drepanaphis granovskyi S-K//Mt. Acer grandidentatum, Spanish Fork, Ut., Aug. 10, 1942, Whitish freen, C F Knowlton//INHS, Insect Collection 1058866—three alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 13)Colour. In life: Pale white, appendages clear; without conspicuous wax [27].Pigmentation of mounted specimens: Head, thorax, ANT pale brown (Figure 9D). Wings clear with palely pigmented pterostigma, with large area inside without pigmentation (Figure 3D). Abdomen, dorsal abdominal tubercles (Figure 1D) and siphunculi pale. Fore femora pale brown (Figure 4D).Morphometric characters: Head setae: two pairs of pointed fronto-orbital setae, 0.02–0.05 mm long; one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.01–0.02 mm long with pointed apices; two pairs of pointed frontal setae on ventral side, 0.02–0.05 mm long. ANT/BL 1.23–1.47; ANT/HW 8.19–10.79; PT/BASE 3.48–8.08. ANT III with 9–13 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 4–6 accessory setae. Other ratios: ANT IV/ANT III 0.55–0.72; ANT V/ANT III 0.5–0.75; ANT VI/ANT III 0.81–1.51; URS/ANT III 0.1–0.14; URS/BASE 0.57–0.96; URS/SIPH 0.33–0.54; HT II/ANT III 0.11–0.18; HT II/BASE 0.82–1.0; TIBIA III/BL 0.46–0.54; SIPH/BL 0.08–0.12; SIPH/CAUDA 1.17–2.62. Dorsal abdominal segments with distinct three pairs of tubercles. DAT I biggest, 0.09–0.12 mm long; DAT II 0.04–0.06 mm long; DAT III 0.03–0.06 mm long. DAT IV inconspicuous (Figure 1D and Figure 11D). Setae at ends of tubercles, 0.02–0.03 mm long. Abdominal dorsal setae 0.02–0.04 mm long with pointed apices. Siphunculi tubular (Figure 5D).Oviparous female—description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Body in general pale brown or with slightly darker hind tibiae and slightly lighter abdomen (Figure 12D).Morphometric characters: Head setae: two pairs of fronto-orbital setae, 0.07–0.09 mm long; one pair of postero-dorsal setae, 0.09 mm long; one pair of latero-dorsal setae, 0.05–0.06 mm long on dorsal side with forked apices. Two pairs of pointed frontal setae on ventral side, 0.04–0.06 mm long. ANT/BL 0.89–0.91. Other ratios: ANT VI/ANT III 1.5–1.83; PT/BASE 5.0–5.82; SIPH/BL 0.08–0.093; FEMUR III/BL 0.2–0.21; TIBIA III/BL 0.39; URS/ANT III 0.19; URS/BASE 0.73; URS/SIPH 0.4. ANT III without secondary rhinaria. URS with four accessory setae. Hind tibiae with 42–45 pseudosensoria, more abundant in middle part and on ends of tibiae. Dorsal setae 0.08–0.11 mm long with forked apices. Siphunculi tubular.Alate male—description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, ANT I dark brown. Pronotum, ANT II, III pale brown; ANT IV–VI brown. Wings clear, pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Abdomen pale, spinal sclerites and siphunculi dark brown. Cauda and anal plate dark brown. Fore femora brown (Figure 13D).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, two pairs of frontal setae on ventral side, 0.02–0.04 mm long with pointed apices. ANT/BL 0.96. Other ratios: SIPH/BL 0.09; URS/SIPH 0.47. ANT III with 66–68 rhinaria, ANT IV with 34–35 rhinaria, ANT V with 19–20 rhinaria. URS with four accessory setae. DAT absent. Dorsal setae 0.02–0.03 mm long with pointed apices. Spinal sclerites on ABD II–V with two pointed setae 0.02 mm long. Marginal sclerites with 1–3 setae, most distinct on ABD IV. Siphunculi tubular. Genitalia with basal part of phallus short, robust, rectangular (Figure 14D).Host plant: Acer grandidentatum.Distribution: USA: Idaho (Birch Creek (Cub River Canyon), Franklin, Mink Creek, Strawberry Creek); Utah (Avon Canyon, Beaver Canyon, Big Cottonwood Canyon, Blacksmith Fork Canyon, Bountiful, Brigham Canyon, East Canyon (Cache County), Eden, Farmington Canyon, Green Canyon, Heber, Liberty—locus typicus, Logan Canyon, Mantua, Mount Nebo, North Ogden, Richmond, Rolapp, Sardine Canyon, Wellsville Canyon, Willow Creek) (Figure 18) [24,27].
3.4.5. Drepanaphis idahoensis Smith & Dillery, 1968
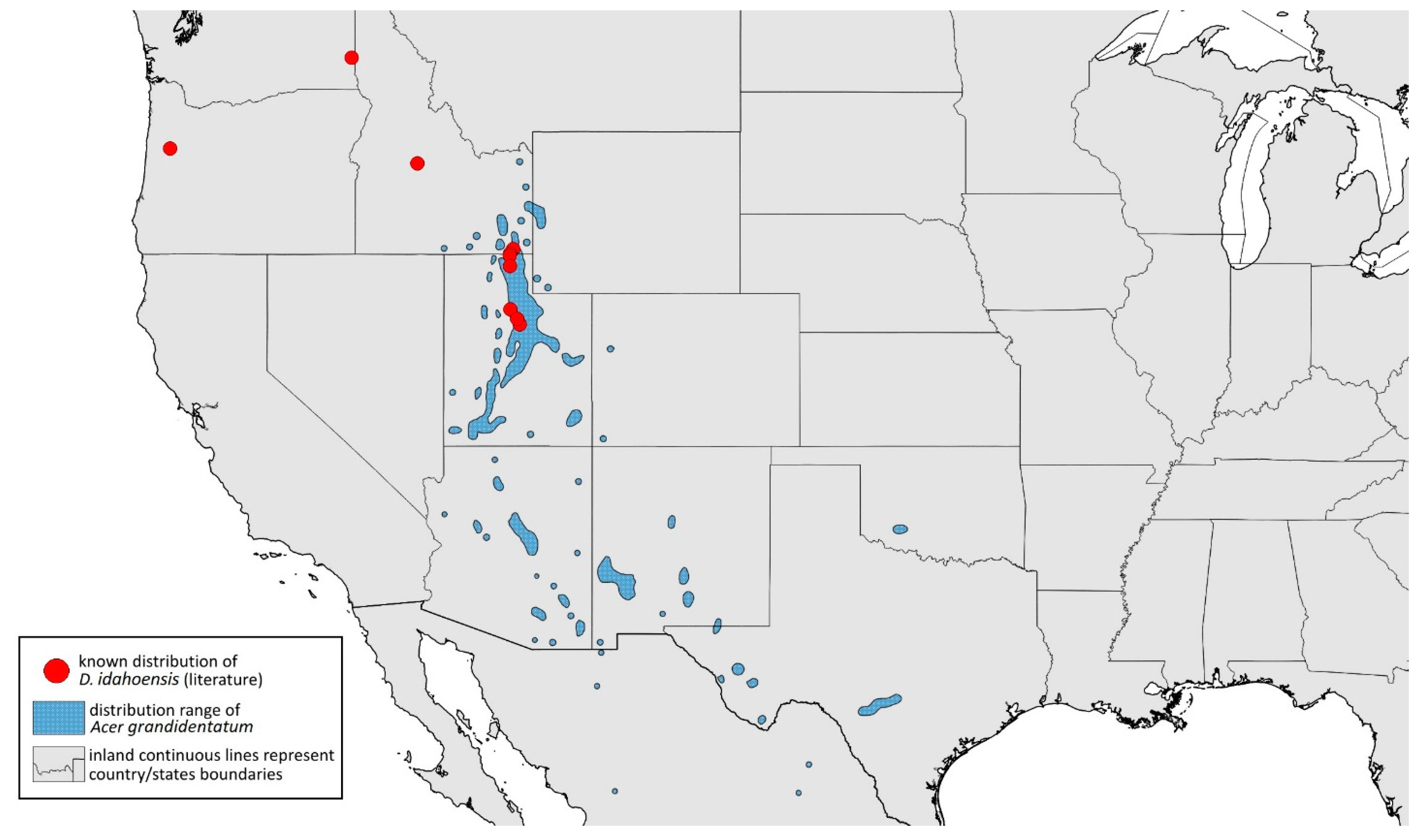
- Drepanaphis idahoensis Smith & Dillery, 1968: 61(1): 186, 193 [27]Figure 1E, Figure 3E, Figure 4E, Figure 5E, Figure 9E, Figure 11E, Figure 12E and Figure 19; Table 1 and Table 2Material examined: Holotype. Drepanaphis idahoensis Smith & Dillery Det. Smith & Dillery, 60-887, Acer grandidentatum, Paratype (blue)//Cub River Cany, Ida., 8•16•60, Al. dK transverse area between cornicles, Holotype (red) K-S—two alate viv. fem. (USNM) Paratype. Drepanaphis idahoensis Smith & Dillery Det. Smith & Dillery, 60-887, Acer grandidentatum, Paratype//Cub River Cany, Ida., 8•16•60, Al. dK transverse area between cornicles//Museum Paris MNHN 25147—two alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 10)Colour. In life: Body entirely frosted with white wax except for dark, U-shaped line more or less connecting DAT III to siphunculi. Legs pale [27].Pigmentation of mounted specimens: Head, thorax, ANT I brown (Figure 9E). ANT II–VI pale brown with slightly darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with distinct area of dark brown pigmentation at end of veins. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3E). Abdomen pale with brown abdominal sclerites. Dorsal abdominal tubercles (Figure 1E) and siphunculi dark brown. Cauda, subgenital and anal plate pale. Fore femora pale brown (Figure 4E).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.03 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.05–0.06 mm long. ANT/BL 1.8–2.54; ANT/HW 11.65–20.98; PT/BASE 5.31–11.99. ANT III with 6–9 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–8 accessory setae. Other ratios: ANT IV/ANT III 0.70–0.85; ANT V/ANT III 0.68–0.99; ANT VI/ANT III 0.83–2.02; URS/ANT III 0.09–0.11; URS/BASE 0.58–0.75; URS/SIPH 0.34–0.49; HT II/ANT III 0.08–0.12; HT II/BASE 0.6–0.86; TIBIA III/BL 0.6–0.81; SIPH/BL 0.11–0.16; SIPH/CAUDA 1.7–2.45. DAT I 0.03–0.06 mm long; DAT II 0.05–0.1 mm long; DAT III biggest, 0.18–0.26 mm long. DAT IV inconspicuous (Figure 11E). Dorsal setae 0.02–0.03 mm long with blunt apices, on ABD I–V on small sclerites. Marginal sclerites with 2–4 blunt setae. Siphunculi flask-shaped (Figure 5E).Oviparous female—description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Body in general pale brown or yellowish with slightly lighter abdomen (Figure 12E).Morphometric characters: Head setae: two pairs of blunt fronto-orbital setae, 0.09–0.1 mm long; one pair of blunt postero-dorsal setae, 0.08 mm long; one pair of blunt latero-dorsal setae on dorsal side, 0.04 mm long; one pair of pointed frontal setae on ventral side, 0.07 mm long. ANT/BL 1.23–1.24. Other ratios: ANT VI/ANT III 0.86–0.92; PT/BASE 3.92–4.58; SIPH/BL 0.08–0.09; FEMUR III/BL 0.25–0.26; TIBIA III/BL 0.51; HT II/ANT VI 0.13–0.16; URS/ANT III 0.14; URS/BASE 0.77; URS/SIPH 0.53. ANT III without secondary rhinaria. URS with eight accessory setae. Hind tibiae with 22–23 pseudosensoria abundant, distributed in central part of tibiae. Dorsal setae 0.08–0.11 mm long. Siphunculi tubular.Male: Unknown.Remarks: Some specimens from Lawrence in Kansas come from the collection of the J.B. Wallis/R.E. Roughley Museum of Entomology, Canada, and the Museum of Zoology, Lund University, Sweden. However, we did not have the opportunity to verify the slides and confirm whether they are indeed individuals representing this species. Moreover, Acer nigrum is listed as a host plant on those slides.Host plant: Acer grandidentatum, occasionally found on Acer negundo and Acer saccharum.Distribution: USA: Idaho (Cub River Canyon—locus typicus, Franklin, Stanley (between Thompson Creek and Tennell Creek‴)); Oregon (Corvallis (Benton County)); Utah (Cub River Canyon, Hobble Creek Canyon, Providence, Salt Lake City, Vivian Park in Provo Canyon); Washington (Pullman (Whitman County)) (Figure 19) ([27]; NMNH Extant Specimen Records (USNM, US) [‴]).
3.4.6. Drepanaphis kanzensis Smith, 1941
- Drepanaphis kanzensis Smith, 1941: 72(2): 228, 232 [23]= Drepanaphis kansensis Leonard, 1959: 32(1): 12 [59]Figure 1F, Figure 3F, Figure 4F, Figure 5F, Figure 9F, Figure 11F, Figure 12F, Figure 13E, Figure 14E and Figure 20; Table 1, Table 2 and Table 3Material examined: Drepanaphis kanzensis Smith Holotype Type No 55838. D.D.N.N.M.//Ka. Aphids, Host Sugar maple, Ft. Scott, Date 6-17 1940, C.F. Smith. Type—five alate viv. fem. (USNM) Paracotype. Drepanaphis kanzensis C. F. Smith//Ka. Aphids, Host Sugar maple, Ft. Scott, Ka, Date 6-17-40, C.F. Smith//08109//Museum Paris MNHN 25148—five alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 17)Colour. In life: Head, thorax and abdomen covered by white wax. ANT and legs pale, eyes red. Dorsal abdominal tubercles dark brown. Wings clear with pale pterostigma.Pigmentation of mounted specimens: Head and pronotum brown, rest of thorax dark brown (Figure 9F). ANT I–II brown, ANT III–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with palely pigmented pterostigma, with large area inside without pigmentation (Figure 3F). Abdomen pale with brown sclerites. Dorsal abdominal tubercles dark brown (Figure 1F). Siphunculi pale brown with darker smudge. Cauda, subgenital and anal plate pale. Fore femora pale brown (Figure 4F).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.08–0.09 mm long. ANT/BL 1.44–2.56; ANT/HW 9.35–14.43; PT/BASE 5.6–12.98. ANT III with 10–15 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–8 accessory setae. Other ratios: ANT IV/ANT III 0.6–0.8; ANT V/ANT III 0.59–0.84; ANT VI/ANT III 0.76–1.086; URS/ANT III 0.087–0.108; URS/BASE 0.56–0.68; URS/SIPH 0.38–0.63; HT II/ANT III 0.16–0.24; HT II/BASE 0.59–0.87; TIBIA III/BL 0.52–0.73; SIPH/BL 0.084–0.137; SIPH/CAUDA 0.94–2.33. Dorsal abdominal segments with distinct three pairs of tubercles. DAT I inconspicuous. DAT II 0.09–0.11 mm long, DAT III biggest 0.2–0.28 mm long (Figure 11F), DAT IV smallest 0.05–0.06 mm long. Pointed setae at end of tubercles 0.03–0.04 mm long. Dorsal setae 0.04–0.05 mm long with pointed apices, on small sclerites. Marginal sclerites with 2–5 setae. ABD VIII with two spinal sclerites, each with two setae. Siphunculi flask-shaped (Figure 5F).Oviparous female—description (n = 5)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, ANT pale brown. ANT with darker apices on ANT III–V. Abdomen and siphunculi pale. Dorsal sclerotisation pale brown. Femora, tibiae pale brown. Tibiae with slightly darker apical areas. Tarsi brown (Figure 12F).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.07–0.1 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.1 mm long. ANT/BL 0.75–1.4; PT/BASE 7.43–10.66. Other ratios: ANT VI/ANT III 1.51–2.17; SIPH/BL 0.05–0.07; FEMUR III/BL 0.21–0.28; TIBIA III/BL 0.38–0.52; HT II/ANT VI 0.07–0.1; URS/ANT III 0.098–0.13; URS/BASE 0.62–0.69; URS/SIPH 0.44–0.53. ANT III without secondary rhinaria. URS with eight accessory setae. Hind tibiae with 41–67 pseudosensoria distributed in central part of tibiae. Dorsal setae 0.09–0.13 mm long. Siphunculi tubular.Alate male—description (n = 3)Colour. In life: Unknown.Pigmentation of mounted specimens: ANT pale with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with palely pigmented pterostigma, with large area inside without pigmentation. Abdomen pale with brown to dark brown sclerotisation. Siphunculi pale; cauda and anal plate brown. Legs pale, tibiae with slightly darker apical parts (Figure 13E).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.04 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.05–0.06 mm long. ANT/BL 1.45–2.36. Other ratios: ANT VI/ANT III 0.92–1.5; PT/BASE 6.38–10.0; SIPH/BL 0.09–0.11; FEMUR III/BL 0.28–0.33; TIBIA III/BL 0.54–0.67; URS/ANT III 0.08–0.09; URS/SIPH 0.35–0.42. ANT III with 94–127 rhinaria, ANT IV with 38–62 rhinaria, ANT V with 18–32 rhinaria. URS with six accessory setae. DAT III 0.13–0.15 mm long with pointed setae 0.03 mm long at end. DAT I, II and III inconspicuous. Dorsal setae 0.03–0.04 mm long with pointed apices. Spinal sclerites with two setae on ABD I, V, VI, VII, ABD VIII with four setae. Marginal sclerites with 3–4 setae. Siphunculi tubular. Genitalia with basal part of phallus elongated, triangular (Figure 14E).Remarks: One slide with two individuals, previously misidentified as Drepanaphis kanzensis from the NHMUK collection (BM 1958-454; NHMUK 014314720) is correctly identified as Drepanaphis idahoensis. The material was collected in Hobble Creek Canyon, Utah, USA, from Acer grandidentatum.Host plant: Acer rubrum, Acer saccharum.Distribution: Canada: New Brunswick (Fredericton); Ontario (Brockville°, Guelph°, Ottawa, Puslinch, Toronto°, Unionville); Quebec (Orsainville (Zoo), Sainte-Foy). USA: Kansas (Fort Scott—locus typicus, Hiawatha); Maine (Presque Isle); Michigan (East Lansing); Missouri (Butler, Columbia); New Jersey (Rahway†); New York (Geneva, Lockport“, Niagara County); Ohio (Columbus); Pennsylvania (State College (Botany Bldg.)); Wisconsin (Sturgeon Bay); Washington DC (Figure 20) ([23,24,27,58]; International Nucleotide Sequence Database Collaboration [°]; Natural History Museum of Denmark Entomology Collection [“]; new record in this publication [†]).

3.4.7. Drepanaphis keshenae Granovsky, 1931
- Drepanaphis keshenae Granovsky, 1931: 19: 246, 248 [21]Figure 1G, Figure 3G, Figure 4G, Figure 5G, Figure 8C, Figure 11G, Figure 13F, Figure 14F, Figure 21A, Figure 22A and Figure 23; Table 1, Table 2 and Table 3Material examined: Lectotype. APHIDIDAE. Drepanaphis keshenae Granovskyi, Det. Granovsky 1929, Sl. 7617, Det. F. C. Hottes, 29, ILL. NAT. HIST. SUR, lectotype//Elizabethtown Ill., VI-20-1929, coll. Frison + Hottes. On Acer saccharum. ILL. NAT. HIST. SUR.//INHS, Insect Collection 459,587—three alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 11)Colour. In life: Head and thorax covered with white wax. Antennae pale with dark apices of segments. Eyes red. Abdomen covered with white wax, apart from ABD V with distinct black dorsal tubercles. Fore femora dark; middle and hind femora, tibiae and tarsi pale brown. Wing veins distinctly brown bordered. Siphunculi dark (Figure 8C).Pigmentation of mounted specimens: Head, thorax, ANT I brown (Figure 21A). ANT II–VI pale brown to brown with darker apices on ANT III–V. Wing veins distinctly brown bordered. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3G). Abdomen pale with brown sclerotisation. DAT dark brown (Figure 1G). Siphunculi pale brown to brown. Cauda, subgenital and anal plate pale. Fore femora darker dorsally (Figure 4G). Middle and hind femora, tibiae and tarsi pale brown to brown. Hind femora with brown smudge.
