Cervicovaginal Microbiome: Physiology, Age-Related Changes, and Protective Role Against Human Papillomavirus Infection
Abstract
:1. Introduction
2. Material and Methods
- What is the composition of a healthy cervicovaginal microbiome?
- What are the age-related changes in the cervicovaginal microbiome?
- Which microbial species and compositions significantly modify the HPV acquisition, persistence, clearance, and development of pre-cancerous/cancerous cervical lesions?
- How does the cervicovaginal microbiome impact immunity against HPV?
3. Physiology of Cervicovaginal Microbiome
3.1. Microbiome Profile Within the Female Reproductive System
3.2. Healthy Vaginal and Cervical Microbiome
4. Sex Hormone Levels and Cervicovaginal Microbiota
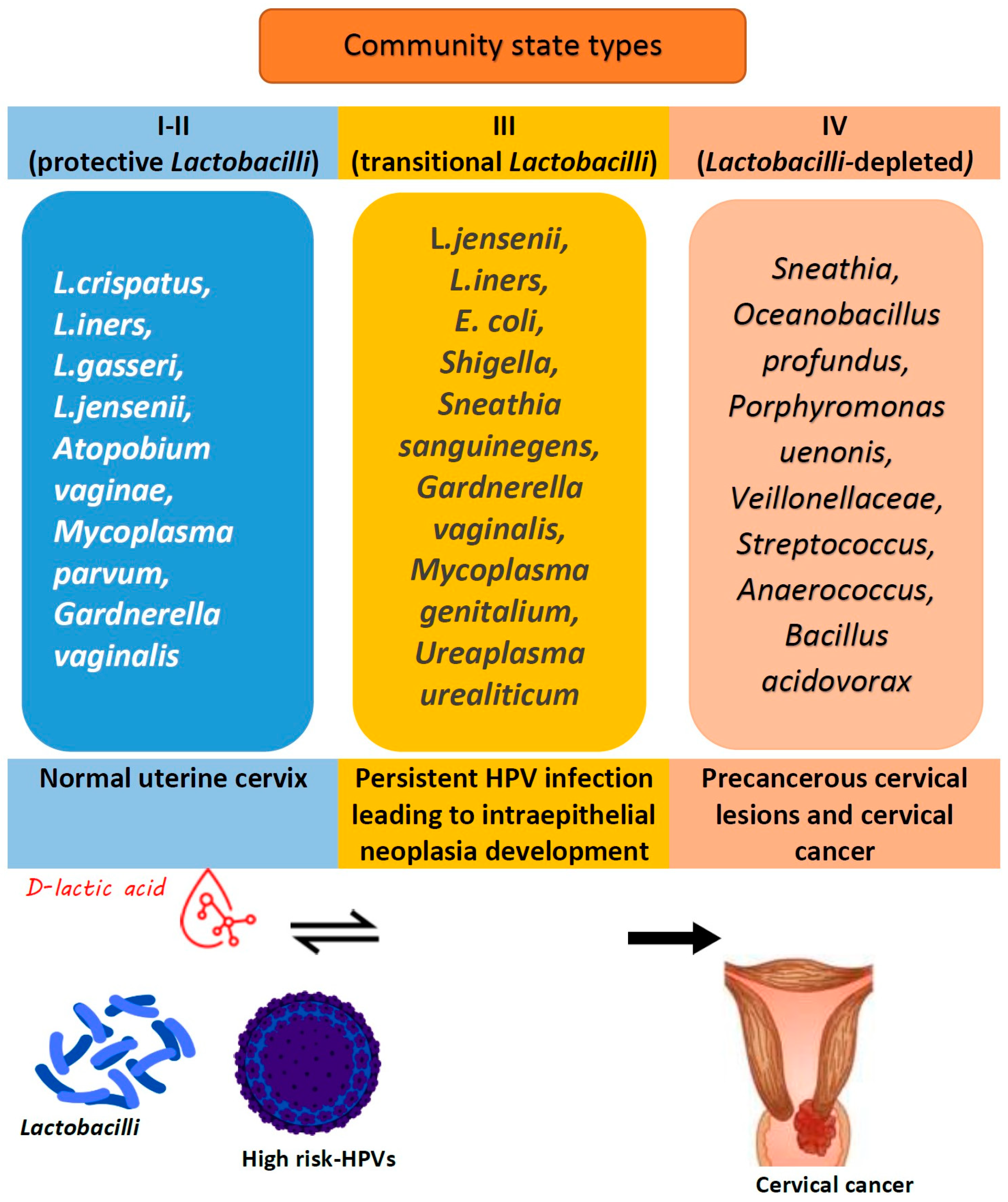
5. Role of the Local Cervicovaginal Microbiome in HPV Infection and Its Persistence
5.1. Human Papillomavirus: Epidemiology, Persistence, and Cancerogenic Properties
5.2. Lactobacillus Species as a Defense Mechanism Against HPV
5.2.1. Lactobacillus iners and Cervicovaginal Microbiome
5.2.2. Lactobacillus gasseri and Cervicovaginal Microbiome
5.2.3. Lactobacillus crispatus and Its Role in Cervicovaginal Microbiome Modulation
5.2.4. Lactobacillus jensenii and Its Role in Cervicovaginal Microbiome
5.2.5. Mechanisms Underlying Defense by Lactobacilli spp.
6. Local Cervicovaginal Immunity
6.1. Mechanisms and Factors Involved in Immune Response
6.2. Local Immune Response to HPV Infection
7. Strengths and Limitations
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Usyk, M.; Zolnik, C.P.; Castle, P.E.; Porras, C.; Herrero, R.; Gradissimo, A.; Gonzalez, P.; Safaeian, M.; Schiffman, M.; Burk, R.D.; et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 2020, 16, e1008376. [Google Scholar] [CrossRef] [PubMed]
- Imankulova, B.; Babi, A.; Issa, T.; Zhumakanova, Z.; Knaub, L.; Yerzhankyzy, A.; Aimagambetova, G. Prevalence of Precancerous Cervical Lesions among Nonvaccinated Kazakhstani Women: The National Tertiary Care Hospital Screening Data (2018). Healthcare 2023, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Aimagambetova, G.; Bapayeva, G.; Ukybassova, T.; Kamzayeva, N.; Sakhipova, G.; Shanazarov, N.; Terzic, M. Risks of Cervical Cancer Recurrence After Fertility-Sparing Surgery and the Role of Human Papillomavirus Infection Types. J. Clin. Med. 2024, 13, 6318. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Azizan, A. Human Papillomavirus Vaccination: Past, Present and Future. Vaccines 2022, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Babi, A.; Issa, T.; Gusmanov, A.; Akilzhanova, A.; Issanov, A.; Makhmetova, N.; Marat, A.; Iztleuov, Y.; Aimagambetova, G. Prevalence of high-risk human papillomavirus infection and genotype distribution among Kazakhstani women with abnormal cervical cytology. Ann. Med. 2024, 56, 2304649. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination-Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Sun, T.; Zhu, L. Cervicovaginal microbiome, high-risk HPV infection and cervical cancer: Mechanisms and therapeutic potential. Microbiol. Res. 2024, 287, 127857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Li, D.; Huang, R.; Deng, X.; Li, M.; Du, F.; Zhao, Y.; Shen, J.; Chen, Y.; et al. HPV-associated cervicovaginal microbiome and host metabolome characteristics. BMC Microbiol. 2024, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Wang, X.; Li, T.; Li, H.; Li, G.; Tan, L.; Chen, Y. Deciphering the role of female reproductive tract microbiome in reproductive health: A review. Front. Cell Infect. Microbiol. 2024, 14, 1351540. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Zhu, N.; Yang, X.; Liu, Q.; Chen, Y.; Wang, X.; Li, H.; Gao, H. “Iron triangle” of regulating the uterine microecology: Endometrial microbiota, immunity and endometrium. Front. Immunol. 2022, 13, 928475. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, L.; Yi, K.; Zhang, B.; Li, C.; Zhou, X. The effects of microbiota on reproductive health: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 1486–1507. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Ma, X.; Du, L.; Jia, Z.; Cui, X.; Yu, L.; Yang, J.; Xiao, L.; Zhang, B.; et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat. Commun. 2021, 12, 4191. [Google Scholar] [CrossRef] [PubMed]
- Recine, N.; Palma, E.; Domenici, L.; Giorgini, M.; Imperiale, L.; Sassu, C.; Musella, A.; Marchetti, C.; Muzii, L.; Panici, P.B. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case-control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obstet. 2016, 293, 101–107. [Google Scholar] [CrossRef]
- Cohen, C.R.; Wierzbicki, M.R.; French, A.L.; Morris, S.; Newmann, S.; Reno, H.; Green, L.; Miller, S.; Powell, J.; Parks, T.; et al. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl. J. Med. 2020, 382, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. Effect of live Lactobacillus capsules andinterferon a-2b suppository combined with LEEP on cervical in-traepithelial neoplasia with high risk human papilloma virus. J. Chin. J. Microecol. 2017, 29, 587–590. [Google Scholar] [CrossRef]
