Does Motor Cortex Engagement During Movement Preparation Differentially Inhibit Nociceptive Processing in Patients with Chronic Whiplash Associated Disorders, Chronic Fatigue Syndrome and Healthy Controls? An Experimental Study
Abstract
:1. Introduction
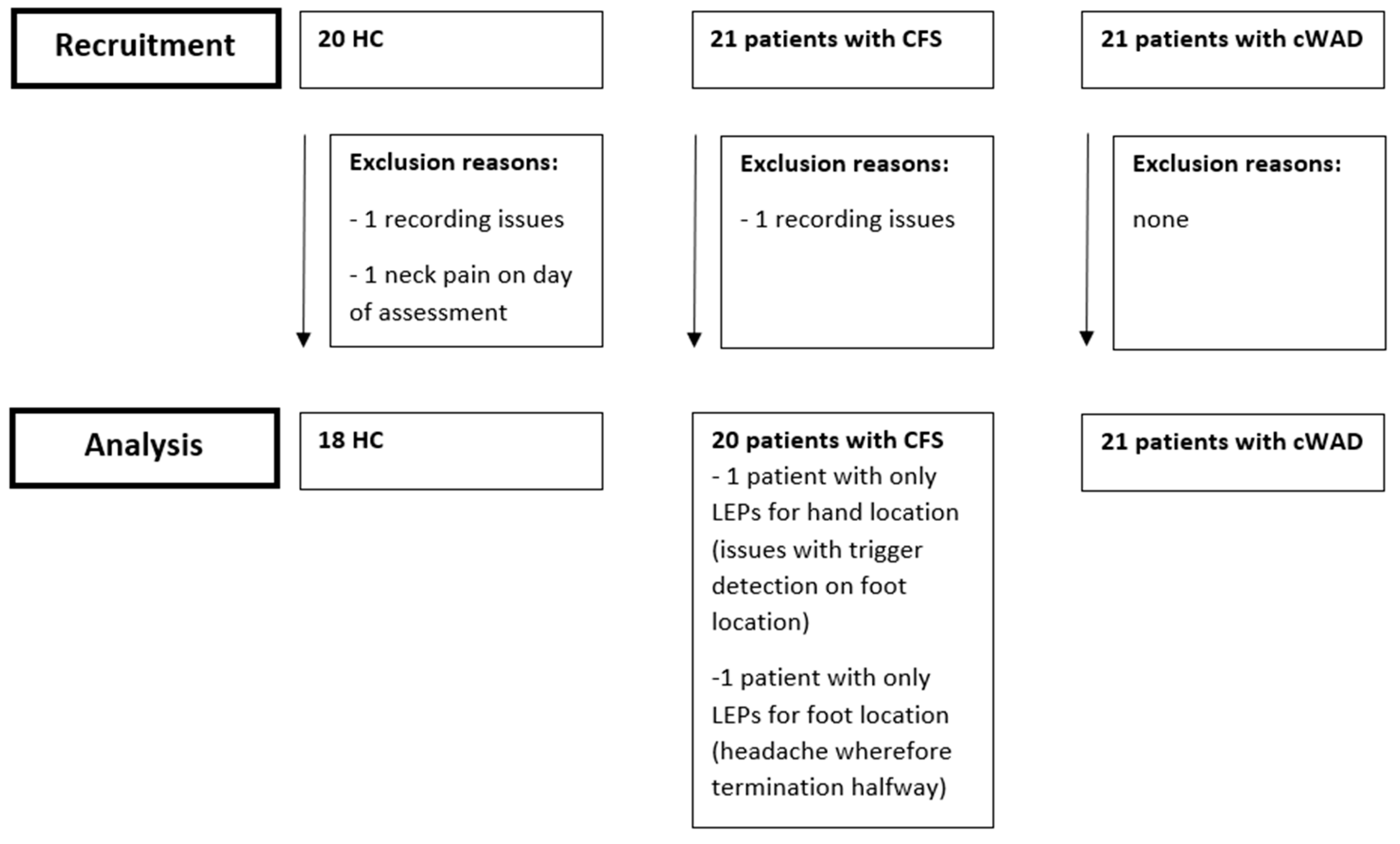
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Sample Size Calculation