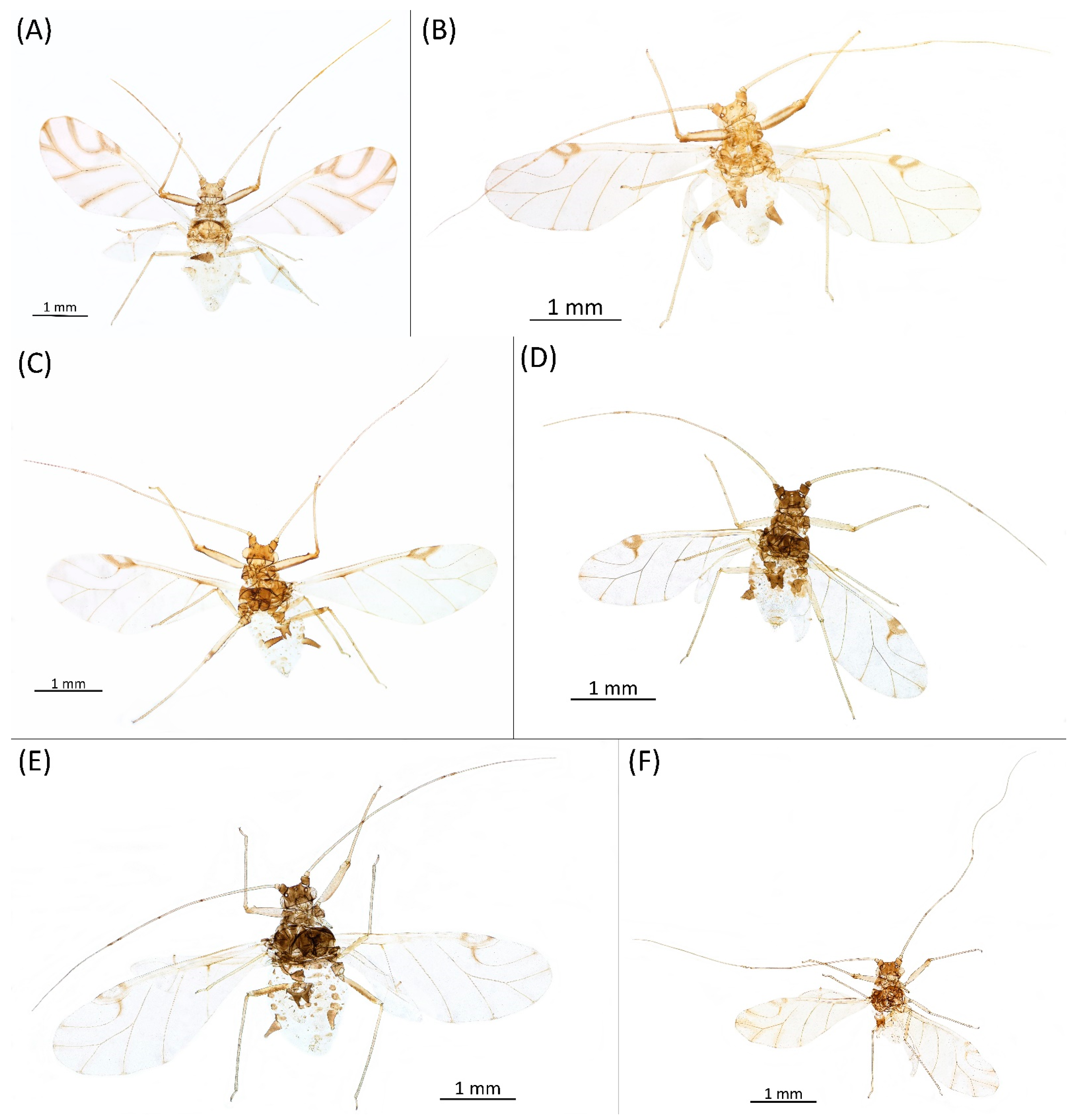
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.04 mm long with pointed apices; one pair of pointed frontal setae on ventral side 0.07 mm long. ANT/BL 1.5–3.12; PT/BASE 7.2–13.82. ANT III with 10–16 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–8 accessory setae. Other ratios: ANT IV/ANT III 0.56–0.79; ANT V/ANT III 0.61–0.81; ANT VI/ANT III 0.99–2.15; URS/ANT III 0.09–0.13; URS/BASE 0.61–0.9; URS/SIPH 0.33–0.64; HT II/ANT III 0.08–0.13; HT II/BASE 0.62–1.00; TIBIA III/BL 0.53–0.82; SIPH/BL 0.096–0.15; SIPH/CAUDA 1.75–3.38. DAT III distinct, 0.24–0.47 mm long (Figure 11G) with pointed setae, 0.02–0.03 mm long at ends. DAT II 0.04 mm long or inconspicuous. DAT I and IV inconspicuous. Dorsal setae 0.02–0.03 mm long, with pointed apices, on ABD I–V on small sclerites. Siphunculi flask-shaped (Figure 5G).Oviparous female—description (n = 2)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, ANT brown. ANT with darker apices on ANT III–V. Abdomen pale with brown sclerotisation. Siphunculi dark brown. Legs brown, tibiae with slightly darker apical parts (Figure 22A).
- Morphometric characters: Head setae: two pairs of blunt fronto-orbital setae 0.09 mm long, one pair of blunt postero-dorsal setae 0.08 mm long, one pair of blunt latero-dorsal setae 0.05 mm long on dorsal side, one pair of pointed frontal setae 0.08 mm long on ventral side. ANT/BL 1.04–1.15. Other ratios: ANT VI/ANT III 1.78–2.08; PT/BASE 7.09–7.83; SIPH/BL 0.06–0.07; FEMUR III/BL 0.22–0.23; TIBIA III/BL 0.44–0.46; HT II/ANT VI 0.11–0.13; URS/ANT III 0.16–0.24; URS/BASE 0.73–1.1; URS/SIPH 0.48–0.86. ANT III without secondary rhinaria. URS with 6–8 accessory setae. Hind tibiae with 56–67 pseudosensoria distributed along almost their entire lengths. Dorsal setae 0.7–0.11 mm long. Siphunculi tubular, 0.14 mm long.Alate male—description (n = 4)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, ANT I, II, IV, V, VI brown. ANT III pale brown with darker apices of segments. Wing veins brown bordered. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Abdomen pale with brown sclerites. Dorsal abdominal tubercles dark brown. Siphunculi, cauda and anal plate brown. Fore femora brown, darker dorsally. Middle and hind femora, tibiae and tarsi brown. Hind femora with brown smudge (Figure 13F).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.03 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.07 mm long. ANT/BL 1.74–2.2. Other ratios: ANT VI/ANT III 1.22–1.93; PT/BASE 9.2–11.1; SIPH/BL 0.1–0.14; FEMUR III/BL 0.3–0.31; TIBIA III/BL 0.59–0.64; URS/ANT III 0.1–0.17; URS/SIPH 0.42–0.76. ANT III with 71–101 rhinaria, ANT IV with 29–46 rhinaria, ANT V with 25–31 rhinaria. URS with six accessory setae. DAT III 0.08–0.24 mm long with pointed setae 0.02–0.03 mm long at end. Dorsal setae 0.02–0.03 mm long with pointed apices. Siphunculi tubular. Genitalia with basal part of phallus elongated, finger-like (Figure 14F).Remarks: Smith and Dillery [27] claimed they could not locate any of the slides from Keshena, Wisconsin. Therefore, they suggested Elizabethtown, Illinois, as locus typicus. Additionally, there is a slide no. 95/58 (Biologické centrum AV ČR) from Logan, Utah, dated 16.09.1962, from Acer grandidentatum, but it is dark and unverifiable.Host plant: Acer saccharum.Distribution: USA: Alabama (Aldridge Gardens in Hoover, Old Rocky Ridge (Jefferson County)); Florida (High Springs, Waccasassa River (Levy County)); Illinois (Bell Smith Springs Scenic Area, Dixon Springs, Eddyville (Bell Smith Springs National Natural Landmark), Elizabethtown); North Carolina (Raleigh); Ohio (Hocking County); Wisconsin (Keshena) (Figure 23) [23,27].

- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): Alabama (Birmingham Botanical Gardens, Hoover); Indiana (Evansville); North Carolina (Asheboro, Durham); Tennessee (vicinity of Ardmore).
3.4.8. Drepanaphis knowltoni Smith & Dillery, 1968
- Drepanaphis knowltoni Smith & Dillery, 1968: 61(1): 186, 195 [27]Figure 1H, Figure 3H, Figure 4H, Figure 5H, Figure 11H, Figure 14G, Figure 21B, Figure 24A and Figure 25; Table 1 and Table 3Material examined: Holotype. Drepanaphis knowltoni Smith & Dillery Det. Smith & Dillery, 60-886, Acer grandidentanum//Cub River Cany., Ida. 8•15•60, Alate whitish, holotype—K-S—one alate viv. fem. (USNM). Paratype. Drepanaphis knowltoni Smith & Dillery Det. Smith & Dillery, 60-886, Acer grandidentanum//Cub River Cany., Ida. 8•15•60, Alate whitish, holotype—K-S//Museum Paris MNHN 25149—one alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 24)Colour. In life: Body white with wax; may be entirely white, or thoracic lobes and V-shaped area connecting siphunculi with DAT III may be exposed and dark. Front femora, DAT III and siphunculi always dark [27].Pigmentation of mounted specimens: Head, thorax, ANT I brown (Figure 21B). ANT II–VI pale brown, with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wing veins clear with small area of dark brown pigmentation on end. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3H). Abdomen pale with brown sclerotisation. DAT III (Figure 1H) and siphunculi dark brown. Cauda, subgenital and anal plate pale. Fore femora brown, darker dorsally (Figure 4H). Middle and hind femora, tibiae and tarsi pale brown to brown. Hind femora with brown stripes at margins.Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side; one pair of pointed frontal setae on ventral side; 0.02–0.05 mm long with pointed apices. ANT/BL 1.73–2.59; PT/BASE 7.1–12.93. ANT III with 10–15 secondary rhinaria, BASE with 4–5 accessory rhinaria. URS with 6–8 accessory setae. Other ratios: ANT IV/ANT III 0.64–0.79; ANT V/ANT III 0.69–0.85; ANT VI/ANT III 1.23–2.20; URS/ANT III 0.09–0.12; URS/BASE 0.47–0.72; URS/SIPH 0.29–0.42; HT II/ANT III 0.09–0.14; HT II/BASE 0.56–0.85; TIBIA III/BL 0.59–0.82; SIPH/BL 0.12–0.18; SIPH/CAUDA 2.2–3.6. DAT III distinct 0.18–0.28 mm long (Figure 11H), with setae 0.03–0.04 mm long at end. Dorsal setae 0.03–0.04 mm long, with pointed apices. ABD VI with marginal sclerites and 2–5 setae each. Siphunculi flask-shaped (Figure 5H).Oviparous female: Unknown.Alate male—re-description (n = 2)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, ANT I, II brown. ANT III–VI pale brown, with darker apices on ANT III–V. Wings clear, pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Abdomen pale with brown sclerotisation. Siphunculi, cauda and anal plate brown. Fore femora pale brown, darker dorsally. Middle and hind femora, tibiae, apices of tarsi pale brown. Hind femora with brown stripes at margins. Tibiae with slightly darker apical parts (Figure 24A).
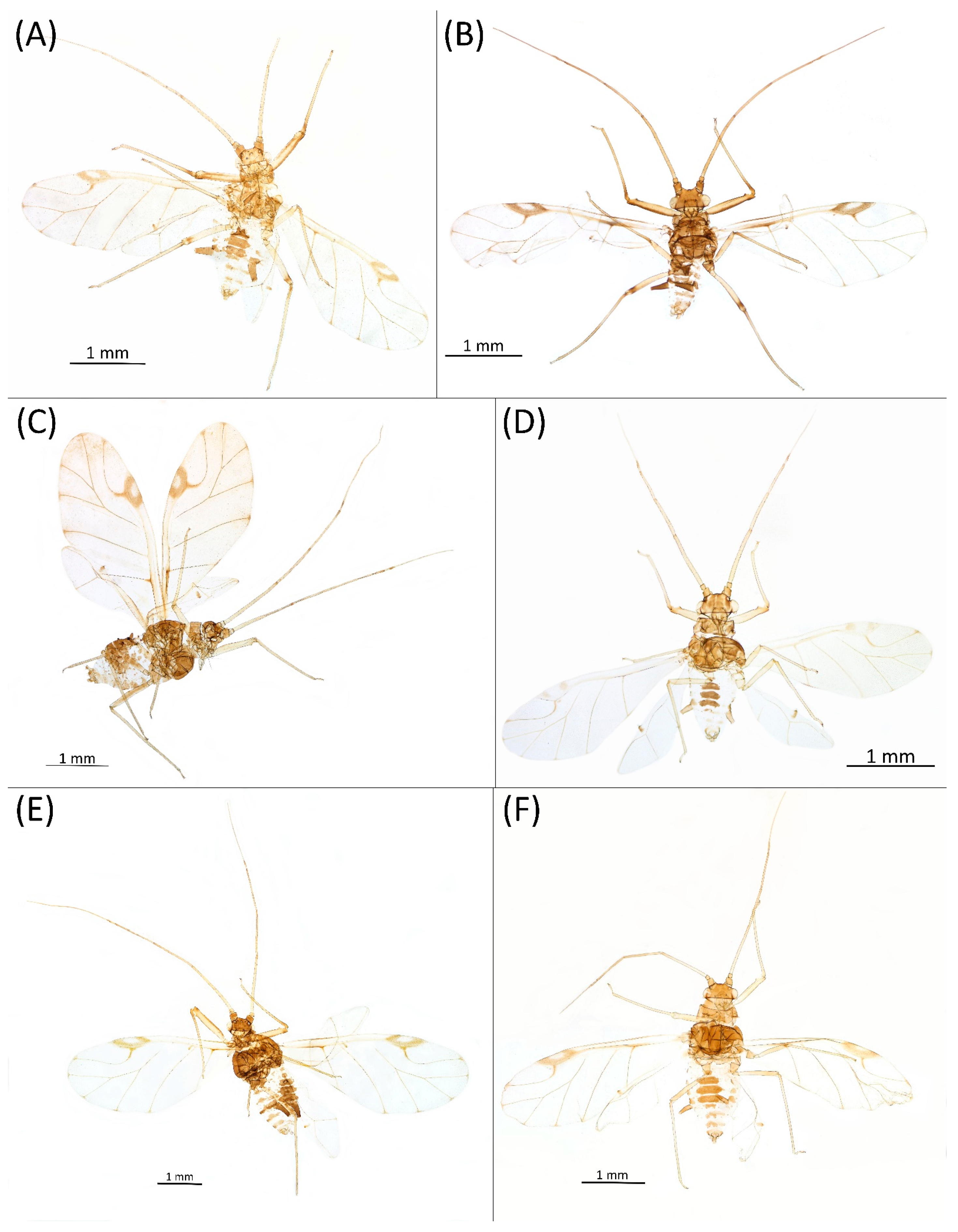
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.04 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.06 mm long. ANT/BL 1.64–1.91. Other ratios: ANT VI/ANT III 1.03–1.78; PT/BASE 6.36–11.65; SIPH/BL 0.12; III FEMUR/BL 0.29–0.31; TIBIA III/BL 0.62–0.65; URS/ANT III 0.1; URS/SIPH 0.37. ANT III with 71–79 rhinaria, ANT IV with 29 rhinaria, ANT V with 22 rhinaria. BASE with five accessory rhinaria. URS with eight accessory setae. DAT III 0.1–0.11 mm long with pointed setae 0.04–0.05 mm long at end. Dorsal setae 0.04–0.05 mm long with pointed apices. Spinal sclerites with 2 setae, marginal sclerites with 3–5 setae. Siphunculi tubular. Genitalia with basal part of phallus elongated, robust with capitate apices (Figure 14G).Male: Unknown.Host plants: Acer grandidentatum, Acer nigrum, Acer rubrum, Acer saccharum.Distribution: Canada: New Brunswick (Fredericton). USA: Connecticut (Wallingford); Idaho (Cub Creak, Cub River Canyon—locus typicus, Deer Cliff Lodge, Franklin, Mink Creek, Stanley, Strawberry Creek, Thomas Spring); Michigan (Midland); Minnesota (Saint Paul); New York (Mount Kisco); North Carolina (Grandfather Mountain); Rhode Island (Providence); Tennessee (vicinity of Cosby* (0.4 mi up Low Mount Cammerer Trail, Great Smoky Mountains National Park)); Utah (Blacksmith Fork Canyon, Cub River Canyon, Daniel’s Canyon, Logan Canyon, Mantua, Parley’s Canyon, Providence, Provo Canyon, Richmond, Smithfield Canyon, Weber Canyon, Wellsville Canyon); Virginia (Richmond) (Figure 25) [27]; Illinois Natural History Survey Insect Collection [*].
- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): New York (Columbia, Syracuse).
3.4.9. Drepanaphis monelli (Davis, 1909)
- = Phymatosiphum monelli Davis, 1909: 2(3): 197 [19]Drepanaphis monelli Gillette, 1910: 3(4): 371 [20]= Drepanosiphum monelli Burnham, 1938: 70(9): 184 [60]Figure 1I, Figure 3I, Figure 4I, Figure 5I, Figure 10B, Figure 11I; Figure 14H, Figure 21C, Figure 22B, Figure 24B and Figure 26; Table 1, Table 2 and Table 3Material examined: Type. Phymatosiphum monelli n.g.e t n.sp., Type, Occ.# 40469. 2n. dt. Zab. Nat. Hist. sz. 3120, John J. Davis.//Phymatosiphum 670 monelli n. sp. -Type- Byckeye, St. Louis, Mo. 30 June’08. J. T. Monell Col. Mounted from alcoholic specimens. John J. Davis.//INHS, Insect Collection 1058879—three alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 11)Colour. In life: Powdery white over entire body, except dark fuscous thoracic lobes. Brownish-yellow, U-shaped line more or less connecting DAT III to siphunculi. Front femora, DAT III and siphunculi dark [27].Pigmentation of mounted specimens: Head, thorax, ANT I dark brown (Figure 21C). ANT II–VI pale brown with darker apices on ANT III–V. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3I). Abdomen pale with brown sclerotisation. Dorsal abdominal tubercles (Figure 1I) and siphunculi dark brown. Cauda, subgenital and anal plate pale. Fore, middle and hind femora pale brown to brown. Fore femora darker dorsally (Figure 4I); hind femora with brown stripes at margins. Fore tibiae with darker apical part.Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.03 mm long; one pair of pointed frontal setae on ventral side, 0.06 mm long. ANT/BL 1.9–2.74; PT/BASE 5.51–13.91. ANT III with 9–12 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–12 accessory setae (Figure 10B). Other ratios: ANT IV/ANT III 0.66–0.94; ANT V/ANT III 0.72–0.92; ANT VI/ANT III 1.11–2.24; URS/ANT III 0.1–0.13; URS/BASE 0.71–0.86; URS/SIPH 0.34–0.48; HT II/ANT III 0.09–0.13; HT II/BASE 0.62–0.86; TIBIA III/BL 0.55–0.82; SIPH/BL 0.11–0.16; SIPH/CAUDA 1.65–2.78. DAT III distinct, 0.16–0.22 mm long (Figure 11I), with setae 0.02–0.03 mm long at end. ABD I–V with dorsal setae 0.02–0.03 mm long with pointed apices, on small sclerites. Marginal sclerites with 3–5 setae. Siphunculi tubular (Figure 5I).Oviparous female—description (n = 5)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax pale brown. ANT brown to dark brown with darker apices on ANT III–V. Abdomen pale with brown sclerotisation. Siphunculi dark brown. Femora and tarsi pale brown. Tibiae dark brown, lighter on ends (Figure 22B).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.07–0.12 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.01 mm long. ANT/BL 1.38–1.83. Other ratios: ANT VI/ANT III 1.64–2.21; PT/BASE 6.29–10.85; SIPH/BL 0.09–0.11; III FEMUR/BL 0.25–0.29; III TIBIAE/BL 0.48–0.55; HT II/ANT VI 0.07–0.1; URS/ANT III 0.14–0.19; URS/BASE 0.75–0.86; URS/SIPH 0.44–0.6. ANT III without secondary rhinaria. URS with 8–10 accessory setae. Hind tibiae with 32–62 pseudosensoria more abundant in middle part of tibiae. Dorsal setae 0.08–0.11 mm long. Siphunculi flask-shaped.Alate male—re-description (n = 4)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, ANT I, II dark brown. ANT III–VI brown with darker apices on ANT III–V. Wings clear with small area of dark brown pigmentation on end. Pterostigma distinct, very darkly pigmented, with small area inside without pigmentation. Abdomen pale with brown sclerotisation. Siphunculi dark brown, cauda and anal plate brown. Fore femora brown, darker dorsally. Middle and hind femora, tibiae and tarsi pale brown. Hind femora with dark brown stripes on ends. Tibiae with slightly darker apical parts (Figure 24B).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.06–0.07 mm long. ANT/BL 1.73–2.13. Other ratios: ANT VI/ANT III 1.36–1.66; PT/BASE 7.16–11.92; SIPH/BL 0.11–0.13; FEMUR III/BL 0.29–0.33; TIBIA III/BL 0.58–0.69; URS/ANT III 0.11–0.12; URS/SIHP 0.48–0.79. ANT III with 91–98 rhinaria, ANT IV with 38–45 rhinaria, ANT V with 21–26 rhinaria. URS with eight accessory setae. DAT III distinct, 0.1–0.12 mm long, with pointed setae, 0.03–0.04 mm long at end. Dorsal setae 0.03 mm long on small sclerites. ABD IV–V with two setae on spinal sclerites. Marginal sclerites with 3–4 setae. Siphunculi tubular. Genitalia with basal part of phallus smooth, elongated, hook-shaped (Figure 14H).Host plants: Aesculus glabra, occasionally found on Aesculus pavia and Acer saccharum.Distribution: Canada: Quebec (Senneville). USA: Florida (Gainesville, High Springs); Illinois (Havana, Kankakee, Mount Carroll, Oakwood, Rock Island, Urbana); Missouri (Columbia, Saint Louis—locus typicus); North Carolina (Bryson City, Cullowhee, Grandfather Mountain, Raleigh (Umstead Park)); Ohio (Columbus); Pennsylvania (State College); Wisconsin (Milwaukee) (Figure 26) [19,21,23,27,49,58].
- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): Alabama (Birmingham Botanical Gardens); Pennsylvania (Penn Hills).
3.4.10. Drepanaphis nigricans Smith, 1941
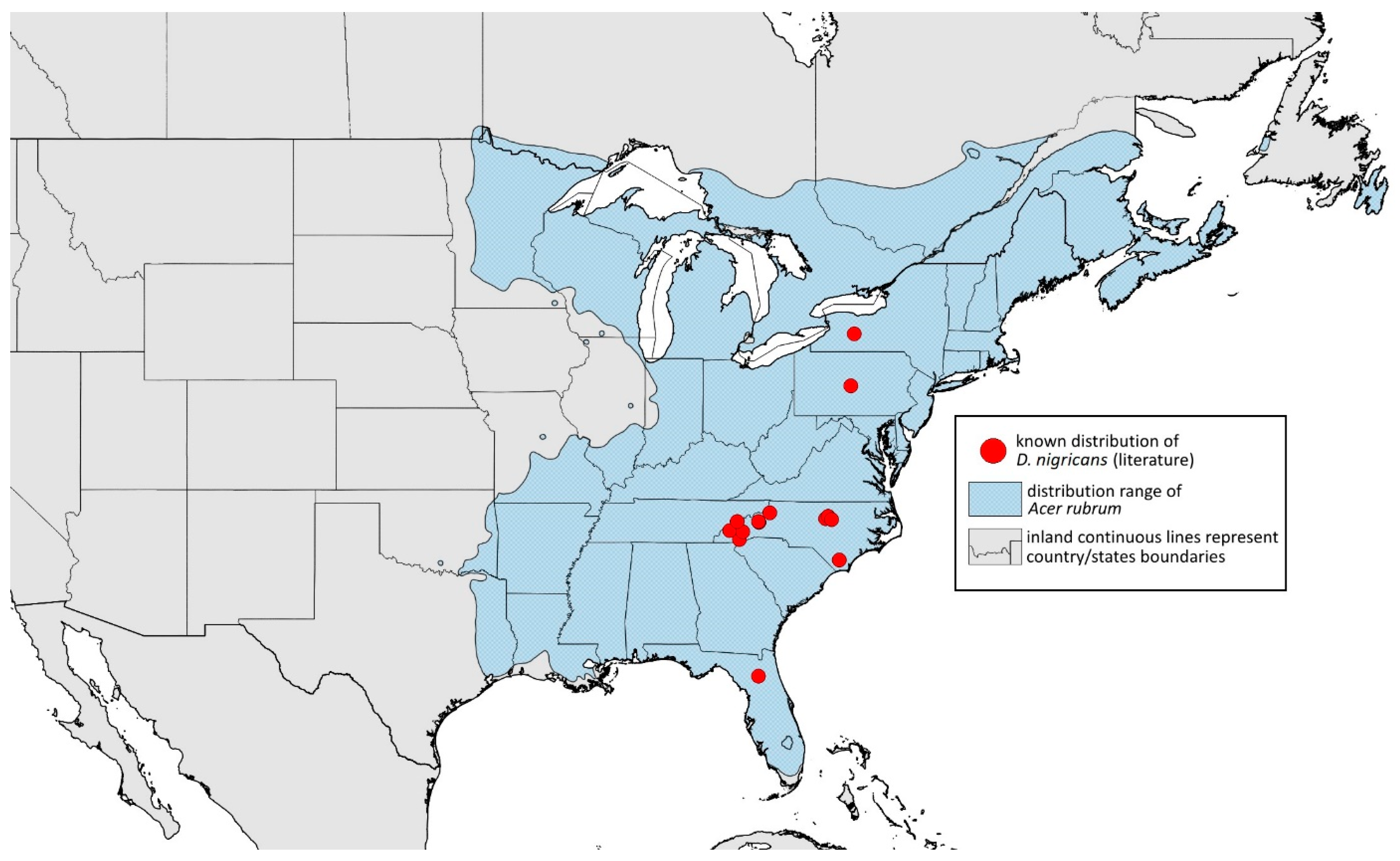
- Drepanaphis nigricans Smith, 1941: 57(2): 228, 236 [23]Figure 1J, Figure 3J, Figure 4J, Figure 5J, Figure 11J, Figure 21D, Figure 22C and Figure 27; Table 2 and Table 4Material examined: Holotype. Drepanaphis nigricans Smith Holotype Type No 55835. D.D.N.N.M.//N.C. 41-151, Acer rubrum, Busick, N.C., (Park Way), 2 July 1941, CF. Smith—five alate viv. fem. (USNM). Paratype. Drepanaphis nigricans C. F. Smith//N. C. Aphids, Host Acer rubrum, Blowing Roak, N. C., Date June 12 1940, C. F. Smith, Black light spots//INHS, Insect Collection 1058449—three alate viv. fem. Paracotype. Drepanaphis nigricans C. F. Smith//N. C. 41-151, Acer rubrum, Busick, NC., Park Way, July 2, 1941, CF Smith//Museum Paris MNHN 25153—six alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 51)Colour. In life: Black with pale legs, wings with noticeable dark spots at ends of veins. Head and pronotum with three longitudinal white wax stripes, median pronotal stripe interrupted, often faint. Mesonotum with two transverse rows of four small wax dots anteriorly (medial pairs sometimes missing) and one pair of elongated dots posteriorly. Metanotum with two wax dots laterally. Abdomen with many wax dots, most dense at posterior end [27].Pigmentation of mounted specimens: Head, thorax, ANT I dark brown (Figure 21D). ANT II–VI pale brown with darker apices on ANT III–V. Wings clear with dark pigmentation at end of veins. Pterostigma distinct, darkly pigmented, oval with small area inside without pigmentation (Figure 3J). Abdomen pale with brown dorsal sclerotisation. Dorsal abdominal tubercles (Figure 1J) and siphunculi dark brown. Cauda, subgenital and anal plate brown. Fore femora pale brown (Figure 4J).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.01–0.02 mm long; one pair of pointed frontal setae on ventral side, 0.06–0.07 mm long. ANT/BL 2–3.38; PT/BASE 7.5–15.8. ANT III with 11–20 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–10 accessory setae. Other ratios: ANT IV/ANT III 0.63–0.79; ANT V/ANT III 0.64–0.84; ANT VI/ANT III 1.18–2.26; URS/ANT III 0.09–0.15; URS/BASE 0.6–0.94; URS/SIPH 0.45–0.7; HT II/ANT III 0.09–0.13; HT II/BASE 0.6–1.02; TIBIA III/BL 0.55–0.86; SIPH/BL 0.077–0.14; SIPH/CAUDA 1.03–2.63. DAT I inconspicuous. DAT II 0.02–0.04 mm long, DAT III biggest 0.14–0.25 mm long (Figure 11J), DAT IV 0.2–0.6 mm long. Ends of tubercles with very short setae, about 0.01 mm long. ABD I–V with dorsal setae 0.01–0.02 mm long, on small sclerites. Marginal sclerites with 3–6 setae. Siphunculi flask-shaped (Figure 5J).Oviparous female—description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Head and thorax pale brown. ANT I–II brown; ANT III–VI pale brown. ANT II–VI with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Siphunculi, cauda, subgenital and anal plate pale brown. Legs pale (Figure 22C).Morphometric characters: Head setae: two pairs of fronto-orbital setae 0.08–0.1 mm long, one pair of postero-dorsal setae 0.08 mm long, one pair of latero-dorsal setae on dorsal side 0.04–0.05 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.09–0.1 mm long. ANT/BL 1.69–1.71. Other ratios: ANT VI/ANT III 2.29–2.45; PT/BASE 12.38–12.72; SIPH/BL 0.08–0.09; FEMUR III/BL 0.26–0.28; TIBIA III/BL 0.51–0.52; HT II/ANT VI 0.06; URS/ANT III 0.14; URS/BASE 0.77; URS/SIPH 0.53. ANT III with 3–4 secondary rhinaria. URS with 12 accessory setae. Hind tibiae with 53–62 pseudosensoria more abundant in middle part of tibiae, closer to distal part of femur. Dorsal setae 0.07–0.09 mm long, on ABD VIII slightly shorter. Siphunculi flask-shaped.Male: Unknown.Host plant: Acer rubrum.Distribution: USA: Florida (Gainesville); New York (Sparta); North Carolina (Blowing Rock, Bolton, Busick—locus typicus, Cashiers, Chapel Hill, Durham, Great Smoky Mountains National Park* (Goldmine Loop Trail), Mount Mitchell, Raleigh (Umstead Park), Sunburst); Pennsylvania (State College); Tennessee (Great Smoky Mountains National Park* (Low Mount Cammerer Trail)) (Figure 27) ([23,27]; Illinois Natural History Survey Insect Collection [*]).
3.4.11. Drepanaphis parva Smith, 1941
- Drepanaphis parva Smith, 1941: 57(2): 228, 237 [23]= Drepanaphis rubrum Smith, 1941: 57(2): 228, 238 [23]= Drepanaphis parvus Smith & Knowlton, 1943: 59(2): 173 [24]Figure 1K, Figure 3K, Figure 4K, Figure 5K, Figure 7C, Figure 11K, Figure 14I, Figure 21E, Figure 24C and Figure 28; Table 3 and Table 4Material examined: Holotype. Drepanaphis parvus Smith Holotype Type No 55836. D.D.N.N.M.//N. C. Aphids, Host Acer, Greensboro, N. C. 193, Date 5-3-40, C. F. Smith—three alate viv. fem. (USNM). Paratype. Drepanaphis parvus C. F. Smith//N. C. Aphids, Host Acer rubrum, Raleigh, NC, Date May 12 1941, C. F. Smith//INHS, Insect Collection 1058907—six alate viv. fem. Paracotype. Drepanaphis parvus C. F. Smith//N. C. Aphids, Host Acer, Greensboro, N. C. 193, Date 5-3-40, C. F. Smith//INHS, Insect Collection 1058906—six alate viv. fem. Paracotype. Drepanaphis parvus C. F. Smith//N. C. Aphids, Host Acer, Greensboro, N. C. 193, Date 5-3-40, C. F. Smith//Museum Paris MNHN 22493—six alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 15)Colour. In life: Head and thorax reddish brown; head and pronotum with five longitudinal white wax stripes; pronotal wax pattern as in D. acerifoliae. Abdomen green–grey–brown with white tip, white wax dots in longitudinal rows in line with those on thorax [27].Pigmentation of mounted specimens: Head, ANT I, thorax brown to dark brown (Figure 21E). ANT II–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wing veins diffusely bordered. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3K and Figure 7C). Abdomen pale with DAT I–IV (Figure 1K) and sclerotisation dark. Siphunculi brown, cauda, subgenital and anal plate pale brown. Fore femora pale brown (Figure 4K). Hind femora with darker smudge.Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.03 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.05–0.06 mm long. ANT/BL 1.41–1.94; PT/BASE 6.36–11.31. ANT III with 9–15 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 4–8 accessory setae. Other ratios: ANT IV/ANT III 0.55–0.79; ANT V/ANT III 0.52–1.16; ANT VI/ANT III 1.05–1.7; URS/ANT III 0.08–0.11; URS/BASE 0.67–0.77; URS/SIPH 0.35–0.43; HT II/ANT III 0.09–0.14; HT II/BASE 0.67–1.08; TIBIA III/BL 0.46–0.59; SIPH/BL 0.08–0.13; SIPH/CAUDA 1.61–2.46. DAT I 0.07–0.11 mm long; DAT II 0.05–0.08 mm long; DAT III biggest, 0.14–0.19 (Figure 11K); DAT IV smallest, 0.04–0.07 mm long. Dorsal setae 0.01–0.03 mm with blunt apices, on small sclerites. ABD VII–VIII with setae pointed and slightly longer, 0.03–0.05 mm long. Siphunculi flask-shaped (Figure 5K).Oviparous female: Unknown.Alate male—re-description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I, thorax brown. ANT II–VI pale brown. ANT III–V with slightly darker apices on ends and dark area with primary rhinarium on ANT VI. Wing veins diffusely bordered. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Abdomen pale with dark tubercles and sclerotisation. Cauda and anal plate brown. Legs pale; hind femora with brown smudge (Figure 24C).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.05–0.06 mm long. ANT/BL 1.83–1.93. Other ratios: ANT VI/ANT III 1.47–1.71; PT/BASE 11.69–13.46; SIPH/BL 0.08–0.09; FEMUR III/BL 0.29–0.3; TIBIA III/BL 0.61; URS/ANT III 0.11; URS/SIPH 0.69. ANT III with 91–97 rhinaria, ANT IV with 38–41 rhinaria, ANT V with 21–26 rhinaria. URS with eight accessory setae. DAT I–II inconspicuous. DAT III 0.13–0.15 mm long, DAT IV 0.04–0.05 mm long. Dorsal setae 0.04–0.06 mm long, with pointed apices. ABD I–V with 2–4 setae on spinal sclerites. Marginal sclerites with 2–6 setae. Genitalia with basal part of phallus robust, elongated, hook-shaped (Figure 14I).Host plants: Acer rubrum, Acer saccharum.Distribution: Canada: New Brunswick (Middle Kouchibouguac‡); Nova Scotia (Jakes Landing^, Kejimkujik Main Parkway‡, Upper Hammonds Plains‡); Ontario (Callander, Cambridge°, Corwhin^, Front of Yonge‡ (44°30′00.0″ N 75°54′00.0″ W), Griffith^, Kitchener‡, Marentette Beach in Wheatley^); Prince Edward Island (Stanhope); Quebec (Shawinigan (Lac Wapizagonke‡)). USA: Florida (Gainesville, Sebring (Highlands Hammock State)); Georgia (Savannah); Maine (Presque Isle); Massachusetts (Mount Grace (Warwick)); North Carolina (Andrew’s Bald in Great Smoky Mountains National Park* (35°32′16.8″ N 83°29′38.4″ W), Blue Ridge Parkway, Greensboro—locus typicus, Purchase Knob in Great Smoky Mountains National Park* (35°35′16.2″ N 83°03′53.4″ W), Raleigh, Sparta‴); Michigan (Chippewa Township° (46°18′00.0″ N 85°06′00.0″ W), Eckerman Corner^); Pennsylvania (Bethayres, Center Hall, Cooksburg, Miquon, Philipsburg, Pleasant Gap, State College); Tennessee (Bote Mountain Trail in Great Smoky Mountains National Park* (35°34′37.1″ N 83°44′02.3″ W), Gatlinburg°); Wisconsin (Sturgeon Bay) (Figure 28) ([23,24,27]; Centre for Biodiversity Genomics—Canadian Specimens [‡]; Illinois Natural History Survey Insect Collection [*]; International Barcode of Life project (iBOL) [^]; International Nucleotide Sequence Database Collaboration [°]; NMNH Extant Specimen Records (USNM, US) [‴]).

- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): Pennsylvania (Wyndmoor); Virginia (Dulles).
3.4.12. Drepanaphis robinsoni Malik sp. nov.
- urn:lsid:zoobank.org:act:3C2B4AAD-E06A-410B-940D-AEA4E96662FCMaterial examined: Holotype. Drepanaphis choanotricha Dillery & Smith [MS], Al. Viv ♀, US/153 HLGS det.//Acer saccharum, Umstead Park, Raleigh N.C., U.S.A, 19•VI•1966, HLGS leg. BM 1982-492//NHMUK 14314713—one alate viv. fem. PARATYPES Drepanaphis choanotricha Dillery & Smith [MS], Al. Viv ♀, US/153 HLGS det.//Acer saccharum, Umstead Park, Raleigh N.C., U.S.A, 19•VI•1966, HLGS leg. BM 1982-492//NHMUK 14314714—one alate viv. fem. Drepanaphis parva Smith, BM 1984-340 Det: Dillery & Smith//N.U.S.A, Acer rubrum, Chapel Hill N.C., 2•VII•1965, Dillery and Smith leg. 65.127//NHMUK 12821447—three alate viv. fem. Drepanaphis parva Smith, BM 1984-340 Det.: C. F. Smith//N.U.S.A, Acer rubrum, Raleigh N.C., 5•VII•1959, Leg: C. F. Smith. 59.394 O//NHMUK 12821448—three alate viv. fem; SA02-385-05-001 DZUS—one alate viv. fem., 22 September 2022, Washington, D.C., USA, Acer rubrum, K. Malik leg. & det.Additional material examined—Table S6.Alate viviparous female—description (n = 11)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I, thorax brown (Figure 21F). ANT II–V pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with smudge-bordered ends of veins. Pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3L). Abdomen pale with DAT I pale, DAT II and IV brown and DAT III dark brown (Figure 1L). Siphunculi, cauda, subgenital and anal plate pale. Legs pale brown, fore femora slightly darker at margins (Figure 4L).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.01–0.02 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.05–0.06 mm long. ANT/BL 2–3.2; PT/BASE 9.39–15.7. ANT III with 9–13 secondary rhinaria, BASE with 4 accessory rhinaria. URS with six accessory setae. Other ratios: ANT IV/ANT III 0.66–0.81; ANT V/ANT III 0.69–0.85; ANT VI/ANT III 1.54–2.67; URS/ANT III 0.09–0.12; URS/BASE 0.67–0.84; URS/SIPH 0.44–0.62; HT II/ANT III 0.09–0.12; HT II/BASE 0.64–0.86; TIBIA III/BL 0.54–0.85; SIPH/BL 0.09–0.17; SIPH/CAUDA 1.63–2.12. DAT I 0.06–0.08 mm long; DAT II 0.05–0.11 mm long; DAT III biggest, 0.14–0.22 mm long (Figure 11L); DAT IV smallest, 0.04–0.06 mm long. Dorsal setae 0.02–0.03 mm long with blunt apices. Marginal sclerites with 3–4 setae. Siphunculi flask-shaped (Figure 5L).Oviparous female: Unknown.Male: Unknown.Remarks: The new species D. robinsoni was designated based on two other incorrectly designated species of the Drepanaphis genus. These species include D. choanotricha (NHMUK 14314713, NHMUK 14314714) and D. parva (NHMUK 12821445, NHMUK 12821447, INHS Insect Collection 1058904). The fresh material of this new species was also collected in Washington, D.C.Diagnosis: Alate viviparous females of the new species can be easily distinguished from the closely related D. parva by their pale siphunculi and lack of sclerotisation on the abdomen and from D. choanotricha by the presence of only four accessory rhinaria.Etymology: We are pleased to name this new species in honour of Arthur Grant Robinson, an eminent Canadian aphidologist, who collected specimens of this species.Host plants: Acer rubrum, Acer saccharum.Distribution: USA: North Carolina (Chapel Hill, Doughton, Raleigh—locus typicus (Umstead Park)); Washington, D.C. (Figure 29).