- Iwami, N.; Kawamata, M.; Ozawa, N.; Yamamoto, T.; Watanabe, E.; Mizuuchi, M.; Moriwaka, O.; Kamiya, H. Therapeutic intervention based on gene sequencing analysis of microbial 16S ribosomal RNA of the intrauterine microbiome improves pregnancy outcomes in IVF patients: A prospective cohort study. J. Assist. Reprod. Genet. 2023, 40, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Łaniewski, P.; Herbst-Kralovetz, M.M. Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix. NPJ Biofilms Microbiomes 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2010, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Integrative, H.M.P. (iHMP) research network consortium. The integrative human microbiome project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The role of viral infection on vaginal microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef]
- Kroon, S.J.; Ravel, J.; Huston, W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil. Steril. 2018, 110, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and their microbes: The unexpected friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef] [PubMed]
- France, M.T.; Mendes-Soares, H.; Forney, L.J. Genomic Comparisons of Lactobacillus crispatus and Lactobacillus iners Reveal Potential Ecological Drivers of Community Composition in the Vagina. Appl. Environ. Microbiol. 2016, 82, 7063–7073. [Google Scholar] [CrossRef]
- Doyle, R.; Gondwe, A.; Fan, Y.-M.; Maleta, K.; Ashorn, P.; Klein, N.; Harris, K. A lactobacillus-deficient vaginal microbiota dominates postpartum women in rural Malawi. Appl. Environ. Microbiol. 2018, 84, e02150-17. [Google Scholar] [CrossRef]
- Brennan, C.; Chan, K.; Kumar, T.; Maissy, E.; Brubaker, L.; Dothard, M.I.; Gilbert, J.A.; Gilbert, K.E.; Lewis, A.L.; Thackray, V.G.; et al. Harnessing the power within: Engineering the microbiome for enhanced gynecologic health. Reprod. Fertil. 2024, 5, e230060. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Theis, K.R.; Gomez-Lopez, N.; Winters, A.D.; Panzer, J.J.; Lin, H.; Galaz, J.; Greenberg, J.M.; Shaffer, Z.; Kracht, D.J.; et al. The Vaginal Microbiota of Pregnant Women Varies with Gestational Age, Maternal Age, and Parity. Microbiol. Spectr. 2023, 11, e0342922. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Campisciano, G.; Zanotta, N.; Ricci, G.; Comar, M. The vaginal community state types microbiome-immune network as key factor for bacterial vaginosis and aerobic vaginitis. Front. Microbiol. 2019, 10, 2451. [Google Scholar] [CrossRef]
- Bommana, S.; Hu, Y.-J.; Kama, M.; Wang, R.; Kodimerla, R.; Jijakli, K.; Read, T.D.; Dean, D. Unique microbial diversity, community composition, and networks among Pacific Islander endocervical and vaginal microbiomes with and without Chlamydia trachomatis infection in Fiji. mBio 2024, 15, e0306323. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Cardile, V.; Petronio Petronio, G.; Furneri, P.M. Biological properties and production of bacteriocins-like-inhibitory substances by Lactobacillus sp. strains from human vagina. J. Appl. Microbiol. 2019, 126, 1541–1550. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Manhanzva, M.T.; Abrahams, A.G.; Gamieldien, H.; Froissart, R.; Jaspan, H.; Jaumdally, S.Z.; Barnabas, S.L.; Dabee, S.; Bekker, L.G.; Gray, G.; et al. Inflammatory and antimicrobial properties differ between vaginal Lactobacillus isolates from South African women with non-optimal versus optimal microbiota. Sci. Rep. 2020, 10, 6196. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.-L.; Mbulawa, Z.Z.A.; Coetzee, D.; Meiring, T.L. The cervical microbiota in reproductive-age South African women with and without human papillomavirus infection. Papillomavirus Res. 2019, 7, 154–163. [Google Scholar] [CrossRef]
- Onywera, H.; Williamson, A.; Mbulawa, Z.Z.; Coetzee, D.; Meiring, T.L. Factors associated with the composition and diversity of the cervical microbiota of reproductive-age Black South African women: A retrospective cross-sectional study. PeerJ 2019, 7, e7488. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Melnikov, V.G.; Kobayashi, R.; Komine-Aizawa, S.; Tsuji, N.M.; Hayakawa, S. Female reproductive tract-organ axes. Front. Immunol. 2023, 14, 1110001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keburiya, L.K.; Smolnikova, V.Y.; Priputnevich, T.V.; Muravieva, V.V.; Gordeev, A.B.; Trofimov, D.Y.; Shubina, E.S.; Kochetkova, T.O.; Rogacheva, M.S.; Kalinina, E.A.; et al. Does the uterine microbiota affect the reproductive outcomes in women with recurrent implantation failures? BMC Womens Health 2022, 22, 168. [Google Scholar] [CrossRef]
- Dong, M.; Dong, Y.; Bai, J.; Li, H.; Ma, X.; Li, B.; Wang, C.; Li, H.; Qi, W.; Wang, Y.; et al. Interactions between microbiota and cervical epithelial, immune, and mucus barrier. Front. Cell. Infect. Microbiol. 2023, 13, 1124591. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Witkin, S.S.; Linhares, I.M. Why do lactobacilli dominate the human vaginal microbiota? BJOG Int. J. Obstet. Gynaecol. 2017, 124, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Wu, F.; Chen, J.; Luo, J.; Wu, C.; Chen, T. Effectiveness of vaginal probiotics Lactobacillus crispatus chen-01 in women with high-risk HPV infection: A prospective controlled pilot study. Aging 2024, 16, 11446–11459. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Mu, B.; Li, R.; Liu, J.; Liang, X.; Su, M. Exploring the vaginal microbiome’s role in HPV infection dynamics: A Prospective Cohort study. Res. Sq. 2024. preprints. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vitro activity of Lactobacillus species against Gardnerella vaginalis. J. Infect. Dis. 2011, 204, 857–863. [Google Scholar] [CrossRef]
- Pendharkar, S.; Skafte-Holm, A.; Simsek, G.; Haahr, T. Lactobacilli and Their Probiotic Effects in the Vagina of Reproductive Age Women. Microorganisms 2023, 11, 636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Marangoni, A.; Laghi, L.; Foschi, C.; Ñahui Palomino, R.A.; Calonghi, N.; Cevenini, R.; Vitali, B. Isolation of Vaginal Lactobacilli and Characterization of Anti-Candida Activity. PLoS ONE 2015, 10, e0131220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiologyopen 2021, 10, e1173. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Lohrasbi, V.; Abdi, M.; Mirkalantari, S.; Esghaei, M.; Kashanian, M.; Oshaghi, M.; Talebi, M. The probiotic properties and potential of vaginal Lactobacillus spp. isolated from healthy women against some vaginal pathogens. Lett. Appl. Microbiol. 2022, 74, 752–764. [Google Scholar] [CrossRef]
- Zárate, G.; Nader-Macias, M.E. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol. 2006, 43, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Ribet, D.; Cossart, P. Pathogen adhesion as a critical step in infection. Nat. Rev. Microbiol. 2015, 13, 310–321. [Google Scholar] [CrossRef]
- Li, J.; Jiang, L.; Wang, C.; Meng, J.; Wang, H.; Jin, H. Investigation of the relationship between the changes in vaginal microecological enzymes and human papillomavirus (HPV) infection. Medicine 2024, 103, e37068. [Google Scholar] [CrossRef]
- Miko, E.; Barakonyi, A. The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants 2023, 12, 1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Thakur, R.; Shen, Q.; He, Y.; Chen, C. Influences of vaginal microbiota on human papillomavirus infection and host immune regulation: What we have learned? Decod. Infect. Transm. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Q.; Zhang, L.; Ma, L.; Wang, D.; Yang, Y.; Jia, P.; Wu, Y.; Wang, F. Human papillomavirus and cervical cancer in the microbial world: Exploring the vaginal microecology. Front. Cell Infect Microbiol. 2024, 14, 1325500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarke, M.A.; Rodriguez, A.C.; Gage, J.C.; Herrero, R.; Hildesheim, A.; Wacholder, S.; Burk, R.; Schiffman, M. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect Dis. 2012, 12, 33. [Google Scholar] [CrossRef]
- Norenhag, J.; Blomberg, M.; Ahrlund-Richter, A. Impact of vaginal pH on HPV infection and disease progression: A systematic review. J. Clin. Virol. 2020, 128, 104431. [Google Scholar] [CrossRef]
- Vitali, D.; Wessels, J.M.; Kaushic, C. Role of sex hormones and the vaginal microbiome in susceptibility and mucosal immunity to HIV-1 in the female genital tract. AIDS Res. Ther. 2017, 14, 39. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, W.; Yuan, Y.; Zhu, W.; Shang, A. Vaginal microecological characteristics of women in different physiological and pathological period. Front. Cell. Infect. Microbiol. 2022, 12, 959793. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Latkowska, M.; Valencia, A.M.; Allana, A.; Soto, J.; Cai, C.L.; Golombek, S.; Hand, I.; Aranda, J.V. Factors Influencing Neonatal Gut Microbiome and Health with a Focus on Necrotizing Enterocolitis. Microorganisms 2023, 11, 2528. [Google Scholar] [CrossRef] [PubMed]
- Dombrowska-Pali, A.; Wiktorczyk-Kapischke, N.; Chrustek, A.; Olszewska-Słonina, D.; Gospodarek-Komkowska, E.; Socha, M.W. Human Milk Microbiome—A Review of Scientific Reports. Nutrients 2024, 16, 1420. [Google Scholar] [CrossRef] [PubMed]
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 2024, 14, 1296447. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.; Aldous, A.; Daniels, J.; Joy, C.; Capozzi, E.; Yang, M.; Moriarty, P.; Emmanuel-Baker, V.; Malcolm, S.; Green, S.J.; et al. Effect of progestin-based contraceptives on HIV-associated vaginal immune biomarkers and microbiome in adolescent girls. PLoS ONE 2024, 19, e0306237. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Brabin, L.; Diallo, S.; Gies, S.; Nelson, A.; Stewart, C.; Swinkels, D.W.; Geurts-Moespot, A.J.; Kazienga, A.; Ouedraogo, S.; et al. Mucosal lactoferrin response to genital tract infections is associated with iron and nutritional biomarkers in young Burkinabé women. Eur. J. Clin. Nutr. 2019, 73, 1464–1472. [Google Scholar] [CrossRef]
- Kaur, H.; Merchant, M.; Haque, M.M.; Mande, S.S. Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological lifecycle. Front. Microbiol. 2020, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Bardos, J.; Fiorentino, D.; Longman, R.E.; Paidas, M. Immunological Role of the Maternal Uterine Microbiome in Pregnancy: Pregnancies Pathologies and Alterated Microbiota. Front. Immunol. 2020, 10, 2823. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Ortashi, O.; Al Khodor, S. Role of the vaginal microbiome in miscarriage: Exploring the relationship. Front. Cell. Infect. Microbiol. 2023, 13, 1232825. [Google Scholar] [CrossRef]
- Oliver, A.; LaMere, B.; Weihe, C.; Wandro, S.; Lindsay, K.L.; Wadhwa, P.D.; Mills, D.A.; Pride, D.T.; Fiehn, O.; Northen, T.; et al. Cervicovaginal Microbiome Composition Is Associated with Metabolic Profiles in Healthy Pregnancy. mBio 2020, 11, e01851-20. [Google Scholar] [CrossRef]
- Jordan, M.M.; Amabebe, E.; Khanipov, K.; Taylor, B.D. Scoping Review of Microbiota Dysbiosis and Risk of Preeclampsia. Am. J. Reprod. Immunol. 2024, 92, e70003. [Google Scholar] [CrossRef]