| Character | D. nigricans n = 51 | D. parva n = 15 | D. robinsoni sp. nov. n = 11 | D. sabrinae n = 14 | D. saccharini n = 15 | D. simpsoni n = 12 | D. spicata n = 19 | D. tissoti n = 25 | D. utahensis n = 26 |
|---|---|---|---|---|---|---|---|---|---|
| BL | 1.04–2.12 | 1.85–2.7 | 1.22–1.73 | 1.75–2.76 | 1.58–2.40 | 1.69–2.41 | 2.12–3 | 0.99–1.8 | 1.55–2.8 |
| HW | 0.2–0.33 | 0.29–0.39 | 0.25–0.29 | 0.33–0.39 | 0.29–0.33 | 0.3–0.36 | 0.3–0.43 | 0.21–0.29 | 0.27–0.39 |
| ANT I-VI | 3.29–5.16 | 3.22–4.9 | 3.15–3.91 | 3.2–5 | 3.65–4.57 | 2.62–3.43 | 4.7–5.86 | 3.11–4.19 | 2.32–4.17 |
| ANT III | 0.6–1.04 | 0.81–1.31 | 0.73–0.88 | 0.77–1.19 | 0.80–1.1 | 0.69–0.85 | 1.04–1.37 | 0.7–1.11 | 0.74–1 |
| ANT IV | 0.52–0.73 | 0.52–0.87 | 0.52–0.67 | 0.59–0.99 | 0.58–0.89 | 0.51–0.61 | 0.78–1.07 | 0.46–0.63 | 0.48–0.78 |
| ANT V | 0.56–0.78 | 0.51–0.84 | 0.57–0.72 | 0.61–0.97 | 0.58–0.85 | 0.44–0.58 | 0.76–1.04 | 0.47–0.71 | 0.44–0.73 |
| ANT VI | 1.02–1.99 | 0.95–1.85 | 1.51–1.95 | 1.04–1.85 | 1.16–1.68 | 0.73–0.97 | 1.54–2.33 | 1.03–2.11 | 0.76–1.5 |
| BASE | 0.1–0.16 | 0.12–0.16 | 0.1–0.14 | 0.13–0.2 | 0.1–0.14 | 0.12–0.15 | 0.12–0.19 | 0.09–0.13 | 0.12–0.17 |
| PT | 0.9–1.84 | 0.83–1.7 | 1.03–1.83 | 0.9–1.66 | 1.04–1.56 | 0.6–0.85 | 1.37–2.17 | 0.9–1.98 | 0.62–1.33 |
| FEMUR I length | 0.45–0.72 | 0.53–0.66 | 0.45–0.55 | 0.6–0.88 | 0.53–0.79 | 0.5–0.59 | 0.74–1.03 | 0.44–0.6 | 0.51–0.87 |
| FEMUR I width | 0.07–0.13 | 0.08–0.15 | 0.09–0.12 | 0.11–0.18 | 0.11–0.15 | 0.14–0.17 | 0.09–0.18 | 0.05–0.09 | 0.1–0.18 |
| FEMUR III | 0.41–0.65 | 0.4–0.67 | 0.39–0.47 | 0.51–0.83 | 0.46–0.7 | 0.49–0.65 | 0.65–0.9 | 0.4–0.59 | 0.5–0.73 |
| TIBIA III | 0.84–1.34 | 1.03–1.45 | 0.9–1.05 | 1.11–1.61 | 1.03–1.51 | 0.92–1.04 | 1.36–1.89 | 0.82–1.16 | 1.05–1.48 |
| HT II | 0.08–0.12 | 0.1–0.13 | 0.08–0.09 | 0.10–0.13 | 0.10–0.12 | 0.10–0.12 | 0.11–0.14 | 0.08–0.1 | 0.1–0.14 |
| URS | 0.08–1 | 0.09–0.11 | 0.08–0.09 | 0.12–0.13 | 0.09–0.11 | 0.08–0.09 | 0.1–0.12 | 0.08–0.1 | 0.08–0.1 |
| SIPH | 0.12–0.22 | 0.21–0.3 | 0.14–0.21 | 0.19–0.34 | 0.19–0.21 | 0.16–0.21 | 0.24–0.43 | 0.13–0.21 | 0.18–0.3 |
| CAUDA | 0.07–0.96 | 0.09–0.15 | 0.08–0.1 | 0.09–0.13 | 0.08–0.14 | 0.1–0.13 | 0.1–0.15 | 0.07–0.11 | 0.1–0.17 |
3.4.13. Drepanaphis sabrinae Miller, 1937
- Drepanaphis sabrinae Miller, 1937: 69(5): 111 [22]Figure 1M, Figure 2C, Figure 3M, Figure 4M, Figure 5M, Figure 11M, Figure 22D, Figure 30A and Figure 31; Table 2 and Table 4Material examined: See Table S6.Alate viviparous female—re-description (n = 14)Colour. In life: Abdomen orange brown, sometimes yellowish. Small flecks of white wax present, three to five on head; four antero-medial, two antero-lateral and two postero-medial on mesonotum; two lateral on metanotum; some on abdomen. Pronotum with three longitudinal wax stripes, median stripe interrupted and faint [27].Pigmentation of mounted specimens: Head, ANT, thorax and legs brown to dark brown (Figure 30A). ANT II–VI with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear, pterostigma distinct, darker pigmentation on ends, with small area inside without pigmentation (Figure 3M). Abdomen pale with dark dorsal tubercles, lighter on bases, darker on tips (Figure 1M), and pale brown sclerotisation. Siphunculi pale brown to brown, slightly darker on ends (Figure 5M). Fore femora dark dorsally (Figure 4M). Tibiae with slightly darker knee apical parts on ends. Cauda, subgenital and anal plate pale.
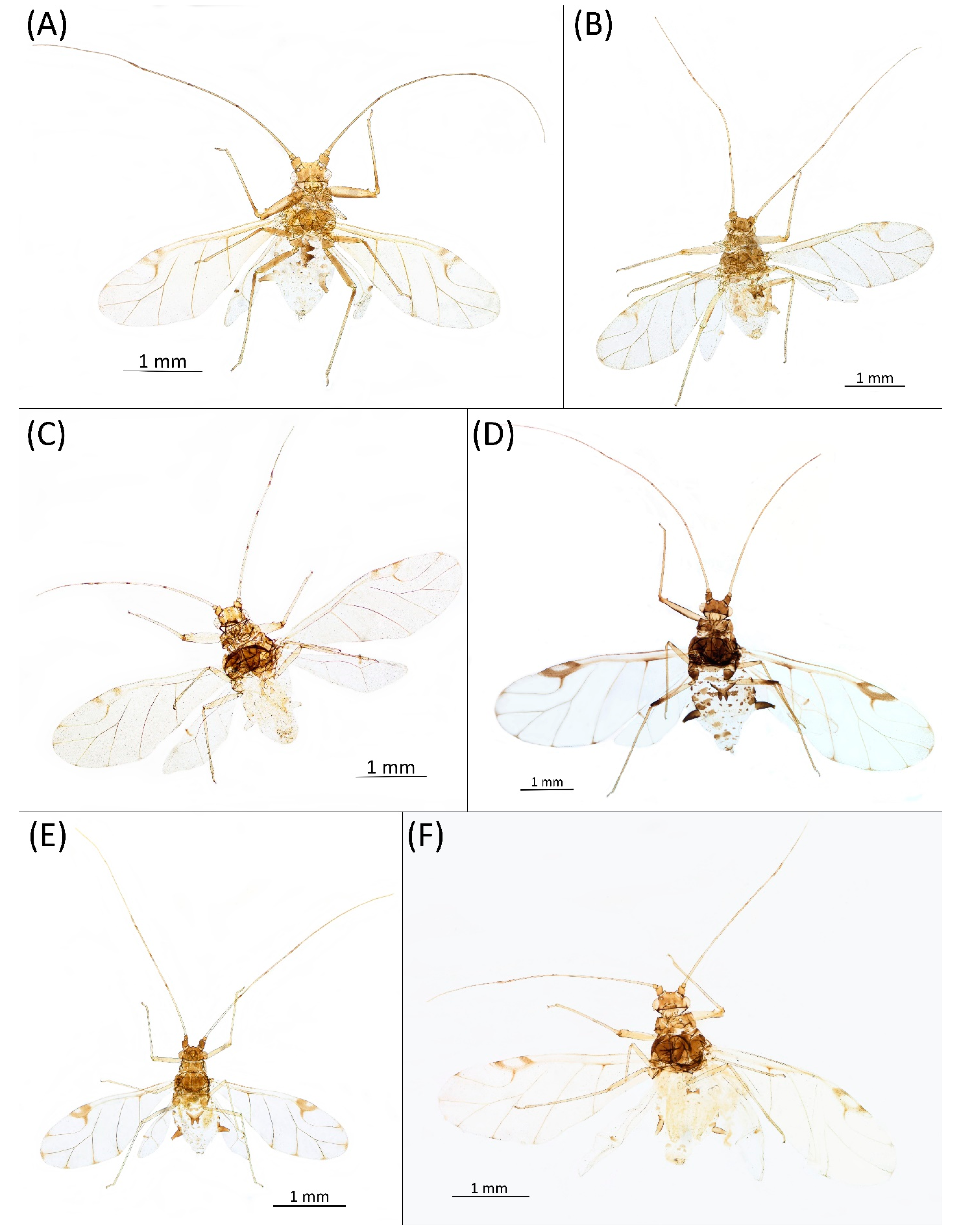
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.04 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.07–0.01 mm long. ANT/BL 1.18–2.51; PT/BASE 5.82–9.66. ANT III with 6–10 secondary rhinaria, BASE with 5–6 accessory rhinaria (Figure 2C). URS with 8–14 accessory setae. Other ratios: ANT IV/ANT III 0.65–0.88; ANT V/ANT III 0.67–0.93; ANT VI/ANT III 1.25–1.9; URS/ANT III 0.11–0.14; URS/BASE 0.68–0.95; URS/SIPH 0.42–0.65; HT II/ANT III 0.09–0.14; HT II/BASE 0.53–0.77; TIBIA III/BL 0.52–0.78; SIPH/BL 0.09–0.16; SIPH/CAUDA 2.01–2.54. DAT I 0.09–0.15 mm long; DAT II and III equal, 0.12–0.23 mm long (Figure 11M); DAT IV smallest, 0.03–0.07 mm long. Dorsal setae 0.03–0.04 mm long with pointed apices, on small sclerites. Marginal sclerites with 2–5 setae. Siphunculi tubular (Figure 5M).Oviparous female—description (n = 2)Colour. In life: Unknown.Pigmentation of mounted specimens: Head and thorax brown, abdomen pale. ANT I–II brown. ANT III–VI dark brown with lighter apices on ANT VI. Abdomen pale with dark sclerotisation. Siphunculi, subgenital, anal plate and cauda brown. Fore, middle, hind femora and siphunculi brown. Tibiae dark brown. Tarsi brown (Figure 22D).Morphometric characters: Head setae: two pairs of fronto-orbital setae 0.08–0.09 mm long, one pair of postero-dorsal setae mm long, one pair of latero-dorsal setae 0.05–0.7 mm long with pointed apices on dorsal side; one pair of pointed frontal setae on ventral side 0.09 mm long. ANT/BL 1.33–1.39. Other ratios: ANT VI/ANT III 1.5–1.76; PT/BASE 7.19–7.57; SIPH/BL 0.08–0.1; FEMUR III/BL 0.24; TIBIA III/BL 0.46–0.47; HT II/ANT VI 0.09; URS/ANT III 0.15–0.16; URS/BASE 0.75–0.86; URS/SIPH 0.5–0.6. ANT III with one or without secondary rhinaria. URS with 8–10 accessory setae. Hind tibiae with 70–76 pseudosensoria distributed along almost their entire lengths. Dorsal setae 0.04 -0.09 mm long. Siphunculi tubular.Male: Unknown.Remarks: Smith [23] lists Hardeeville as a place of occurrence, along with Raleigh and Chapel Hill, and located all of them in North Carolina. However, the locality of Hardeeville occurs only in South Carolina and is thus classified in this work as such a locality for this species.Host plant: Acer saccharum.Distribution: USA: Maine (Orono); Massachusetts (Amherst—locus typicus, Groton); Michigan (Albion (College Campus)); Minnesota (Saint Paul); New York (Ithaca); North Carolina (Chapel Hill, Laurinburg (Jaycee Park†), Raleigh (Fred Fletcher Park)); Pennsylvania (State College); South Carolina (Hardeeville) (Figure 31) ([23,27]; new record in this publication [†]).

3.4.14. Drepanaphis saccharini Smith & Dillery, 1968
- Drepanaphis saccharini Smith & Dillery, 1968: 61(1): 186, 198 [27]Material examined: Holotype. Drepanaphis saccharini S & D Det. Smith & Dillery, 60-887, Silver Maple//St. James, Minn, 8•3•60, Holotype, S•S—one alate viv. fem. (USNM). Paratype. Drepanaphis saccharini S & D Det. Smith & Dillery, 60-887, Silver Maple, Paratype//St. James, Minn, 8•3•60, S•S//INHS, Insect Collection 1058912—one alate viv. fem. Paratype. Drepanaphis saccharini Smith & Dillery, paratype, Det: Smith & Dillery, BM 1984-340//N.U.S.A., Pl. Silver maple, Loc. St. James, Minn., Date 3.VIII.1960, Leg. S. S. S//NHMUK 12821470—three alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 15)Colour. In life: Abdomen greenish with white tip [27].Pigmentation of mounted specimens: Head, ANT I and thorax brown (Figure 30B). ANT II–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear, pterostigma palely pigmented, with large area inside without pigmentation (Figure 3N). Abdomen pale with dorsal abdominal tubercles dark (Figure 1N) and sclerotisation pale brown. Siphunculi, cauda, subgenital and anal plate pale brown. Legs pale brown. Fore femora slightly darker on margins (Figure 4N), hind femora with smudge.Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.08 mm long. ANT/BL 1.2–2.4; PT/BASE 8.68–14.04. ANT III with 6–10 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–8 accessory setae. Other ratios: ANT IV/ANT III 0.69–0.87; ANT V/ANT III 0.69–0.83; ANT VI/ANT III 1.27–1.97; URS/ANT III 0.11–0.13, URS/BASE 0.78–0.91; URS/SIPH 0.46–0.57; HT II/ANT III 0.1–0.14; HT II/BASE 0.8–1.1; TIBIA III/BL 0.56–0.66. SIPH/BL 0.09–0.12; SIPH/CAUDA 1.48–2.34. DAT I and II equal 0.03–0.08 mm long; DAT III biggest, 0.08–0.17 mm long (Figure 11N); DAT IV smallest, 0.02–0.04 mm long. ABD I–V with dorsal setae 0.03–0.04 mm long with pointed apices, on small sclerites. Marginal sclerites with 2–6 setae. Siphunculi flask-shaped (Figure 5N).Remarks: Sexual generation remains unknown.Host plants: Acer saccharinum, Acer rubrum.Distribution: Canada: Ontario (Toronto). USA: Georgia (Oakwood); Illinois (Chemung, Oakwood); Iowa (Decorah); Kansas (Wathena); Maryland (Beltsville, Plummers Island); Minnesota (Cannon Falls, Saint James—locus typicus, Savage); New York (Chemung); North Carolina (Burlington, Hendersonville, Moravian Falls); Ohio (Gallipolis) (Figure 32) [27].
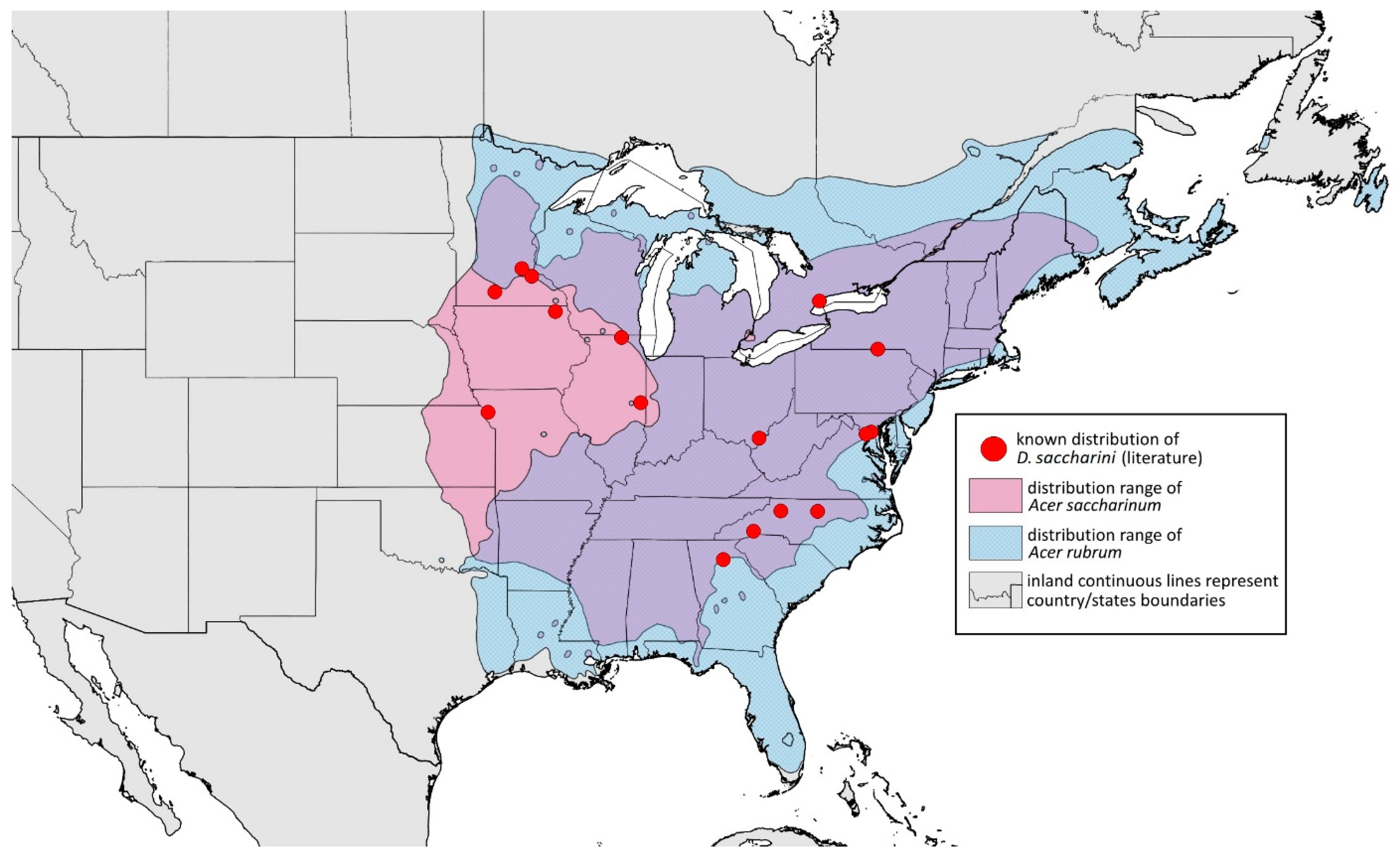
3.4.15. Drepanaphis simpsoni Smith, 1959
- Drepanaphis simpsoni Smith, 1959: 52(6): 647 [26]= Drepanaphis pallida Richards, 1969: 101(1): 107 [28]Figure 1O, Figure 3O, Figure 4O, Figure 5O, Figure 11O, Figure 14J, Figure 24D, Figure 30C, Figure 33A and Figure 34; Table 2, Table 3 and Table 4Material examined: Holotype. Drepanaphis simpsoni Smith, Holotype + Allotype C. F. S. Det: D. H. R. L. C. F. SMith//N. U. S. A. Pl. Acer saccharum, Loc. Presque Isle (Maine), Date 10.IX.1956, Leg. Simpson & H. R. L. 465, BM1984-340—two alate viv. fem., one alate viv. male. Paratype. Drepanaphis simpsoni Smith + kanzenis Smith, oviparae, Det. C. F. Smith//N. U. S. A. Pl. Acer saccharum, Loc. Presque Isle (Maine), Date 10.IX.1956, Leg. Simpson & H. R. L. 465, BM1984-340—three Viviparous ♀ ♀. Paratype. Drepanaphis simpsoni Smith, Acer saccharum//Presque Isle, Me., Oct. 9, 1958, Simpson//INHS, Insect Collection 1058916—one alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 12)Colour. In life: Head and thorax reddish brown, somewhat darker dorsally with short, fine, longitudinal, white wax stripes. Abdomen light yellow to white with DAT I reddish brown [27].Pigmentation of mounted specimens: Head, ANT I–II and pronotum brown (Figure 30C). Rest of thorax dark brown. ANT II–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear, pterostigma distinct, darkly pigmented, with small area inside without pigmentation (Figure 3O). DAT I dark; DAT II, III and IV pale brown on apices (Figure 1O). Cauda, subgenital and anal plate brown. Legs, abdomen and siphunculi pale. Fore femora darker on ends (Figure 4O).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.01–0.03 mm long with pointed apices; two pairs of pointed frontal setae on ventral side, 0.04–0.05 mm long. ANT/BL 1.17–1.61; PT/BASE 4.62 -7.08. ANT III with 8–13 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 4–6 accessory setae. Other ratios: ANT IV/ANT III 0.66–0.83; ANT V/ANT III 0.58–0.76; ANT VI/ANT III 0.98–1.33; URS/ANT III 0.1–0.12; URS/BASE 0.59–0.67; URS/SIPH 0.42–0.48; HT II/ANT III 0.13–0.17; HT II/BASE 0.77–1.0; TIBIA III/BL 0.42–0.56; SIPH/BL 0.07–0.11; SIPH/CAUDA 0.63–2.16. DAT I biggest, 0.17–0.22 mm long, DAT II 0.1–0.16 mm long, DAT III 0.08–0.16 mm long (Figure 11O), DAT IV smallest, 0.05–0.11 mm long. End of tubercles with setae 0.02 mm long. Dorsal setae 0.02–0.03 mm long with pointed apices. Siphunculi tubular (Figure 5O).Oviparous female—description (n = 3)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, thorax, siphunculi pale. ANT and legs pale brown, ANT II–VI with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Cauda, subgenital and anal plate brown (Figure 33A).