- Shardell, M.; Gravitt, P.E.; Burke, A.E.; Ravel, J.; Brotman, R.M. Association of Vaginal Microbiota With Signs and Symptoms of the Genitourinary Syndrome of Menopause Across Reproductive Stages. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Gliniewicz, K.; Schneider, G.M.; Ridenhour, B.J.; Williams, C.J.; Song, Y.; Farage, M.A.; Miller, K.; Forney, L.J. Comparison of the Vaginal Microbiomes of Premenopausal and Postmenopausal Women. Front. Microbiol. 2019, 10, 193. [Google Scholar] [CrossRef]
- de Oliveira, N.S.; de Lima, A.B.F.; de Brito, J.C.R.; Sarmento, A.C.A.; Gonçalves, A.K.S.; Eleutério, J., Jr. Postmenopausal Vaginal Microbiome and Microbiota. Front. Reprod. Health 2022, 3, 780931. [Google Scholar] [CrossRef]
- Głowienka-Stodolak, M.; Bagińska-Drabiuk, K.; Szubert, S.; Hennig, E.E.; Horala, A.; Dąbrowska, M.; Micek, M.; Ciebiera, M.; Zeber-Lubecka, N. Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota—Evidence from Next-Generation Sequencing Studies. Cancers 2024, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Jin, B.; Zhang, Y.; Huang, Y.; Luo, Z.; Su, C.; Li, J.; Ma, L.; Zhou, J. Vaginal microbiota, menopause, and the use of menopausal hormone therapy: A cross-sectional, pilot study in Chinese women. Menopause 2024, 31, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Gutierrez, P.; Garza, J.; Arispe, R.; Galloway, M.; Ventolini, G. Lactobacillus species and inflammatory cytokine profile in the vaginal milieu of pre-menopausal and post-menopausal women. GREM—Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 180–187. [Google Scholar] [CrossRef]
- Yoshikata, R.; Yamaguchi, M.; Mase, Y.; Tatsuzuki, A.; Myint, K.Z.Y.; Ohta, H. Age-Related changes, influencing factors, and crosstalk between vaginal and gut microbiota: A Cross-Sectional Comparative Study of pre- and postmenopausal women. J. Women’s Health 2022, 31, 1763–1772. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203, Erratum in: Lancet Glob Health. 2022, 10, e41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wu, Q.; Hao, Y.; Hu, J.; Gao, Y.; Zhou, S.; Han, L. Global, regional, and national burden of cervical cancer for 195 countries and territories, 2007–2017: Findings from the Global Burden of Disease Study 2017. BMC Women’s Health 2021, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Mitra, A.; Moscicki, A. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef]
- Zhou, Z.; Long, H.; Cheng, Y.; Luo, H.; Wen, D.; Gao, L. From microbiome to inflammation: The key drivers of cervical cancer. Front. Microbiol. 2021, 12, 767931. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Aswathy, S.; Reshma, J.; Avani, D. Epidemiology of cervical cancer with special focus on India. Int. J. Women’s Health 2015, 7, 405–414. [Google Scholar] [CrossRef]
- Caruso, S.; Bruno, M.T.; Cianci, S.; Di Pasqua, S.; Minona, P.; Cianci, A. Sexual behavior of women with diagnosed HPV. J. Sex Marital. Ther. 2019, 45, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.K.; Alvarez, R.D.; Huh, W.K. Human papillomavirus: What every provider should know. Am. J. Obstet. Gynecol. 2014, 208, 169–175. [Google Scholar] [CrossRef]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: A pilot study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Rapisarda, A.M.C.; Vitale, S.G.; Cianci, S.; Caggia, C.; Randazzo, C.L.; Cianci, A. A clinical pilot study on the effect of the probiotic Lacticaseibacillus rhamnosus TOM 22.8 strain in women with vaginal dysbiosis. Sci. Rep. 2021, 11, 2592. [Google Scholar] [CrossRef] [PubMed]
- Frąszczak, K.; Barczyński, B.; Kondracka, A. Does lactobacillus exert a protective effect on the development of cervical and endometrial cancer in women? Cancers 2022, 14, 4909. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Carter, K.A.; Ravel, J.; Brotman, R.M. Lactobacillus iners and Genital Health: Molecular Clues to an Enigmatic Vaginal Species. Curr. Infect. Dis. Rep. 2023, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Beghini, J.; Linhares, I.; Giraldo, P.; Ledger, W.; Witkin, S. Differential expression of lactic acid isomers, extracellular matrix metalloproteinase inducer, and matrix metalloproteinase-8 in vaginal fluid from women with vaginal disorders. BJOG Int. J. Obstet. Gynaecol. 2014, 122, 1580–1585. [Google Scholar] [CrossRef]
- Ojala, T.; Kankainen, M.; Castro, J.; Cerca, N.; Edelman, S.; Westerlund-Wikström, B.; Paulin, L.; Holm, L.; Auvinen, P. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genom. 2014, 15, 1070. [Google Scholar] [CrossRef] [PubMed]
- Leizer, J.; Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; Boester, A.; Witkin, S.S. Properties of Epithelial Cells and Vaginal Secretions in Pregnant Women When Lactobacillus crispatus or Lactobacillus iners Dominate the Vaginal Microbiome. Reprod. Sci. 2017, 25, 854–860. [Google Scholar] [CrossRef]
- Nasioudis, D.; Witkin, S.S. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med. Microbiol. Immunol. 2015, 204, 471–479. [Google Scholar] [CrossRef]
- Abtin, A.; Eckhart, L.; Gläser, R.; Gmeiner, R.; Mildner, M.; Tschachler, E. The antimicrobial heterodimer S100A8/S100A9 (Calprotectin) is upregulated by bacterial flagellin in human epidermal keratinocytes. J. Investig. Dermatol. 2010, 130, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Vaneechoutte, M. Lactobacillus iners, the unusual suspect. Res. Microbiol. 2017, 168, 826–836. [Google Scholar] [CrossRef]
- Kwak, W.; Han, Y.; Seol, D.; Kim, H.; Ahn, H.; Jeong, M.; Kang, J.; Kim, H.; Kim, T.H. Complete Genome of Lactobacillus iners KY Using Flongle Provides Insight Into the Genetic Background of Optimal Adaption to Vaginal Econiche. Front. Microbiol. 2020, 11, 1048. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, I.M.; Ng, Q.J.; Koo, S.; Tan, M.; Wang, J.; Chin, F.H.X.; Qi, M.; Ho, W.Y.; Ng, Z.Y.; Tan, E.C. Lactobacillus iners is the predominant species in the vaginal microbiome of women with high-risk HPV-infection: Experience from a tertiary referral colposcopy centre in Singapore. Int. J. Gynecol. Cancer 2023, A119–A120. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; Van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; Van De Wijgert, J.H.H.M. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2015, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Zhu, J.; Qu, S.; Chen, J.; Zhang, L.; Mu, X. Cervicovaginal microbiota dysbiosis correlates with HPV persistent infection. Microb. Pathog. 2021, 152, 104617. [Google Scholar] [CrossRef]
- Wan, B.; Wei, L.J.; Tan, T.M.; Qin, L.; Wang, H. Inhibitory effect and mechanism of Lactobacillus crispatus on cervical precancerous cells Ect1/E6E7 and screening of early warning factors. Infect. Agents Cancer 2023, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Shirzad, M.; Akrami, S.M.; Mousavi, A.; Mirsalehian, A.; Modarressi, M.H. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J. Med. Microbiol. 2013, 62, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.D.; Xu, D.J.; Wang, B.Y.; Yan, D.H.; Lv, Z.; Su, J.R. Inhibitory Effect of Vaginal Lactobacillus Supernatants on Cervical Cancer Cells. Probiotics Antimicrob Proteins 2018, 10, 236–242. [Google Scholar] [CrossRef]
- Buchta, V. Vaginal microbiome. Ceska Gynekol. 2018, 83, 371–379. (In English) [Google Scholar] [PubMed]
- Nicolò, S. Interplay Among Microbial Communities, Epithelial Cells, and Immune System in Vaginal Mucosa of Women with High-Risk Human Papillomavirus Infection. 2022. Available online: https://hdl.handle.net/2158/1275958 (accessed on 1 September 2024).
- Nicolò, S.; Antonelli, A.; Tanturli, M.; Baccani, I.; Bonaiuto, C.; Castronovo, G.; Rossolini, G.M.; Mattiuz, G.; Torcia, M.G. Bacterial Species from Vaginal Microbiota Differently Affect the Production of the E6 and E7 Oncoproteins and of p53 and p-Rb Oncosuppressors in HPV16-Infected Cells. Int. J. Mol. Sci. 2023, 24, 7173. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Gao, Q.; Fan, T.; Luo, S.; Zheng, J.; Zhang, L.; Cao, L.; Zhang, Z.; Li, L.; Huang, Z.; Zhang, H.; et al. Lactobacillus gasseri LGV03 isolated from the cervico-vagina of HPV-cleared women modulates epithelial innate immune responses and suppresses the growth of HPV-positive human cervical cancer cells. Transl. Oncol. 2023, 35, 101714. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Weng, J.; Gao, Y.; Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect. Dis. 2013, 13, 271. [Google Scholar] [CrossRef]
- Xiao, B.; Niu, X.; Han, N.; Wang, B.; Du, P.; Na, R.; Chen, C.; Liao, Q. Predictive value of the composition of the vaginal microbiota in bacterial vaginosis, a dynamic study to identify recurrence-related flora. Sci. Rep. 2016, 6, 26674. [Google Scholar] [CrossRef] [PubMed]
- Mortaki, D.; Gkegkes, I.D.; Psomiadou, V.; Blontzos, N.; Prodromidou, A.; Lefkopoulos, F.; Nicolaidou, E. Vaginal microbiota and human papillomavirus: A systematic review. J. Turk. -Ger. Gynecol. Assoc. 2019, 21, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cerdeira, C.; Sanchez-Blanco, E.; Alba, A. Evaluation of Association between Vaginal Infections and High-Risk Human Papillomavirus Types in Female Sex Workers in Spain. ISRN Obstet. Gynecol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghaniabadi, R.; Hashemi, S.; Bajgiran, M.S.; Javadi, S.; Mohammadzadeh, N.; Masjedian, F. Distribution of Lactobacillus species in Iranian women with both human papillomavirus (HPV) infection and bacterial vaginosis (BV). Meta Gene 2020, 26, 100791. [Google Scholar] [CrossRef]
- Atassi, F.; Pho Viet Ahn, D.L.; Lievin-Le, M.o.a.l.V. Diverse Expression of Antimicrobial Activities Against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus gasseri and Lactobacillus crispatus. Front. Microbiol. 2019, 10, 2900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Xu, X.; Yu, L.; Shi, X.; Min, M.; Xiong, L.; Pan, J.; Zhang, Y.; Liu, P.; Wu, G.; et al. Vaginal microbiota changes caused by HPV infection in Chinese women. Front. Cell. Infect. Microbiol. 2022, 12, 814668. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zheng, J.; Lin, L.; Cong, Q.; Qiao, L. Perspective on human papillomavirus infection treatment by vaginal microbiota. Deleted J. 2023, 1, e20220020. [Google Scholar] [CrossRef]
- Nunn, K.L.; Wang, Y.; Harit, D.; Humphrys, M.S.; Ma, B.; Cone, R.; Ravel, J.; Lai, S.K. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6, e01084-15. [Google Scholar] [CrossRef] [PubMed]
- Reimers, L.L.; Mehta, S.D.; Massad, L.S.; Burk, R.D.; Xie, X.; Ravel, J.; Cohen, M.H.; Palefsky, J.M.; Weber, K.M.; Xue, X.; et al. The cervicovaginal microbiota and its associations with human papillomavirus detection in HIV-Infected and HIV-Uninfected women. J. Infect. Dis. 2016, 214, 1361–1369. [Google Scholar] [CrossRef]
- Ghanavati, R.; Asadollahi, P.; Shapourabadi, M.B.; Razavi, S.; Talebi, M.; Rohani, M. Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microb. Pathog. 2020, 139, 103829. [Google Scholar] [CrossRef]
- Hyun, C.J. The Research for Association Between Vaginal Microbiome and High-Risk Human Papillomavirus in Young Korean Women. 2024. Available online: https://s-space.snu.ac.kr/handle/10371/210377 (accessed on 10 October 2024).