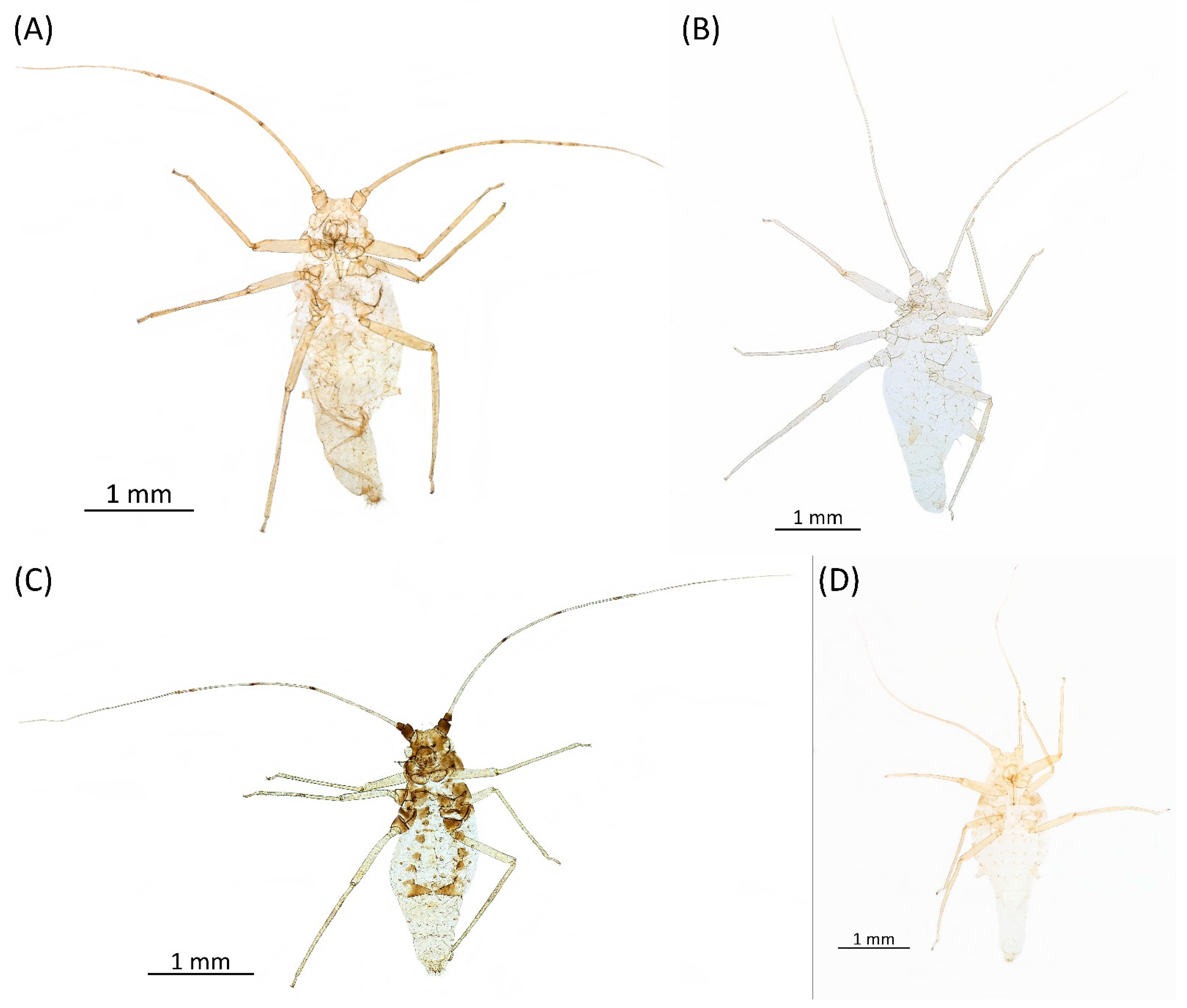
- Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.07–0.1 mm long with blunt apices; two pairs of pointed frontal setae on ventral side, 0.08–0.1 mm long. ANT/BL 1.04–1.17. Other antennal ratios: ANT VI/ANT III 0.93–1.82; PT/BASE 4.07–9.92; SIPH/BL 0.05–0.06; III FEMUR/BL 0.21–0.22; TIBIA III/BL 0.4–0.41; HT II/ANT VI 0.08–0.17; URS/ANT III 0.11–0.12; URS/BASE 0.57–0.75; URS/SIPH 0.53. ANT III without secondary rhinaria. URS with 6–9 accessory setae. Hind tibiae with 43 pseudosensoria more abundant in middle part of tibiae, closer to distal part. Dorsal setae 0.08–0.12 mm long, on ABD VIII slightly shorter. Siphunculi tubular.Alate male—re-description (n = 3)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I–II, thorax and siphunculi brown. ANT III–VI pale brown, ANT II–V with slightly darker apices on ends of segments and dark area with primary rhinarium on ANT VI. Wings clear, pterostigma palely pigmented, with large area inside without pigmentation. Cauda and anal plate brown. Abdomen pale with dark sclerotisation. Legs pale brown (Figure 24D).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.01–0.03 mm long with pointed apices; two pairs of pointed frontal setae on ventral side, 0.03–0.04 mm long. ANT/BL 1.33–1.62. Other ratios: ANT VI/ANT III 0.99–1.14; PT/BASE 5.16–5.85; SIPH/BL 0.07–0.09; III FEMUR/BL 0.24–0.31; TIBIA III/BL 0.45–0.56; URS/ANT III 0.55–0.56; URS/SIPH 0.64–0.73. ANT III with 92–118 rhinaria, ANT IV with 62–82 rhinaria, ANT V with 30–38 rhinaria. URS with six accessory setae. DAT inconspicuous. Dorsal setae 0.02–0.03 mm long, with pointed apices. ABD II–VI with distinct spinal sclerites, each with 2–4 setae. Marginal sclerites with 1–3 setae. Siphunculi tubular. Genitalia with basal part of phallus rectangular, with broadly rounded apices (Figure 14J).Remarks: The analysis of type material of D. pallida confirmed its identity with D. simpsoni.Host plant: Acer saccharum.
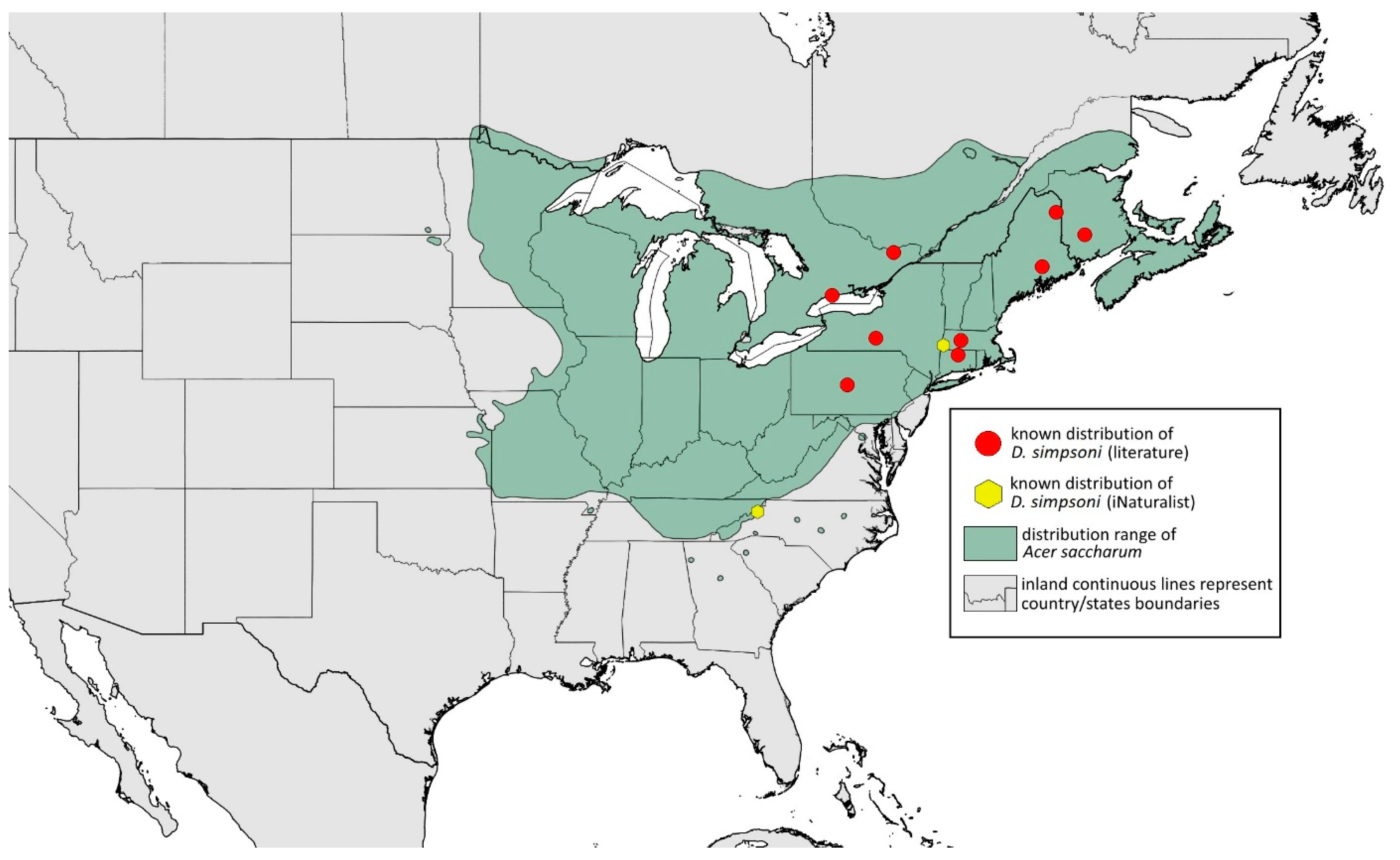
- Additional distribution from iNaturalist (www.inaturalist.org, accessed on 12 June 2024): Massachusetts (Great Barrington); Tennessee (Roan Mountain).
3.4.16. Drepanaphis spicata Smith, 1941
- Drepanaphis spicatum Smith, 1941: 57(2): 228, 241 [23]= Drepanaphis spicata Palmer, 1952: 5:87 [51]Figure 1P, Figure 3P, Figure 4P, Figure 5P, Figure 11P, Figure 14K, Figure 24E, Figure 30D, Figure 33B and Figure 35; Table 2 and Table 4Material examined: Holotype. Drepanaphis spicatum Smith Holotype Type No 55839. D.D.N.N.M.//N. C. 41-150, Acer spicatum, Mt. Mitchell, (Camp Alice), July 2, 1941, C. F. Smith, Remounted June 7,00—two alate viv. fem. (USNM). Paratype. Drepanaphis spicatum C. F. Smith//N. C. 41-188, Acer spicatum, On Park Way, Busic, N. C. 7-31-41, C. F. S.//INHS, Insect Collection 1058917—five alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 19)Colour. In life: Entire body powdery white except dark fuscous thoracic lobes, DAT III, siphunculi and brownish-yellow, U-shaped line more or less connecting DAT III to siphunculi. Fore femora dark [27].Pigmentation of mounted specimens: Head, ANT I–II and pronotum brown (Figure 30D). Rest of thorax dark brown. ANT II–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wing veins clear, pterostigma distinct, very darkly pigmented, with small area inside without pigmentation (Figure 3P), radius veins dark brown. Abdomen pale with DAT III dark (Figure 1P) and dorsal sclerotisation dark. Cauda, subgenital and anal plate pale brown to brown. Fore femora brown, darker dorsally (Figure 4P). Middle femora pale brown, hind femora pale brown with dark stripes at margins.Morphometric characters: Head setae: two pairs of fronto-orbital setae 0.05–0.07 mm long, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.09–0.012 mm long. ANT/BL 1.77–2.35; PT/BASE 7.86–14.63. ANT III with 9–15 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–8 accessory setae. Other ratios: ANT IV/ANT III 0.67–0.95; ANT V/ANT III 0.6–0.88; ANT VI/ANT III 1.19–2.06; URS/ANT III 0.08–0.1; URS/BASE 0.6–0.8; URS/SIPH 0.26–0.41; HT II/ANT III 0.08–0.11; HT II/BASE 0.63–0.85; TIBIA III/BL 0.54–0.73; SIPH/BL 0.1–0.18; SIPH/CAUDA 1.87–3.17. DAT III significant, 0.22–0.31 mm long (Figure 11P). Dorsal setae with pointed apices, 0.04–0.06 mm long, on small sclerites. Marginal sclerites with 3–6 setae. Siphunculi flask-shaped (Figure 5P).Oviparous female—description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Body in general pale brown, with slightly darker hind tibiae and ends of siphunculi (Figure 33B).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.08–0.12 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.11 mm long. ANT/BL 1.2. Other ratios: ANT VI/ANT III 1.19; PT/BASE 6.85; SIPH/BL 0.08–0.09; FEMUR III/BL 0.24–0.25; TIBIA III/BL 0.48–0.49; HT II/ANT VI 0.12; URS/ANT III 0.12; URS/BASE 0.77; URS/SIPH 0.43. URS with 10 accessory setae. Hind tibiae with 59–71 pseudosensoria distributed in central part of tibiae. Dorsal setae 0.1–0.13 mm long, on ABD VIII slightly shorter, to 0.09 mm long. Siphunculi flask-shaped.Alate male—re-description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I–II, thorax and siphunculi brown. ANT III–VI pale brown, ANT II–V with slightly darker apices on ends of segments and dark area with primary rhinarium on ANT VI. Wings clear, pterostigma distinct, darkly pigmented, with small area inside without pigmentation. Abdomen pale with dark sclerotisation. Cauda and anal plate brown. Legs pale, fore femora darker dorsally, hind femora with dark stripes on the apical parts (Figure 24E).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.05 mm long with pointed apices; one pair of pointed frontal setae on ventral side, 0.07 mm long. ANT/BL 1.76–1.94. Other ratios: ANT VI/ANT III 1.58–1.24; PT/BASE 9.47–12.6; SIPH/BL 0.14; FEMUR III/BL 0.29; TIBIA III/BL 0.62; URS/ANT III 0.09; URS/SIPH 0.73. ANT III with 106–107 rhinaria, ANT IV with 46 rhinaria, ANT V with 23–25 rhinaria. URS with 12 accessory setae. Dorsal tubercles inconspicuous. Dorsal setae 0.04–0.06 mm long, with pointed apices. ABD I–VI with spinal sclerites and 2–4 setae. Marginal sclerites with 1–3 setae. Siphunculi flask-shaped. Genitalia with basal part of phallus short, robust and almost square-shaped (Figure 14K).Remarks: Smith and Knowlton [24] note in their article that individuals from Utah and Idaho are slightly different from other representatives of this species. They note the difference in abdominal dorsal tubercule III, usually longer, and with fewer and smaller dark areas around the hairs on the dorsal side of the abdomen.Host plant: Acer spicatum, specimens from Utah and Idaho known from Acer grandidentatum.Distribution: Canada: Manitoba (Caddy Lake, Camp Morton, Grand Beach); Quebec (Chute Panet, Saint-Nicolas). USA: Idaho (Franklin, Mink Creek); North Carolina (Buck Creek Gap, Grandfather Mountain, Mount Mitchell—locus typicus); Pennsylvania (Philipsburg, Pleasant Gap, Sprace Creek (Colerain Park), State College (Poe Paddy Laken Tussey Tower), Woodward); Utah (Big Cottonwood Canyon, Blacksmith Fork Canyon, City Creek Canyon, Logan Canyon, Mantua, Mount Nebo, Mount Sterling in Smithfield Canyon, Provo Canyon, Strawberry Creek, Weber Canyon) (Figure 35) [23,24,27].
3.4.17. Drepanaphis tissoti Smith, 1944 stat. rev.
- Drepanaphis tissoti Smith, 1944: 27(3): 55 [25]Figure 1Q, Figure 2D, Figure 3Q, Figure 4Q, Figure 5Q, Figure 11Q, Figure 30E, Figure 33C and Figure 36; Table 2 and Table 3Material examined: Holotype. Drepanaphis tissoti Smith, Det. CFSmith, Acer rubrum//Gainesville, Florida, Hatchetcreek, 5-5-1941, A. N. Tissot coll. F-2207-41. Paracotype. Drepanaphis tissoti Smith, Acer rubrum,//Gainesville, Florida, Hatchetcreek, 5-5-1941, A. N. Tissot coll. F-2207-41//Museum Paris MNHN 25154—one alate viv. fem. Paracotype. Drepanaphis tissoti Smith, Acer rubrum,//Gainesville, Florida, Hatchetcreek, 5-5-1941, A. N. Tissot coll. B. M.1967-340. F-2207-41//NHMUK 12821452—one alate viv. fem.Additional material examined—Table S6Alate viviparous female—re-description (n = 25)Colour. In life: Black with pale legs, wings with noticeable dark spots at end of veins. Head with three, pronotum with two longitudinal white wax stripes. Mesonotum with two antero-lateral wax dots; two anteromedial dots usually connected to two postero-medial dots to make two longitudinal stripes. Metanotum with two wax dots laterally. Abdomen with many wax dots, most dense at posterior end [27].Pigmentation of mounted specimens: Head, thorax, ANT I–II dark brown (Figure 30E). ANT III–VI pale brown with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with dark pigmentation of ends of veins, radius veins dark brown. Pterostigma distinct, darkly pigmented, oval with small area inside without pigmentation (Figure 3Q). Abdomen pale with brown dorsal sclerotisation. Dorsal abdominal tubercles (Figure 1Q) and siphunculi dark brown. Cauda, subgenital and anal plate pale. Femora (Figure 4Q), tibiae and tarsi pale brown.Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.01–0.02 mm long with blunt apices. One pair of pointed frontal setae on ventral side, 0.05–0.06 mm long. ANT/BL 1.95–3.64; PT/BASE 7.2–16.8. ANT III with 8–11 secondary rhinaria, BASE with 5–11 accessory rhinaria (Figure 2D). URS with 6–9 accessory setae. Other ratios: ANT IV/ANT III 0.61–0.73; ANT V/ANT III 0.53–0.83; ANT VI/ANT III 1.18–2.72; URS/ANT III 0.11–0.13; URS/BASE 0.67–0.9; URS/SIPH 0.44–0.69; HT II/ANT III 0.1–0.13; HT II/BASE 0.65–0.85; TIBIA III/BL 0.57–0.88; SIPH/BL 0.09–0.18; SIPH/CAUDA 1.4–2.45. DAT I, II and IV inconspicuous. DAT III 0.15–0.19 mm long (Figure 11Q). Dorsal setae 0.01–0.02 mm long with blunt apices, on small sclerites. Siphunculi flask-shaped (Figure 5Q).Oviparous female—description (n = 1)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, ANT I–II, thorax, siphunculi dark brown. ANT and legs pale brown, ANT II–VI with darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Abdomen pale with dark sclerotisation. Cauda, subgenital and anal plate pale brown (Figure 33C).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.06-.0.07 mm long with blunt apices; one pair of pointed frontal setae on ventral side, 0.09 mm long. ANT/BL 1.6. Other ratios: ANT VI/ANT III 1.78–1.82; PT/BASE 12.5–12.8; SIPH/BL 0.074–0.086; FEMUR III/BL 0.23; TIBIA III/BL 0.42; HT II/ANT VI 0.05–0.06; URS/ANT III 0.088; URS/BASE 0.67; URS/SIPH 0.44. URS with eight accessory setae. Hind tibiae with 30–31 pseudosensoria more abundant on the middle part of tibiae, closer to knee area. Dorsal setae 0.08–0.1 mm long, marginal sclerites with 1–4 setae each. Siphunculi flask-shaped.Male: Unknown.Remarks: Remaudière and Remaudière, based on personal communication from F.W. Quednau [29], establish the synonymisation of D. tissoti with D. nigricans without sufficient morphological study. However, the analysis and comparison of both species indicate differences (particularly the significant differences between oviparous females of these species). The main features that differentiate the alate viviparous females of these species are the number of secondary rhinaria on ANT III (in the case of D. nigricans, more than 11, in the case of D. tissoti, never more than 11) and the number of accessory rhinaria on BASE (D. nigricans always with 4, D. tissoti with 5–11). However, the features between oviparous females are also significant: hind tibiae with 53–62 pseudosensoria (D. nigricans) and 30–31 pseudosensoria (D. tossoti). Differences are also present in the respective ratios: ANT VI/ANT III, TIBIA III/BL, URS/BASE.Host plants: Acer rubrum, occasionally on Acer saccharum.