- Irina, P.; Alena, V.; Arsene, M.M.J.; Milana, D.; Alla, P.; Lyudmila, K.; Boris, E. Comparison of Vaginal microbiota in HPV-negative and HPV-positive pregnant women using a culture-based approach. Diagn Microbiol Infect Dis. 2024, 110, 116419. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, Q.; Wan, Z.; OuYang, Z.; Zhang, Q. Exploring the association between cervical microbiota and HR-HPV infection based on 16S RRNA gene and metagenomic sequencing. Front. Cell. Infect. Microbiol. 2022, 12, 922554. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hao, Y.; Zhang, X.; Yang, Y.; Liu, M.; Wang, N.; Zhang, T.; He, H. Lacticaseibacillus casei LH23 Suppressed HPV Gene Expression and Inhibited Cervical Cancer Cells. Probiotics Antimicrob. Proteins 2021, 15, 443–450. [Google Scholar] [CrossRef]
- Mosleh, I.S.; Karami, F.; Salahshourifar, I.; Ebrahimi, M.T.; Marvibaigi, M. Investigating the effects of Lactobacillus acidophilus and Lactobacillus paracasei supernatant on cervical cancer cells. Deleted J. 2023, 27, 426–434. [Google Scholar] [CrossRef]
- Chao, X.; Sun, T.; Wang, S.; Fan, Q.; Shi, H.; Zhu, L.; Lang, J. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int. J. Gynecol. Cancer 2019, 29, 28–34. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and microbiota crosstalk: Should we pay attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiu, X.; Wang, W.; Li, D.; Wu, A.; Hong, Z.; Di, W.; Qiu, L. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect. Dis. 2020, 20, 629. [Google Scholar] [CrossRef]
- Campisciano, G.; Gheit, T.; De Seta, F.; Cason, C.; Zanotta, N.; Delbue, S.; Ricci, G.; Ferrante, P.; Tommasino, M.; Comar, M. Oncogenic Virome Benefits from the Different Vaginal Microbiome-Immune Axes. Microorganisms 2019, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.S.; Carter, K.A.; Bassis, C.; Young, V.B.; Reed, B.; Harper, D.M.; Iv, M.T.R.; Bell, J.D. The vaginal microbiota, high-risk human papillomavirus infection, and cervical cytology: Results from a population-based study. Gynecol. Pelvic Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, Q.; Chen, Y.; Dong, B.; Xue, H.; Lei, H.; Lu, Y.; Wei, X.; Sun, P. Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: A cross-sectional analysis. Sci. Rep. 2022, 12, 2812. [Google Scholar] [CrossRef]
- Kang, G.; Jung, D.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J. Potential Association between Vaginal Microbiota and Cervical Carcinogenesis in Korean Women: A Cohort Study. Microorganisms 2021, 9, 294. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Wei, X.; Zhu, J.; Wang, X.; Xie, X.; Lu, W. The alterations of vaginal microbiome in HPV16 infection as identified by shotgun metagenomic sequencing. Front. Cell. Infect. Microbiol. 2020, 10, 286. [Google Scholar] [CrossRef]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Farahmandi, F.; Parhizgar, P.; Tape, P.M.K.; Bizhannia, F.; Rohani, F.S.; Bizhanzadeh, M.; Alhosseini, Z.S.M.; Hosseinzade, M.; Farsi, Y.; Nasiri, M.J. Implications and Mechanisms of antiviral effects of lactic acid Bacteria: A Systematic review. Int. J. Microbiol. 2023, 2023, 9298363. [Google Scholar] [CrossRef]
- Lithgow, K.V.; Buchholz, V.C.H.; Ku, E.; Konschuh, S.; D’Aubeterre, A.; Sycuro, L.K. Protease activities of vaginal Porphyromonas species disrupt coagulation and extracellular matrix in the cervicovaginal niche. Npj Biofilms Microbiomes 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Hearps, A.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.; Gugasyan, R.; Anderson, D.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.R.; De Melo, C.M.L.; Barros, M.L.C.M.G.; De Cássia Pereira De Lima, R.; De Freitas, A.C.; Venuti, A. Activities of stromal and immune cells in HPV-related cancers. J. Exp. Clin. Cancer Res. 2018, 37, 137. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Williams, P. Immunological properties of the cervix and its microbiome. J. Infect. Dis. 2022, 226, 1121–1128. [Google Scholar] [CrossRef]
- De Tomasi, J.B.; Opata, M.M.; Mowa, C.N. Immunity in the Cervix: Interphase between Immune and Cervical Epithelial Cells. J. Immunol. Res. 2019, 2019, 7693183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguezgarcia, M. Mucosal Immunity in the Human Female Reproductive Tract. N.p., 2015. Mucosal Immunol. 2015, 2097–2124. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhu, M.; Ge, L.; Liu, X.; Su, K.; Chen, Z.; Zhao, W. The Interplay Between Cervicovaginal Microbial Dysbiosis and Cervicovaginal Immunity. Front Immunol. 2022, 13, 857299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitchell, C.; Marrazzo, J. Bacterial vaginosis and the cervicovaginal immune response. Am. J. Reprod Immunol. 2014, 71, 555–563. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Don, K.R.; Padma, K.R.; Chandana, B.V.S.; Anjum, M.R.; Mohan, S. Influence of Vaginal Microbiota on Sexual and Reproductive Health: A Mini Review. Entomol. Appl. Sci. Lett. 2023, 10, 11–28. [Google Scholar] [CrossRef]
- Villa, P.; Cipolla, C.; D’Ippolito, S.; Amar, I.D.; Shachor, M.; Ingravalle, F.; Scaldaferri, F.; Puca, P.; Di Simone, N.; Scambia, G. The interplay between immune system and microbiota in gynecological diseases: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5676–5690. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Caselli, E.; D’Accolti, M.; Santi, E.; Soffritti, I.; Conzadori, S.; Mazzacane, S.; Greco, P.; Contini, C.; Bonaccorsi, G. Vaginal Microbiota and Cytokine Microenvironment in HPV Clearance/Persistence in Women Surgically Treated for Cervical Intraepithelial Neoplasia: An Observational Prospective Study. Front. Cell. Infect. Microbiol. 2020, 10, 540900. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Leclaire, S.; Bandekar, M.; Rowe, M.; Ritari, J.; Jokiniemi, A.; Partanen, J.; Allinen, P.; Kuusipalo, L.; Kekäläinen, J. Female reproductive tract microbiota varies with MHC profile. Proc. Biol. Sci. 2024, 291, 20241334. [Google Scholar] [CrossRef]