3.4.18. Drepanaphis utahensis Smith & Knowlton, 1943
- Drepanaphis utahensis Smith & Knowlton, 1943: 59(2): 172, 174 [24]Figure 1R, Figure 3R, Figure 4R, Figure 5R, Figure 10C, Figure 11R, Figure 14L, Figure 25F, Figure 30F, Figure 33D and Figure 37; Table 2, Table 3 and Table 4Material examined: Holotype. Drepanaphis utahensis K.-S.//Type Utah Aphids, Host maple, Drepanaphis utahensis K.-S. Brigham Canyon, Date 7-1-1937, C. F. Smith, C.K.S.—five alate viv. fem. (USNM). Paratype. Mt. Maple—Paratype., APHIDIDAE OF UTAH, Host Acer glabrum, Aphid Drepanaphis utahensis K-S, Locality Ogden Cany; Ut., May-20-1930, GEORGE F. KNOWLTON, Coll. very active. Heavy whitish pruinose, over head, thorax + abdomen. Ground color of abdomen green (not to dark)////INHS, Insect Collection 1058919—one alate viv. fem.Additional material examined—Table S6.Alate viviparous female—re-description (n = 26)Colour. In life: Body yellow, head and pronotum edged in black, prescutum and thoracic lobes brown, DAT III dark; entire body frosted with white wax [27].Pigmentation of mounted specimens: Head, ANT I, pronotum and siphunculi brown (Figure 30F). Rest of thorax dark brown. ANT II–VI pale brown with slightly darker apices on ANT III–V and dark area with primary rhinarium on ANT VI. Wings clear with small area of dark brown pigmentation at end. Wings clear, pterostigma distinct, pigmented darker on ends, with small area inside without pigmentation (Figure 3R). Abdomen pale. DAT III dark (Figure 1R). Cauda, subgenital and anal plate pale brown. Legs pale brown. Fore femora darker on ends (Figure 4R).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.03–0.04 mm long with pointed apices; two pairs of pointed frontal setae on ventral side 0.06–0.08 mm long. ANT/BL 0.94–2.05; PT/BASE 4.27–9.04. ANT III with 12–22 secondary rhinaria, BASE with 4 accessory rhinaria. URS with 6–10 accessory setae (Figure 10C). Other ratios: ANT IV/ANT III 0.57–0.88; ANT V/ANT III 0.5–0.87; ANT VI/ANT III 0.87–1.68; URS/ANT III 0.09–0.13; URS/BASE 0.49–0.73; URS/SIPH 0.33–0.46; HT II/ANT III 0.12–0.18; HT II/BASE 0.69–0.95; TIBIA III/BL 0.41–0.66; SIPH/BL 0.08–0.13; SIPH/CAUDA 1.51–2.36. DAT I, II and IV inconspicuous. DAT III distinct, 0.09–0.14 mm long (Figure 11R). Dorsal setae 0.03–0.04 mm long with pointed apices. Siphunculi tubular (Figure 5R).Oviparous female—description (n = 5)Colour. In life: Unknown.Pigmentation of mounted specimens: Body generally pale brown with abdomen slightly lighter. Legs and dorsal sclerotisation slightly darker (Figure 33D).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae 0.07–0.01 mm long, one pair of latero-dorsal setae on dorsal side 0.05–0.07 mm long with blunt apices; two pairs of pointed frontal setae on ventral side, 0.05–0.07 mm long. ANT/BL 0.88–1.04. Other ratios: ANT VI/ANT III 1.31–1.2; PT/BASE 5.92–7.77; SIPH/BL 0.05–0.066; FEMUR III/BL 0.19–0.21; TIBIA III/BL 0.36–0.42; HT II/ANT VI 0.1–0.12; URS/ANT III 0.12–0.17; URS/BASE 0.69–0.83; URS/SIPH 0.47–0.64. ANT III without secondary rhinaria. URS with 6–8 accessory setae. Hind tibiae with 13–52 pseudosensoria more abundant in middle part of tibiae. Dorsal setae 0.08–0.09 mm long. ABD I–II with large marginal sclerites. Siphunculi tubular.Alate male—re-description (n = 4)Colour. In life: Unknown.Pigmentation of mounted specimens: Head, pronotum, ANT and siphunculi brown. Rest of thorax dark brown. Wings clear with closed, wide, brown pterostigma. Abdomen pale with dark sclerotisation. Cauda, anal plate and genitalia brown. Legs pale, hind femora with light brown smudge (Figure 24F).Morphometric characters: Head setae: two pairs of fronto-orbital setae, one pair of postero-dorsal setae, one pair of latero-dorsal setae on dorsal side, 0.02–0.03 mm long with pointed apices; two pairs of pointed frontal setae on ventral side, 0.03–0.06 mm long. ANT/BL 1.29–1.68. Other antennal ratios: ANT VI/ANT III 1.2–1.44; PT/BASE 6.14–7.3; SIPH/BL 0.09–0.11; FEMUR III/BL 0.26–0.3; TIBIA III/BL 0.51–0.64; URS/ANT III 0.11–0.12; URS/SIPH 0.47–0.53. ANT III with 78–83 rhinaria, ANT IV with 46–52 rhinaria, ANT V with 27–30 rhinaria. URS with eight accessory setae. DAT inconspicuous. Dorsal setae 0.02–0.03 mm long, with pointed apices. ABD II–V with spino-pleural sclerites with two setae each. Marginal sclerites with 3–6 setae. Siphunculi tubular. Genitalia with basal part of phallus short, robust, with broadly rounded apices (Figure 14L).Remarks: Archibald [61] reported an individual of this species from Nova Scotia on sugar maple, A. saccharum [27]. However, when analysing its range of occurrence, Archibald probably incorrectly identified this individual. The databases analysed also included records from Illinois and North Carolina. But the host plant was never A. grandidentatum, and we could not analyse these individuals, so we do not include their occurrence on the map. Smith and Knowlton [24] also mention that they had over 100 other slides from Utah and Idaho, but these specimens were brighter. However, they did not notice any other significant differences apart from colour. These individuals come from the following locations: Idaho (Boise (Minidoka National Forest), Franklin, Mink Creek); Utah (American Fork Canyon, Beaver Canyon, Big Cottonwood Canyon, Brigham Canyon, Eden, Emigration Canyon, Lakota, Mantua, Millville, Mt. Nebo, Oak Creek Canyon, Parley’s Canyon, Providence Canyon, Provo Canyon, Richmond, Sardine Canyon, Uinta).Host plant: Acer grandidentatum.Distribution: USA: Idaho (Bench^, Caribou-Targhee National Forest, Cub River Canyon); New Mexico (Cibola National Forest); Utah (Abajo Mountains, Blacksmith Fork Canyon, Bountiful (Skyline Drive), Brigham Canyon—locus typicus, City Creek Canyon, Cutler Dam, Daniels Canyon, Farmington Canyon, Honeyville, Huntsville, Liberty, Logan (Green Canyon), Mantua, Mount Nebo, Mount Timpanogos, Muller’s Park (Davis County), North Ogden, Ogden Canyon, Peterson, Pinecrest, Providence Canyon, Provo Canyon, Salt Lake City‴, Santaquin, Smithfield Canyon, Weber Canyon, Wellsville Canyon (also Mount Sterling), West Hodges Canyon) (Figure 37) [24,27]; International Barcode of Life project (iBOL) [^]; NMNH Extant Specimen Records (USNM, US) [‴].
3.5. Results of the Statistical Analysis
4. Discussion
4.1. Morphological Groups within the Genus Drepanaphis
4.2. Morphometric Similarities in the Genus Drepanaphis
4.3. Host Plant Ambiguity
4.4. Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favret, C.; Aphid Species File. Version 5.0/5.0. 2024. Available online: http://Aphid.SpeciesFile.org (accessed on 12 June 2024).
- Nováková, E.; Hypša, V.; Klein, J.; Foottit, R.; von Dohlen, C.; Moran, N. Reconstructing the phylogeny of aphids (Hemiptera: Aphididae) using DNA of the obligate symbiont Buchnera aphidicola. Mol. Phylogenet. Evol. 2013, 68, 42–54. [Google Scholar] [CrossRef]
- von Dohlen, C.D.; Moran, N.A. Molecular data support a rapid radiation of aphids in the Cretaceous and multiple origins of host alternation. Biol. J. Linn. Soc. 2000, 71, 689–717. [Google Scholar] [CrossRef]
- Völkl, W.; Woodring, J.; Fischer, M.; Lorenz, M.; Hoffmann, K. Ant-aphid mutualisms: The impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 1999, 118, 483–491. [Google Scholar] [CrossRef]
- Kamiński, P.; Barczak, T.; Bennewicz, J.; Jerzak, L.; Kavanagh, B.; Tkachenko, H.; Stuczyński, T.; Baszyński, J.; Szady-Grad, M.; Woźniak, A. The role of aphids in the transfer of chemical elements in disturbed Polish saline environments. Sci. Total Environ. 2021, 776, 145980. [Google Scholar] [CrossRef]
- Chan, C.K.; Forbes, A.R.; Raworth, D.A. Aphid-Transmitted Viruses and Their Vectors of the World, 1st ed.; Research Station/Research Branch/Agriculture Canada: Vancouver, BC, Canada, 1991; pp. 1–216. [Google Scholar]
- Davatchi, A.; Hille Ris Lambers, D.; Remaudière, G. On some near-eastern aphids. Tijdschr. Entomol. 1957, 100, 125–128. [Google Scholar]
- Koch, C.L. Die Pflanzenläuse Aphiden Getreu Nach Dem Leben Abgebildet und Beschrieben, 1st ed.; J.L. Lotzbeck: Nürnberg, Germany, 1855; Volume 7, pp. 201–208. [Google Scholar]
- Matsumura, S. A list of the Aphididae of Japan, with description of new species and genera. J. Coll. Agric. Tohoku. Imp. 1917, 7, 351–414. [Google Scholar]
- Hottes, F.C.; Frison, T.H. The plant lice, or Aphiidae, of Illinois. Bull. Ill. Nat. Hist. Surv. 1931, 19, 121–447. [Google Scholar] [CrossRef]
- Wieczorek, K.; Kanturski, M.; Junkiert, Ł. Shenahweum minutum (Hemiptera, Aphidoidea: Drepanosiphinae)—Taxonomic position and description of sexuales. Zootaxa 2013, 3731, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, K.; Kanturski, M.; Junkiert, Ł.; Bugaj-Nawrocka, A. A comparative morphometric study of the genus Drepanosiphoniella Davatchi, Hille Ris Lambers & Remaudière (Hemiptera: Aphididae: Drepanosiphinae). Zool. Anz. 2015, 257, 39–53. [Google Scholar] [CrossRef]
- Wieczorek, K.; Junkiert, Ł.; Kanturski, M. Taxonomical implications of the comparative study of the genus Drepanosiphum Koch, 1855 (Hemiptera: Aphididae, Drepanosiphinae). Zool. Anz. 2016, 263, 92–117. [Google Scholar] [CrossRef]
- Wieczorek, K.; Lachowska-Cierlik, D.; Kajtoch, Ł.; Kanturski, M. The relationships within the Chaitophorinae and Drepanosiphinae (Hemiptera, Aphididae) inferred from molecular-based phylogeny and comprehensive morphological data. PLoS ONE 2017, 12, e0173608. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S. Revision of the genus Yamatocallis Matsumura in Japan (Hemiptera: Aphididae). Insecta Matsumurana 2017, 73, 39–63. [Google Scholar]
- Del Guercio, G. Intorno ad un nuovo genere di Macrosifonidi americani [Gen. Drepanaphis. n.]. Riv. Patol. Veg. 1909, 2, 49–50. [Google Scholar]
- Thomas, C. A List of the Species of the Tribe Aphidini, family Aphidae, found in the United States, which have been heretofore named, with descriptions of some New Species. Bull. Ill. Nat. Hist. Surv. 1878, 1, 3–16. [Google Scholar] [CrossRef]
- Malik, K.; Miller, G.L.; Jensen, A.S.; Wieczorek, K. Key characteristics of selected Drepanaphis Del Guercio, 1909 (Hemiptera: Aphididae) species based on various identification methods. Bonn. Zool. Bull. 2023, 72, 185–199. [Google Scholar] [CrossRef]
- Davis, J.J. Two new genera and species of Aphididae. Ann. Entomol. Soc. Am. 1909, 2, 196–200. [Google Scholar] [CrossRef]
- Gillette, C.P. Plant Louse Notes, Family Aphididae. J. Econ. Entomol. 1910, 3, 367–371. [Google Scholar] [CrossRef]
- Granovsky, A.A. The Plant Lice, or Aphiidae, of Illinois; Hottes, F.C., Frison, T.H., Eds.; Schnepp and Barnes: Springfield, IL, USA, 1931; Volume 19, pp. 246–248. [Google Scholar] [CrossRef]
- Miller, F.W. Two new aphids from Massachusetts. Can. Entomol. 1937, 69, 111–113. [Google Scholar] [CrossRef]
- Smith, C.F. The genus Drepanaphis Del Guercio east of the Rocky Mountains. J. Elisha Mitchell Sci. Soc. 1941, 57, 226–242. [Google Scholar]
- Smith, C.F.; Knowlton, G.F. The aphid genus Drepanaphis Del Guercio. J. Elisha Mitchell Sci. Soc. 1943, 59, 171–176. [Google Scholar]
- Smith, C.F. Drepanaphis tissoti, a new species of aphid from Florida. Flo. Entomol. 1944, 27, 55–57. [Google Scholar] [CrossRef]
- Smith, C.F. A new species of aphid on sugar maple (Aphidae: Homoptera). Ann. Entomol. Soc. Am. 1959, 52, 647–649. [Google Scholar] [CrossRef]
- Smith, C.F.; Dillery, D.G. The genus Drepanaphis Del Guercio (Homoptera: Aphididae). Ann. Entomol. Soc. Am. 1968, 61, 185–204. [Google Scholar] [CrossRef]
- Richards, W.R. Drepanaphis pallida, a new aphid collected on Acer saccharum in Ontario (Homoptera: Aphididae). Can. Entomol. 1969, 101, 107–108. [Google Scholar] [CrossRef]
- Remaudière, G.; Remaudière, M. Catalogue of the World’s Aphididae, 1st ed.; INRA: Paris, France, 1997; pp. 1–478. [Google Scholar]
- Wieczorek, K.; Chłond, D. Hop-on, hop-off: The first record of the alien species crescent-marked lily aphid (Neomyzus circumflexus) (Insecta, Hemiptera, Aphididae) in Greenland. Polar Res. 2020, 39, 1–4. [Google Scholar] [CrossRef]
- Ilharco, F.A.; van Harten, A. Systematics. In Aphids: Their Biology, Natural Enemies and Control, 1st ed.; Minks, A.K., Harrewijn, P., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; pp. 51–77. [Google Scholar]
- WFO Plant List. 2024. Available online: https://wfoplantlist.org (accessed on 12 June 2024).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- iNaturalist. Available online: https://www.inaturalist.org. (accessed on 12 June 2024).
- Global Biodiversity Information Facility (GBIF). 2024. GBIF Occurrence Download. Available online: https://occurrence-download.gbif.org/ (accessed on 12 June 2024).
- Google Inc. Google Earth, Version 7.3.2. 2024. Mountain View, CA, USA. Available online: http://www.google.com/earth/index.html (accessed on 12 June 2024).
- Conservation Biology Institute (CBI). Data Basin Conservation Biology Institute 136 SW Washington Ave., Ste. 202 Corvallis, OR 97333. 2024. Available online: http://databasin.org (accessed on 12 June 2024).
- Little, E.L., Jr. Atlas of United States Trees. Volume 1. Conifers and Important Hardwoods; Miscellaneous Publication 1146; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1971; p. 9, 313 maps. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open-Source Geospatial Foundation Project. 2024. Available online: http://qgis.osgeo.org (accessed on 12 June 2024).
- Monell, J. Notes on the Aphididae of the United States, with Descriptions of Species Occurring West of the Mississippi; Riley, C.V., Monell, J., Eds.; Bulletin of the United States Geological and Geographical Survey of the Territories: Washington, DC, USA, 1879; Volume 5, p. 27. [Google Scholar]
- Davidson, W.M. Notes on Aphididae collected in the vicinity of Stanford University. J. Econ. Entomol. 1909, 2, 299–305. [Google Scholar] [CrossRef]
- Davis, J.J. A list of the Aphididae of Illinois, with notes on some of the species. J. Econ. Entomol. 1910, 3, 407–420. [Google Scholar] [CrossRef]
- Miller, F.W. Three new species of aphids. Can. Entomol. 1936, 68, 81–82. [Google Scholar] [CrossRef]
- Baker, A.C. Tribe Callipterini [In: Family Aphididae by Patch E.M.]. In Guide to the Insects of Connecticut. Part IV. The Hemiptera or Sucking Insects of Connecticut; Britton, W.E., Ed.; Bulletin of the Connecticut State Geological and Natural History Survey: Hartford, CT, USA, 1923; Volume 34, pp. 271–290. [Google Scholar]
- Barbagallo, S.; Limonta, L.; Süss, L. Reperti faunistici e bioecologici sugli afidi della regione Lombardia (Italia settentrionale). Boll. Zool. Agrar. Bachic. Ser. II 2008, 40, 155–224. [Google Scholar]
- Barbagallo, S.; Cocuzza, G.E.M. A survey of the aphid fauna in the Italian regions of Latium and Campania. Redia 2014, 97, 19–47. [Google Scholar]
- Barbagallo, S.; Pollini, A. Additional notes on the aphid fauna of the Emilia Romagna region in Italy. Redia 2014, 97, 71–82. [Google Scholar]
- Clarke, W.T. A list of California Aphididae. Can. Entomol. 1903, 35, 247–254. [Google Scholar] [CrossRef]
- Guyton, T.L. A taxonomic, ecologic and economic study of Ohio Aphididae. Ohio. J. Sci. 1924, 24, 1–30. [Google Scholar]
- Lozzia, G.C.; Binaghi, P. Entomofauna delle alberate cittadine e studio di un metodo di protezione integrate. Seconda parte. Disinfestazione 1992, 9, 35–41. [Google Scholar]
- Palmer, M.A. Aphids of the Rocky Mountain Region. Thomas Say Found. 1952, 5, 452. [Google Scholar]
- Pérez Hidalgo, N.; Pons, X.; Mier Durante, M. Detection of Drepanaphis acerifoliae (Thomas) (Hemiptera: Aphididae: Drepanosiphinae) on sugar maple trees, Acer saccharinum, in Spain. Bol. SEA 2008, 43, 441–444. [Google Scholar]
- Petrović-Obradović, O.; Šćiban, M.; Tomić, M. Presence of North American aphid Drepanaphis acerifoliae (Thomas, 1878) (Hemiptera: Aphididae: Drepanosiphinae) in Serbia. Acta Entomol. Serbica 2021, 26, 9–15. [Google Scholar] [CrossRef]
- Ripka, G. Occurrence of a new alien aphid species, Drepanaphis acerifoliae in Hungary. Növėnyvėdelem Övényvédelem 2010, 46, 413–415. (In Hungarian) [Google Scholar]
- Sanborn, C.E. Kansas Aphididae, with catalogue of North American Aphididae, and host-plant and host-plant list. Univ. Kans Sci. Bull. 1904, 3, 3–82. [Google Scholar] [CrossRef]
- Smith, C.F.; Parron, C.S. An annotated list of Aphididae (Homoptera) of North America. NC Agric. Exp. Stn. Tech. Bull. 1978, 255, 428. [Google Scholar]
- Williams, T.A. The Aphididae of Nebraska. Univ. Stud. Univ. Neb. 1911, 10, 85–175. [Google Scholar]
- Leonard, M.D. Additional records of Missouri aphids. J. Kans Entomol. Soc. 1963, 36, 65–84. [Google Scholar]
- Leonard, M.D. A preliminary list of the aphids of Missouri. J. Kans Entomol. Soc. 1959, 32, 9–18. [Google Scholar]
- Burnham, J.C. A contribution to a list of the Aphididae of the Maritime provinces of Canada. Can. Entomol. 1938, 70, 180–188. [Google Scholar] [CrossRef]
- Archibald, K.D. Forest Aphidae of Nova Scotia. Proc. Nova Scotian. Inst. Sci. 1958, 24, 254. [Google Scholar]
- Kim, H.; Lee, W.; Lee, S. Morphometric relationship, phylogenetic correlation, and character evolution in the species-rich genus Aphis (Hemiptera: Aphididae). PLoS ONE 2010, 5, e11608. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.; Lee, S.; Kim, H. A cryptic species of Aphis gossypii (Hemiptera: Aphididae) complex revealed by genetic divergence and different host plant association. Bull. Entomol. Res. 2014, 105, 1–12. [Google Scholar] [CrossRef]
- Hille Ris Lambers, D. Polymorphism in Aphididae. Ann. Rev. Entomol. 1966, 11, 47–78. [Google Scholar] [CrossRef]
- Kanturski, M.; Karcz, J.; Wieczorek, K. Morphology of the European species of the aphid genus Eulachnus (Hemiptera: Aphididae: Lachninae)—A SEM comparative and integrative study. Micron 2015, 76, 23–36. [Google Scholar] [CrossRef]
- Langhof, M.; Gathmann, A.; Poehling, H.M. Insecticide drift deposition on noncrop plant surfaces and its impact on two beneficial nontarget arthropods, Aphidius colemani Viereck (Hymenoptera, Braconidae) and Coccinella septempunctata L. (Coleoptera, Coccinellidae). Environ. Toxicol. Chem. 2005, 24, 2045–2054. [Google Scholar] [CrossRef]
- Heie, O.E. The Aphidoidea of Fennoscandia and Denmark III. Family Aphididae: Subfamily Pterocommatinae & Tribe Aphidini of Subfamily Aphidinae, 1st ed.; Brill (Fauna Entomologica Scandinavica): Leiden, The Netherlands, 1986; Volume 17, p. 314. [Google Scholar]
- Malik, K.; Bugaj-Nawrocka, A.; Wieczorek, K. Distribution of Drepanaphis acerifoliae—Aphid pest of Acer trees—Faced with global climate change. Folia Biol. 2023, 71, 115–130. [Google Scholar] [CrossRef]
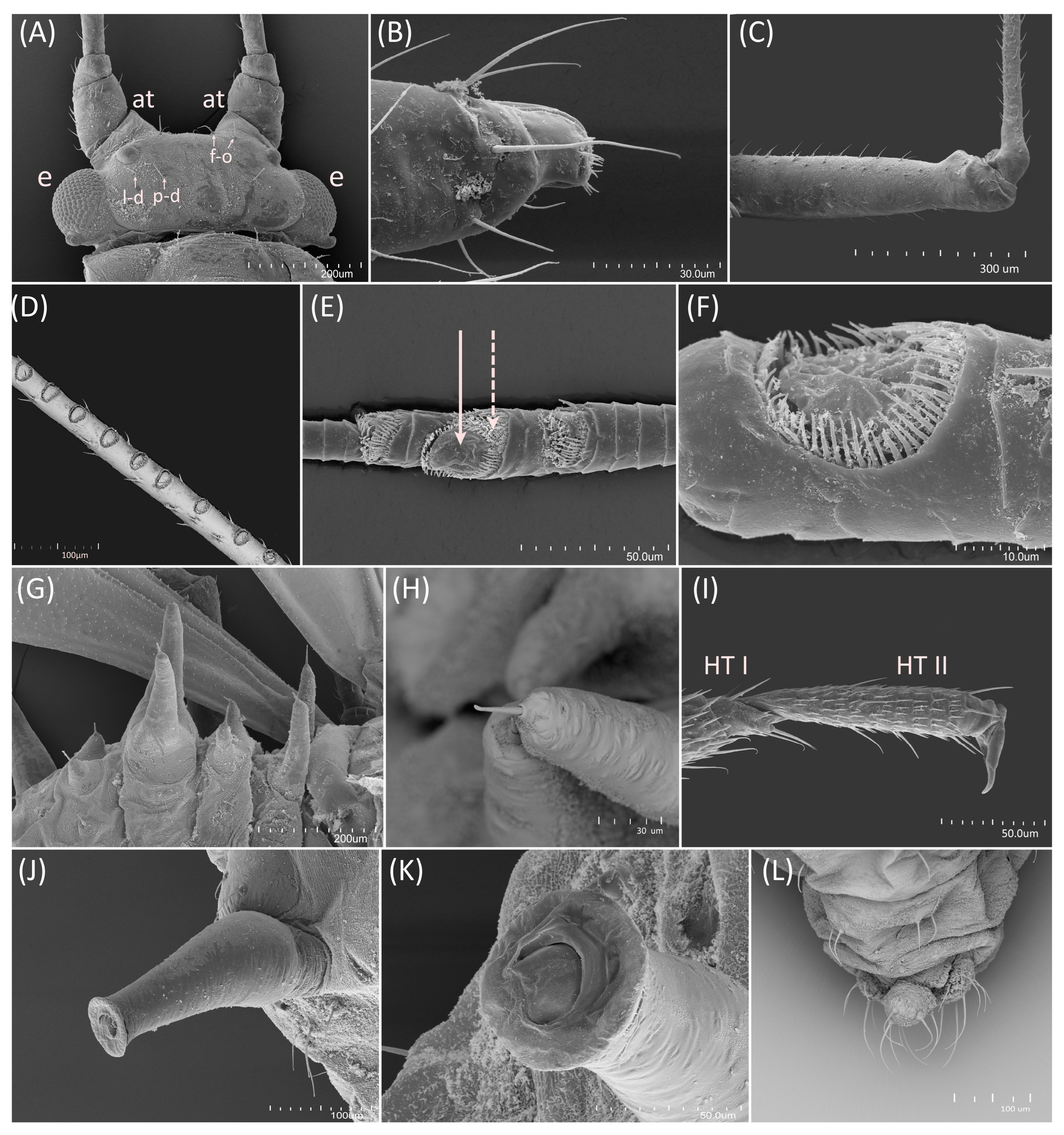

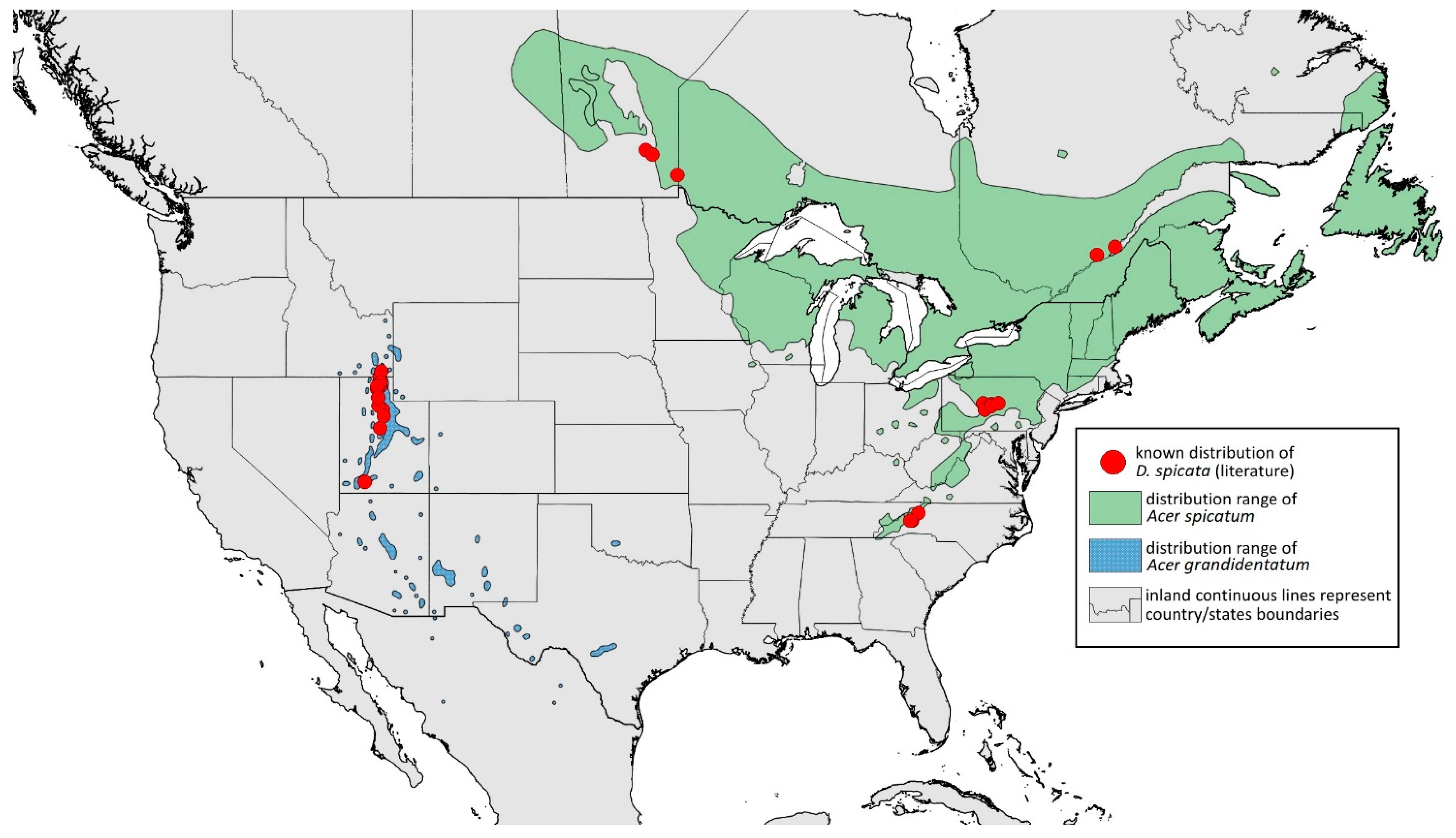
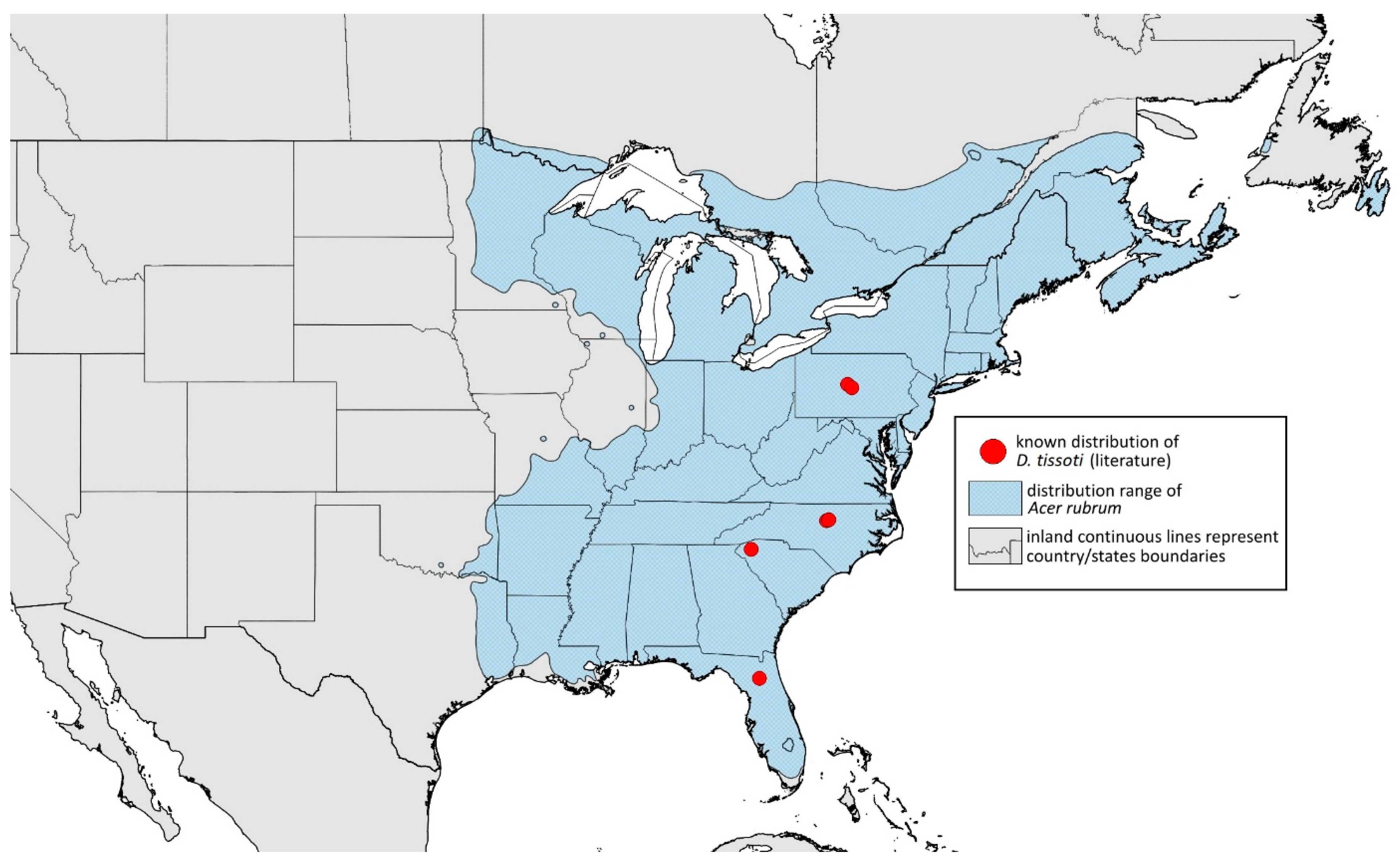

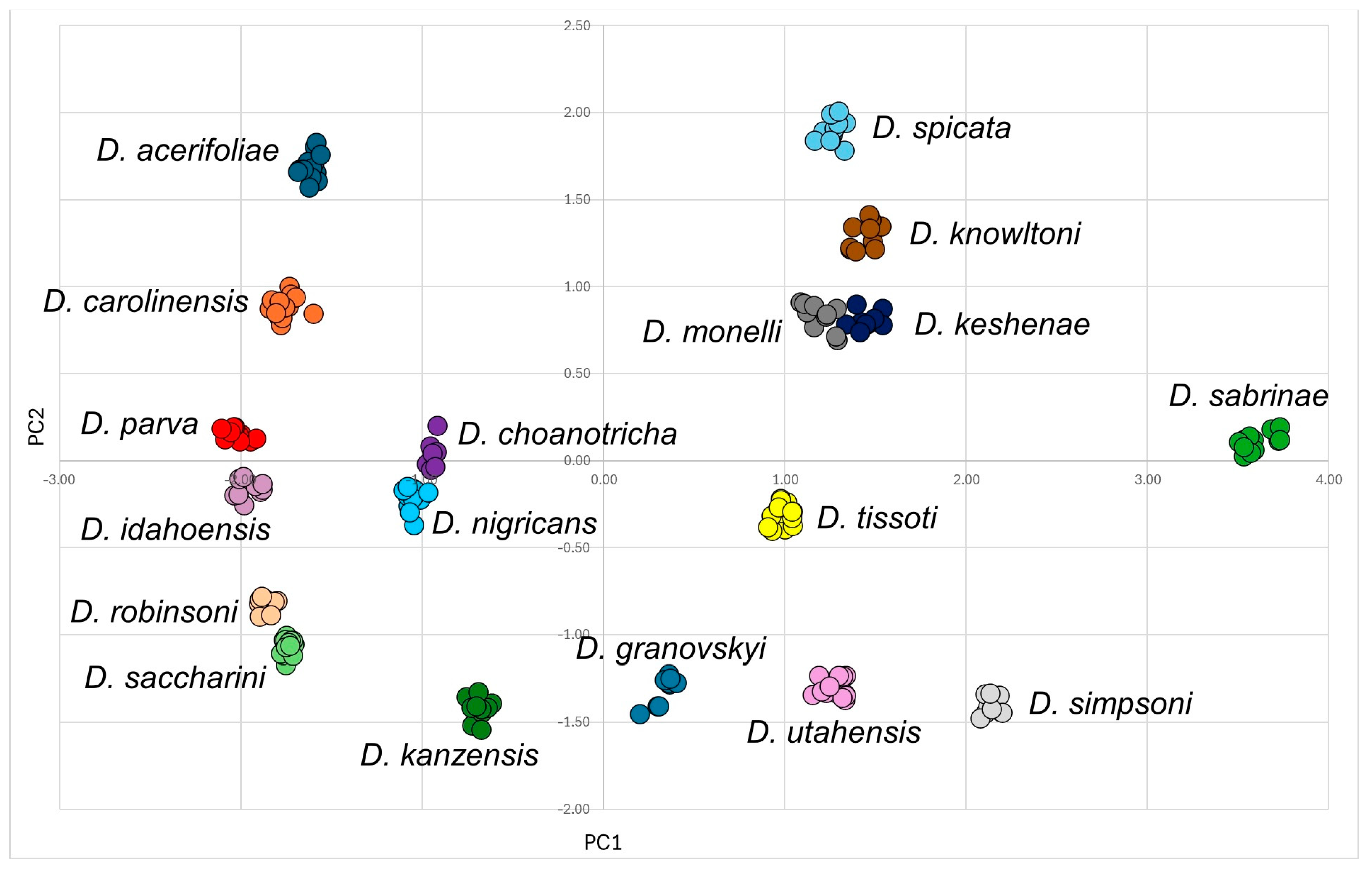

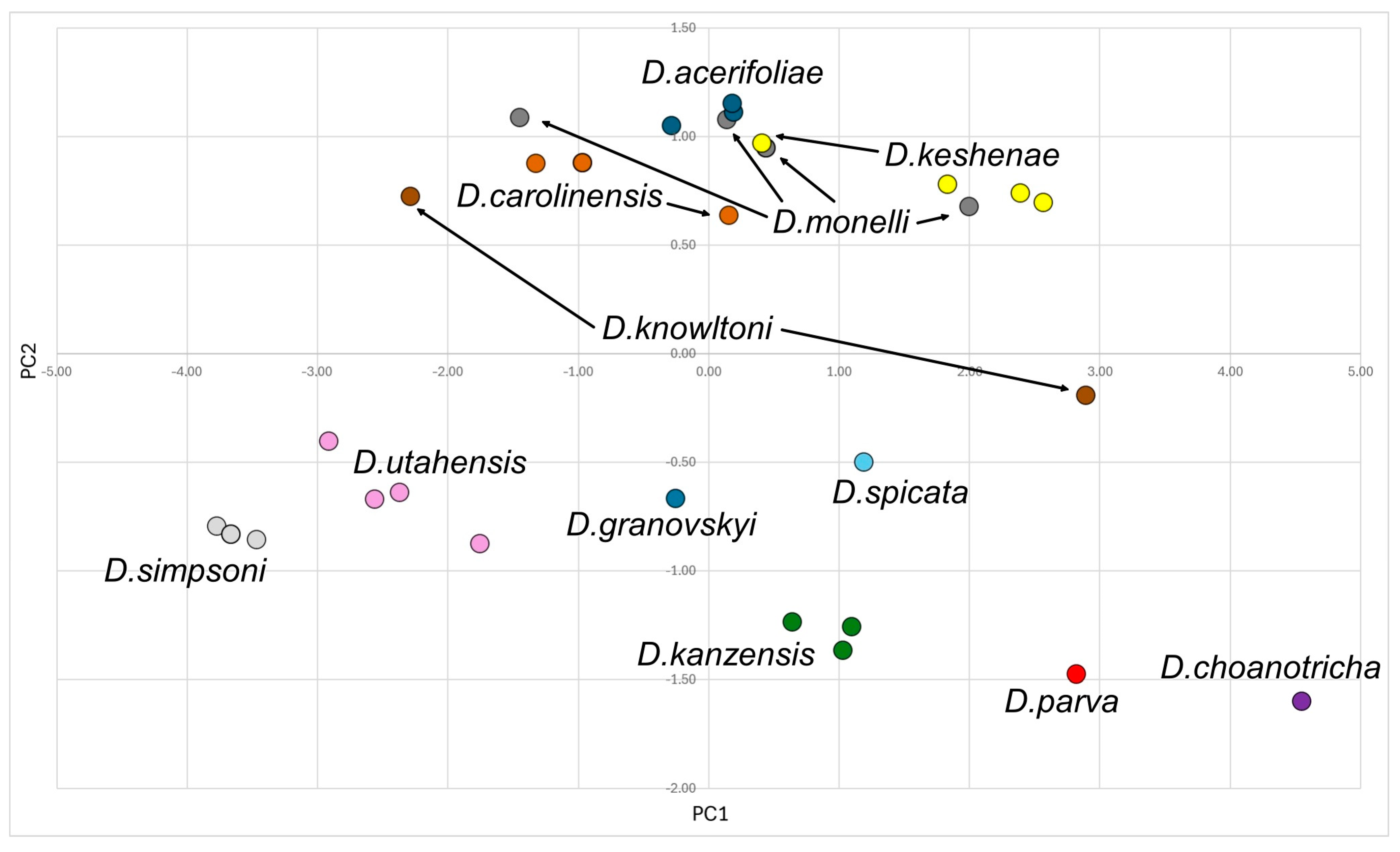
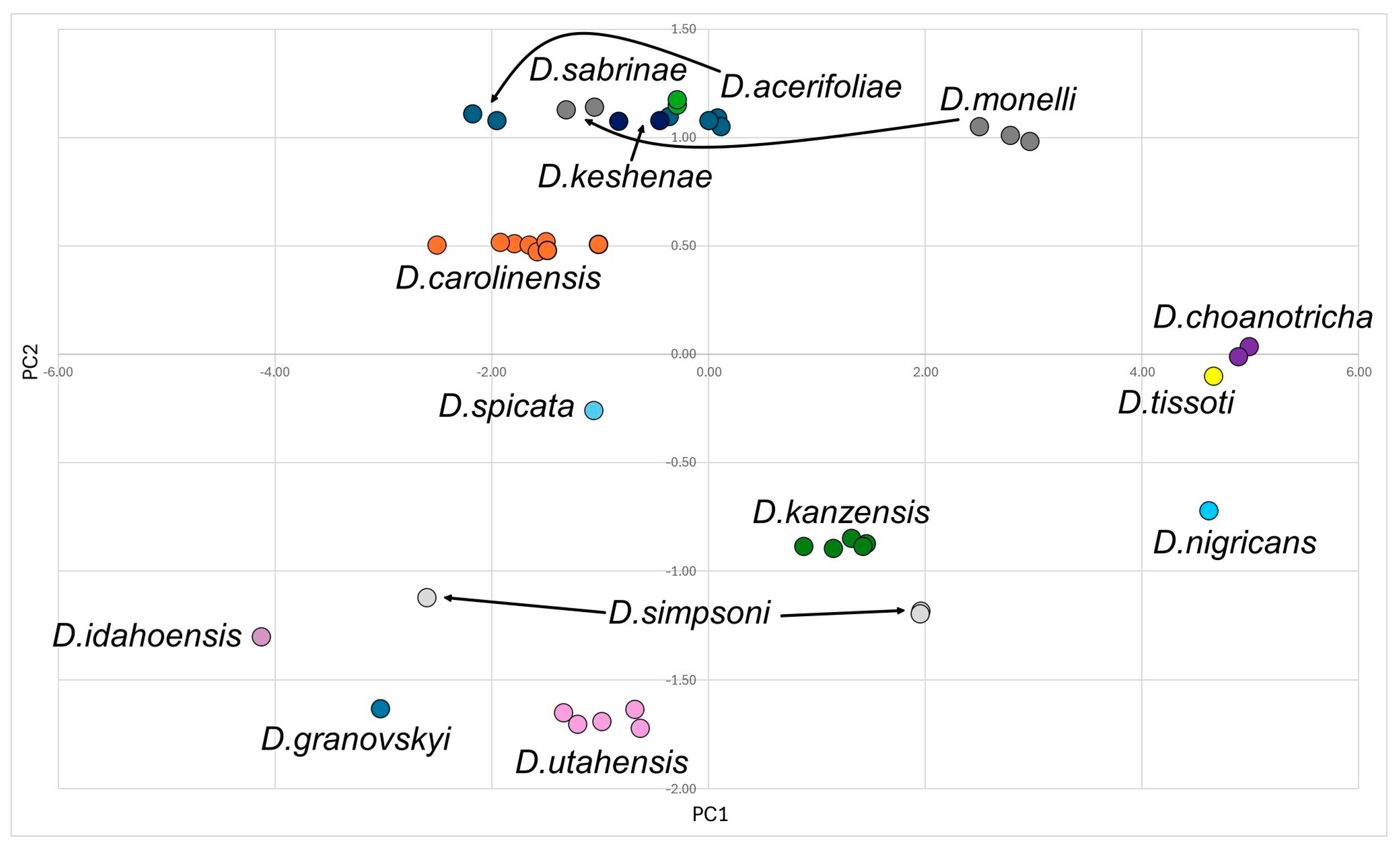
| Character | D. acerifoliae n = 26 | D. carolinensis n = 16 | D. choanotricha n = 13 | D. granovskyi n = 13 | D. idahoensis n = 10 | D. kanzensis n = 17 | D. keshenae n = 11 | D. knowltoni n = 24 | D. monelli n = 11 |
|---|---|---|---|---|---|---|---|---|---|
| BL | 1.55–2.68 | 1.57–2.5 | 1.15–1.63 | 1.37–2.21 | 1.41–2.19 | 1.24–2.83 | 1.05–2.43 | 1.4–2.46 | 1.58–2.46 |
| HW | 0.28–0.4 | 0.28–0.39 | 0.2–0.27 | 0.21–0.31 | 0.22–0.3 | 0.2–0.38 | 0.25–0.35 | 0.25–0.35 | 0.28–0.35 |
| ANT I-VI | 3.19–5.29 | 3.02–4.11 | 2.04–4.1 | 1.77–2.94 | 3.17–4.68 | 3.26–4.7 | 3–4.75 | 3.54–4.8 | 3.34–4.76 |
| ANT III | 0.71–1.11 | 0.75–1.01 | 0.56–0.76 | 0.53–0.77 | 0.82–1.03 | 0.77–1.17 | 0.7–1.1 | 0.68–1.16 | 0.83–1.1 |
| ANT IV | 0.47–0.90 | 0.53–0.73 | 0.36–0.6 | 0.32–0.52 | 0.65–0.79 | 0.51–0.9 | 0.45–0.86 | 0.49–0.8 | 0.67–0.8 |
| ANT V | 0.5–0.87 | 0.53–0.7 | 0.41–0.57 | 0.32–0.53 | 0.62–0.92 | 0.51–0.87 | 0.47–0.85 | 0.49–0.85 | 0.66–0.8 |
| ANT VI | 1.06–1.76 | 0.71–1.36 | 1.35–2.11 | 0.44–1.03 | 0.83–1.88 | 0.97–2 | 1.04–1.8 | 1.3–2.1 | 0.97–2.06 |
| BASE | 0.11–0.16 | 0.13–0.18 | 0.11–0.14 | 0.08–0.14 | 0.12–0.17 | 0.12–0.19 | 0.1–0.15 | 0.11–0.17 | 0.14–0.16 |