- Taddei, C.R.; Cortez, R.V.; Mattar, R.; Torloni, M.R.; Daher, S. Microbiome in normal and pathological pregnancies: A literature overview. Am. J. Reprod. Immunol. 2018, 80, e12993. [Google Scholar] [CrossRef] [PubMed]
- Mall, A.S.; Habte, H.; Mthembu, Y.; Peacocke, J.; De Beer, C. Mucus and Mucins: Do they have a role in the inhibition of the human immunodeficiency virus? Virol. J. 2017, 14, 192. [Google Scholar] [CrossRef]
- Taherali, F.; Varum, F.; Basit, A.W. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2017, 124, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The interplay between reproductive tract microbiota and immunological system in human reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Doerflinger, S.Y.; Throop, A.L.; Herbst-Kralovetz, M.M. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a Species-Specific manner. J. Infect. Dis. 2014, 209, 1989–1999. [Google Scholar] [CrossRef]
- Pandit, H.; Gopal, S.; Sonawani, A.; Yadav, A.K.; Qaseem, A.S.; Warke, H.; Patil, A.; Gajbhiye, R.; Kulkarni, V.; Al-Mozaini, M.A.; et al. Surfactant Protein D Inhibits HIV-1 Infection of Target Cells via Interference with gp120-CD4 Interaction and Modulates Pro-Inflammatory Cytokine Production. PLoS ONE 2014, 9, e102395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kannan, A.; Nunn, K.L.; Murphy, M.A.; Subramani, D.B.; Moench, T.; Cone, R.; Lai, S.K. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol. 2014, 7, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-kB pathway. Biomed. Pharmacother. 2020, 123, 109790. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K.; Bodily, J.M. Human papillomavirus 16 E5 inhibits interferon signaling and supports episomal viral maintenance. J. Virol. 2020, 94, e01582-19. [Google Scholar] [CrossRef]
- De Matos, L.G.; Cândido, E.B.; Vidigal, P.V.; Bordoni, P.H.; Lamaita, R.M.; Carneiro, M.M.; Da Silva-Filho, A.L. Association between Toll-like Receptor and Tumor Necrosis Factor Immunological Pathways in Uterine Cervical Neoplasms. Tumori J. 2016, 103, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.; Li, C. The role of TLRs in cervical cancer with HPV infection: A review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dai, T.; He, X.; Zhang, Z.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The interactions between cGAS-STING pathway and pathogens. Signal Transduct. Target. Ther. 2020, 5, 91. [Google Scholar] [CrossRef]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.a.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and cervical cancer. Pathobiology 2020, 88, 187–197. [Google Scholar] [CrossRef]
- Langers, I.; Renoux, V.; Reschner, A.; Touzé, A.; Coursaget, P.; Boniver, J.; Koch, J.; Delvenne, P.; Jacobs, N. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur. J. Immunol. 2014, 44, 3585–3595. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Duluc, D.; Banchereau, R.; Gannevat, J.; Thompson-Snipes, L.; Blanck, J.; Zurawski, S.; Zurawski, G.; Hong, S.; Rossello-Urgell, J.; Pascual, V.; et al. Transcriptional fingerprints of antigen-presenting cell subsets in the human vaginal mucosa and skin reflect tissue-specific immune microenvironments. Genome Med. 2014, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Shannon, B.; Yi, T.; Perusini, S.; Gajer, P.; Ma, B.; Humphrys, M.; Thomas-Pavanel, J.; Chieza, L.; Janakiram, P.; Saunders, M.; et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017, 10, 1310–1319. [Google Scholar] [CrossRef]
- Xue, J.; Wang, Y.; Chen, C.; Zhu, X.; Zhu, H.; Hu, Y. Effects of Th17 cells and IL-17 in the progression of cervical carcinogenesis with high-risk human papillomavirus infection. Cancer Med. 2017, 7, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, C.; Mattarollo, S.R.; Bridge, J.A.; Frazer, I.H.; Blumenthal, A. IL-17 suppresses immune effector functions in human Papillomavirus-Associated epithelial hyperplasia. J. Immunol. 2014, 193, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Passmore, J.S.; Williamson, A. Host Immune Responses Associated with Clearance or Persistence of Human Papillomavirus Infections. Curr. Obstet. Gynecol. Rep. 2016, 5, 177–188. [Google Scholar] [CrossRef]
- Thurman, A.R.; Kimble, T.; Herold, B.; Mesquita, P.M.; Fichorova, R.N.; Dawood, H.Y.; Fashemi, T.; Chandra, N.; Rabe, L.; Cunningham, T.D.; et al. Bacterial vaginosis and subclinical markers of genital tract inflammation and mucosal immunity. AIDS Res. Hum. Retroviruses 2015, 31, 1139–1152. [Google Scholar] [CrossRef]
- Leo, P.J.; Madeleine, M.M.; Wang, S.; Schwartz, S.M.; Newell, F.; Pettersson-Kymmer, U.; Hemminki, K.; Hallmans, G.; Tiews, S.; Steinberg, W.; et al. Correction: Defining the genetic susceptibility to cervical neoplasia—A genome-wide association study. PLoS Genet. 2018, 14, e1007257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef] [PubMed]

| Age Group | Predominant Microbial Genera | Description of Changes | References |
|---|---|---|---|
| Infancy and childhood | Staphylococcus, Streptococcus, Enterobacteriaceae, Corynebacterium | Leads to the dominance of a wide range of aerobes and facultative anaerobes. | [66,67,68,69,70,71] |
| Adolescent | Transition towards Lactobacillus spp. dominance, including L. crispatus, L. iners; presence of Streptococcus, Staphylococcus, Corynebacterium | The vaginal pH of a young girl changes from birth until pre-puberty to become neutral or slightly alkaline, followed by a low abundance of lactobacilli. | [66,68,72] |