| PT | 0.94–1.61 | 0.53–1.21 | 1.21–1.99 | 0.35–0.9 | 0.70–1.72 | 0.82–1.84 | 0.93–1.66 | 1.16–1.94 | 0.83–1.92 |
| FEMUR I length | 0.51–0.85 | 0.54–0.72 | 0.31–0.54 | 0.34–0.59 | 0.54–0.79 | 0.53–0.78 | 0.47–0.79 | 0.48–0.87 | 0.62–0.77 |
| FEMUR I width | 0.11–0.18 | 0.1–0.15 | 0.05–0.08 | 0.09–0.15 | 0.1–0.13 | 0.09–0.37 | 0.1–0.15 | 0.1–0.16 | 0.1–0.16 |
| FEMUR III | 0.42–0.76 | 0.43–0.65 | 0.31–0.48 | 0.31–0.54 | 0.49–0.7 | 0.45–0.80 | 0.37–0.66 | 0.41–0.8 | 0.52–0.7 |
| TIBIA III | 0.84–1.54 | 0.96–1.28 | 0.71–0.99 | 0.65–1.1 | 1.1–1.40 | 1–1.5 | 0.9–1.40 | 1–1.69 | 1.2–1.4 |
| HT II | 0.07–0.14 | 0.09–0.14 | 0.08–0.09 | 0.07–0.12 | 0.08–0.12 | 0.1–0.13 | 0.08–0.12 | 0.09–0.12 | 0.09–0.11 |
| URS | 0.09–0.11 | 0.09–0.10 | 0.09–0.1 | 0.07–0.09 | 0.08–0.10 | 0.08–0.1 | 0.08–0.09 | 0.07–0.1 | 0.1–0.12 |
| SIPH | 0.17–0.38 | 0.16–0.26 | 0.12–0.19 | 0.13–0.24 | 0.18–0.28 | 0.15–0.26 | 0.14–0.3 | 0.2–0.39 | 0.21–0.3 |
| CAUDA | 0.08–0.13 | 0.09–0.17 | 0.04–0.08 | 0.09–0.14 | 0.09–0.12 | 0.09–0.18 | 0.07–0.13 | 0.07–0.13 | 0.09–0.18 |
| Character | Drepanaphis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acerifoliae n = 6 | carolinensis n = 8 | choanotricha n = 2 | granovskyi n = 1 | idahoensis n = 1 | kanzensis n = 5 | keshenae n = 2 | monelli n = 5 | nigricans n = 1 | sabrinae n = 2 | simpsoni n = 3 | spicata n = 1 | tissoti n = 1 | utahensis n = 5 | |
| BL | 2.74–3.08 | 1.82–2.81 | 1.77–2 | 2.15 | 2.12 | 2.44–3.32 | 2.22–2.3 | 1.99–2.7 | 2.14 | 2.42–2.44 | 2.78–2.85 | 2.87 | 2.43 | 2.64–3.24 |
| HW | 0.36–0.41 | 0.29–0.38 | 0.28–0.3 | 0.3 | – | 0.31–0.4 | 0.3–0.36 | 0.28–0.38 | 0.31 | 0.35–0.36 | 0.35–0.38 | – | 0.3 | 0.3–0.4 |
| ANT I-VI | 2.65–3.1 | 2.47–2.75 | 3.29–3.41 | 1.92–1.96 | 2.6–2.63 | 2.17–3.61 | 1.61–2.55 | 1.38–1.83 | 3.62–3.67 | 3.24–3.37 | 2.11–.3.3 | 3.44 | 3.87–3.92 | 2.56–3.24 |
| ANT III | 0.59–0.7 | 0.52–0.64 | 0.61–0.68 | 0.41–0.44 | 0.73–0.74 | 0.7–0.84 | 0.49–0.51 | 0.62–0.8 | 0.71–0.72 | 0.74–0.8 | 0.72–0.78 | 0.86–0.87 | 0.91 | 0.52–0.74 |
| ANT IV | 0.42–0.53 | 0.42–0.46 | 0.41–0.45 | 0.32–0.33 | 0.5–0.51 | 0.51–0.62 | 0.36–0.42 | 0.49–0.6 | 0.47–0.5 | 0.51–0.58 | 0.54–0.61 | 0.66–0.68 | 0.57–0.58 | 0.46–0.52 |
| ANT V | 0.46–0.58 | 0.43–0.49 | 0.47–0.49 | 0.35 | 0.58 | 0.56–0.61 | 0.41–0.45 | 0.57–0.64 | 0.6 | 0.55–0.59 | 0.51–0.67 | 0.66–0.7 | 0.6 | 0.5–0.55 |
| ANT VI | 0.88–1.2 | 0.86–1.05 | 1.59–1.72 | 0.66–0.75 | 0.64–0.67 | 1.18–1.52 | 0.89–1.06 | 1.17–1.7 | 1.65–1.74 | 1.2–1.31 | 0.71–1.31 | 1.02 | 1.62–1.66 | 0.9–1.16 |
| BASE | 0.12–0.14 | 0.13–0.15 | 0.12 | 0.11 | 0.12–0.13 | 0.13–0.15 | 0.11–0.12 | 0.13–0.16 | 0.12–0.13 | 0.14–0.16 | 0.12–0.14 | 0.13 | 0.12 | 0.12–0.14 |
| PT | 0.75–1.08 | 0.72–0.91 | 1.47–1.6 | 0.55–0.64 | 0.51–0.55 | 1.04–1.39 | 0.78–0.94 | 1.01–1.55 | 1.53–1.61 | 1.06–1.15 | 0.57–1.19 | 0.89 | 1.5–1.54 | 0.77–1.02 |
| URS | 0.1–0.11 | 0.09–0.1 | 0.09–0.1 | 0.08 | 0.1 | 0.08–0.09 | 0.08–0.12 | 0.11–0.12 | 0.1 | 0.12 | 0.08–0.09 | 0.1 | 0.08 | 0.09–0.1 |
| FEMUR III | 0.59–0.65 | 0.46–0.6 | 0.5–0.54 | 0.42–0.45 | 0.53–0.55 | 0.64–0.72 | 0.51–0.52 | 0.55–0.72 | 0.55–0.59 | 0.58–0.59 | 0.61–0.62 | 0.68–0.73 | 0.56–0.57 | 0.53–0.64 |
| TIBIA III | 1.08–1.26 | 0.91–1.12 | 0.9–0.95 | 0.84 | 1.08–1.09 | 1.21–1.38 | 1–.1.03 | 1.1–1.37 | 1.1 | 1.12–1.15 | 1.12–1.16 | 1.39–1.41 | 1.02 | 1.03–1.23 |
| HT II | 0.12–0.13 | 0.1–0.13 | 0.09–0.1 | 0.09 | 0.09–0.1 | 0.1–0.13 | 0.11–0.12 | 0.11–0.12 | 0.1 | 0.11–0.12 | 0.11–0.12 | 0.11–0.12 | 0.09–0.1 | 0.11–0.12 |
| SIPH | 0.2–0.25 | 0.13–0.19 | 0.21–0.24 | 0.17–0.2 | 0.17–0.19 | 0.17–0.19 | 0.14–0.17 | 0.2–0.27 | 0.18–0.19 | 0.2–0.24 | 0.15–0.18 | 0.23–0.27 | 0.18–0.21 | 0.14–0.21 |
| Character | Drepanaphis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acerifoliae n = 3 | carolinensis n = 3 | choanotricha n = 1 | granovskyi n = 1 | kanzensis n = 3 | keshenae n = 4 | knowltoni n = 2 | monelli n = 4 | parva n = 1 | simpsoni n = 3 | spicata n = 1 | utahensis n = 4 | |
| BL | 2.09–2.21 | 1.75–2.26 | 1.44 | 1.82 | 1.85–2.44 | 1.67–2.11 | 2.01–2.1 | 1.96–2.16 | 2.48 | 1.62–2.19 | 2.82 | 1.7–2.47 |
| HW | 0.33–0.38 | 0.33–0.37 | 0.24 | 0.28 | 0.33–0.37 | 0.33–0.34 | 0.28–0.34 | 0.3–0.35 | – | 0.28–0.36 | 0.38 | 0.27–0.35 |
| ANT I-VI | 2.33–3.5 | 2.82–3.51 | 3.37–3.4 | 1.74–1.75 | 3.04–3.89 | 3.37–3.81 | 3.45–3.97 | 3.73–4.28 | 4.54–4.79 | 2.39–3.04 | 4.97–5.48 | 2.74–3.2 |
| ANT III | 0.72–0.94 | 0.73–1 | 0.69–0.71 | 0.71–0.73 | 0.86–1.05 | 0.77–1.05 | 0.92–1.0 | 0.96–1.02 | 1.12–1.1 | 0.66–0.87 | 1.27–1.29 | 0.75–0.82 |
| ANT IV | 0.59–0.64 | 0.5–0.67 | 0.43–0.44 | 0.41–0.42 | 0.53–0.71 | 0.53–0.64 | 0.6–0.64 | 0.66–0.78 | 0.79–0.81 | 0.47–0.63 | 0.98–1.0 | 0.46–0.62 |
| ANT V | 0.55–0.65 | 0.42–0.61 | 0.41–0.43 | 0.37–0.38 | 0.48–0.66 | 0.51–0.6 | 0.63–0.65 | 0.63–0.75 | 0.8–0.82 | 0.37–0.49 | 0.94 | 0.47–0.58 |
| ANT VI | 1.21 | 1.01–1.08 | 1.67–1.73 | – | 0.96–1.73 | 1.28–1.49 | 1.03–1.64 | 1.3–1.68 | 1.65–1.88 | 0.73–0.9 | 1.57–2.04 | 0.9–1.14 |
| BASE | 0.13–0.14 | 0.12–0.15 | 0.12 | 0.1–0.11 | 0.12–0.13 | 0.11–0.13 | 0.13–0.14 | 0.13–0.16 | 0.13 | 0.11–0.14 | 0.15 | 0.12–0.14 |
| PT | 1.08 | 0.88–0.95 | 1.55–1.61 | – | 0.83–1.61 | 1.17–1.36 | 0.89–1.51 | 1.15–1.55 | 1.52–1.75 | 0.62–0.76 | 1.42–1.89 | 0.78–1.0 |
| URS | 0.09 | 0.08–0.09 | 0.09 | 0.08 | 0.08 | 0.09–0.13 | 0.09–0.1 | 0.11–0.12 | 0.09 | 0.08–0.09 | 0.11 | 0.09 |
| FEMUR III | 0.55–0.63 | 0.46–0.63 | 0.41–0.44 | – | 0.56–0.7 | 0.49–0.65 | 0.58–0.65 | 0.63–0.67 | 0.73–0.74 | 0.43–0.59 | 0.82 | 0.48–0.64 |
| TIBIA III | 1.14–1.28 | 1.01–1.2 | 0.83–0.85 | – | 1.1–1.35 | 1.01–1.32 | 1.24–1.37 | 1.23–1.41 | 1.52 | 0.81–1.09 | 1.75 | 1.06–1.27 |
| HT II | 0.09–0.12 | 0.1–0.12 | 0.09 | – | 0.1–0.12 | 0.1–0.11 | 0.1–0.11 | 0.1–0.12 | 0.12 | 0.1–0.11 | 0.12 | 0.1–0.12 |
| SIPH | 0.23–0.27 | 0.16–0.23 | 0.17 | 0.16–0.17 | 0.19–0.23 | 0.17–0.28 | 0.25–0.26 | 0.25–0.28 | 0.19–0.23 | 0.15–0.16 | 0.39–0.4 | 0.17–0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, K.; Bugaj-Nawrocka, A.; Wieczorek, K. Taxonomic Revision of the Nearctic Genus Drepanaphis Del Guercio (Hemiptera, Aphididae: Drepanosiphinae). Insects 2024, 15, 553. https://doi.org/10.3390/insects15070553
Malik K, Bugaj-Nawrocka A, Wieczorek K. Taxonomic Revision of the Nearctic Genus Drepanaphis Del Guercio (Hemiptera, Aphididae: Drepanosiphinae). Insects. 2024; 15(7):553. https://doi.org/10.3390/insects15070553
Chicago/Turabian StyleMalik, Kamila, Agnieszka Bugaj-Nawrocka, and Karina Wieczorek. 2024. "Taxonomic Revision of the Nearctic Genus Drepanaphis Del Guercio (Hemiptera, Aphididae: Drepanosiphinae)" Insects 15, no. 7: 553. https://doi.org/10.3390/insects15070553