| Reproductive period | Predominance of Lactobacillus spp. (L. crispatus, L. iners, L. gasseri, L. jensenii); presence of Gardnerella, Prevotella, Atopobium | Depending on the phases of the menstrual cycle, the overall increase in estrogen level leads to the dominance of Lactobacilli spp. and lower vaginal pH. | [73,74,75,76] |
| Pregnancy | Increased abundance of Lactobacillus spp., Bifidobacteriaceae, particularly L. crispatus; reduced microbial diversity | Both estrogen and progesterone contribute to the increased dominance of lactobacilli during pregnancy, which stimulates glycogen accumulation in the vaginal epithelial cells favoring Lactobacilli spp. colonization. | [77,78,79,80] |
| Menopause | Decreased Lactobacillus spp.; increased prevalence of Gardnerella, Atopobium, Prevotella, Mobiluncus, Streptococcus, Staphylococcus | Hormonal changes during menopause lead to a decrease in Lactobacillus dominance, resulting in increased microbial diversity. A higher vaginal pH increases the risks of infections. | [68,73,81,82,83] |
| Author | Country | Study Type | Sample Size | Participants’ Age (Years) | Test Technique Used | Findings | Reference |
|---|---|---|---|---|---|---|---|
| Campisciano et al., 2019 | Italy | Cohort study | 90 women | 20–40 | Species-specific multiplex genotyping assay | Lactobacillus crispatus increased in CST I while Prevotella timonensis and Sneathia sanguinegens increased in CST IV. An efficient viral clearance was observed only in women from CST I, dominated by Lactobacillus crispatus. | [144] |
| Chao et al., 2019 | China | Cohort study | 151 women | 20–65 | Sequencing barcoded 16S rDNA gene fragments (V4) on Illumina HiSeq2500 | Bacteroides plebeius, Acinetobacter lwoffii, and Prevotella buccae were found significantly more frequently in HPV-positive women. | [141] |
| Onywera et al., 2019 | South Africa | Retrospective cross-sectional study | 62 women | Average 34.5 | Bacterial 16S rRNA gene | Lactobacillus, Gardnerella, Prevotella, and Sneathia were the most predominant genera in the phyla Firmicutes, Actinobacteria, Bacteriodetes, and Fusobacteria, respectively. | [40,41] |
| Chen et al., 2020 | China | Cohort study | 229 women | 25–69 | Deep sequencing barcoded 16s rRNA ThinPrep cytology test, colposcopy examination | The highest microbial diversity was observed in cervical cancer patients when compared to other CIN/lesion-statused groups. HPV contributed to the reduction in the abundance of species of Prevotella, Bacillus, Anaerococcus, Sneathia, Megasphaera, Streptococcus, and Anaerococcus. | [143] |
| McKee et al., 2020 | Appalachia, United States | Population study | 308 women | 21–39 | Illumina MiSeq sequencing of 16S rRNA gene amplicons | Women who were determined to have abnormal cervical cytology or high-risk HPV possessed increased relative abundance of G. vaginalis and reduced relative abundance of L. gasseri. | [145] |
| Yang et al., 2020 | China | Exploratory and validation cohort study | 2251 women | 25–50 | Metagenome sequencing and HPV genotyping | Lactobacillus, followed by the Gardnerella genus, was highly dominant in both HPV-16-infected women and healthy groups. | [148] |
| Kang et al., 2021 | South Korea | Cohort study | 23 women, 4 groups: healthy individuals, patients with CIN 2, 3, and ICC | Average 47.4 | Amplicon sequencing was performed using the Ion Torrent PGM | Gardnerella and Prevotella were abundant in the CIN group and only one genus was abundant in the healthy control group (Lactobacillus). Gardnerella and Streptococcus were the only microorganisms that differed significantly between each group. | [147] |
| Lin et al., 2022 | China | Population-based cohort study | 448 women | 20–74 | Sequencing the region of the bacterial 16S V4 rRNA gene | The proportion of Gardnerella and Prevotella were markedly increased in HPV (+) patients. Gardnerella and Prevotella are the most high-risk combination for the development of HPV (+) in women. | [146] |
| Liu et al., 2024 | China | Prospective observational cohort study | 802 women | Age was not reported | High-throughput 16S rRNA sequencing technology | Infected group exhibited a lower abundance of Lactobacillus and a significantly higher abundance of Pseudomonas, Bifidobacterium, Limosilactobacillus, Peptostreptococcus, Gardnerella, Prevotella, and Dialister. | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizhan, D.; Ukybassova, T.; Bapayeva, G.; Aimagambetova, G.; Kongrtay, K.; Kamzayeva, N.; Terzic, M. Cervicovaginal Microbiome: Physiology, Age-Related Changes, and Protective Role Against Human Papillomavirus Infection. J. Clin. Med. 2025, 14, 1521. https://doi.org/10.3390/jcm14051521
Alizhan D, Ukybassova T, Bapayeva G, Aimagambetova G, Kongrtay K, Kamzayeva N, Terzic M. Cervicovaginal Microbiome: Physiology, Age-Related Changes, and Protective Role Against Human Papillomavirus Infection. Journal of Clinical Medicine. 2025; 14(5):1521. https://doi.org/10.3390/jcm14051521
Chicago/Turabian StyleAlizhan, Diana, Talshyn Ukybassova, Gauri Bapayeva, Gulzhanat Aimagambetova, Kuralay Kongrtay, Nazira Kamzayeva, and Milan Terzic. 2025. "Cervicovaginal Microbiome: Physiology, Age-Related Changes, and Protective Role Against Human Papillomavirus Infection" Journal of Clinical Medicine 14, no. 5: 1521. https://doi.org/10.3390/jcm14051521
APA StyleAlizhan, D., Ukybassova, T., Bapayeva, G., Aimagambetova, G., Kongrtay, K., Kamzayeva, N., & Terzic, M. (2025). Cervicovaginal Microbiome: Physiology, Age-Related Changes, and Protective Role Against Human Papillomavirus Infection. Journal of Clinical Medicine, 14(5), 1521. https://doi.org/10.3390/jcm14051521