2.4. Demographic Characteristics
2.5. Questionnaires
2.6. Laser Stimulation
2.7. LEP Recording
2.8. Time-Domain Analysis of LEPs
2.9. Time-Frequency Analysis of LEPs
3. Results
3.1. Group characteristics
3.2. Pain Intensity Ratings
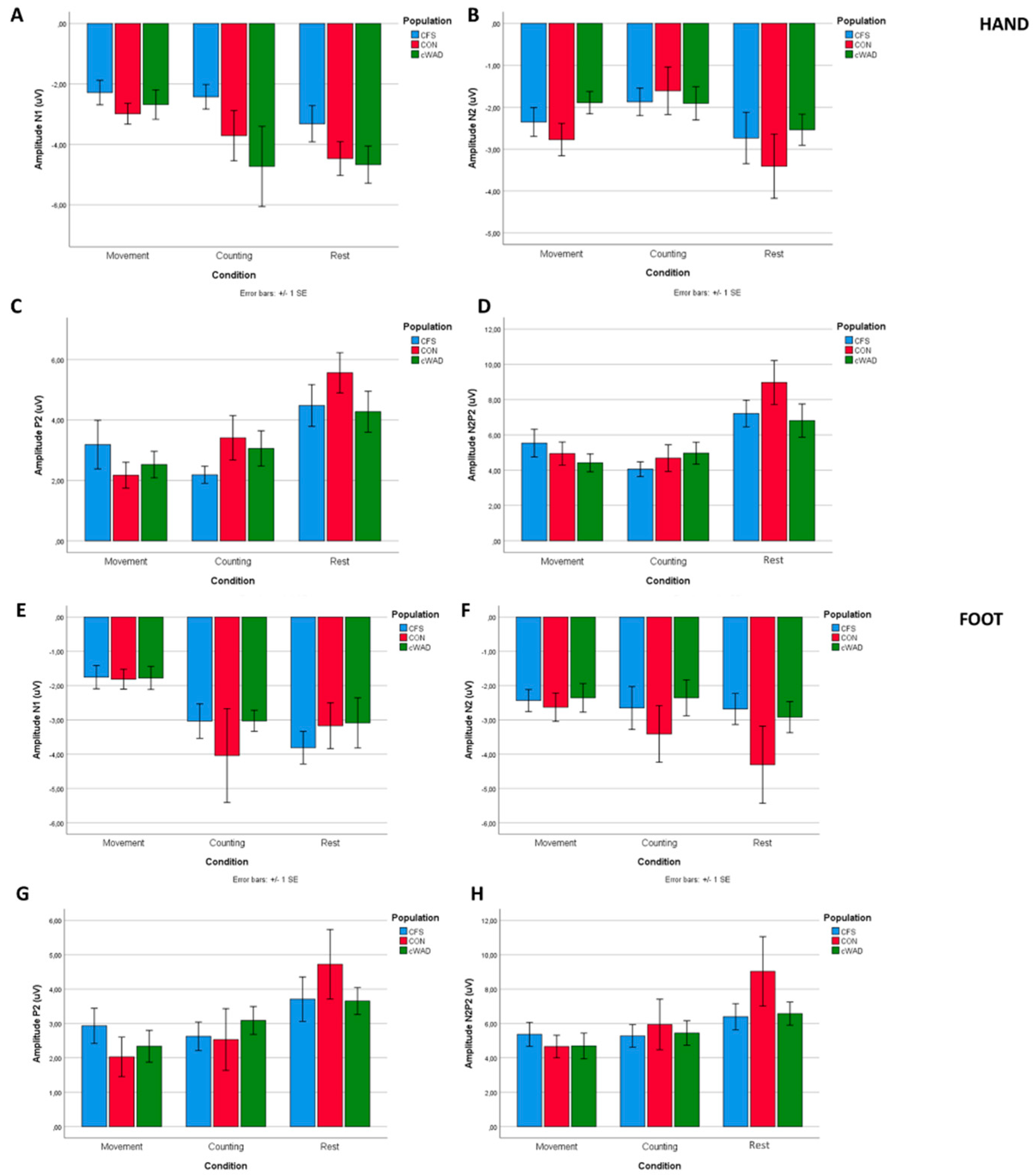
3.3. Laser-Evoked Brain Potentials: Time Domain Analysis
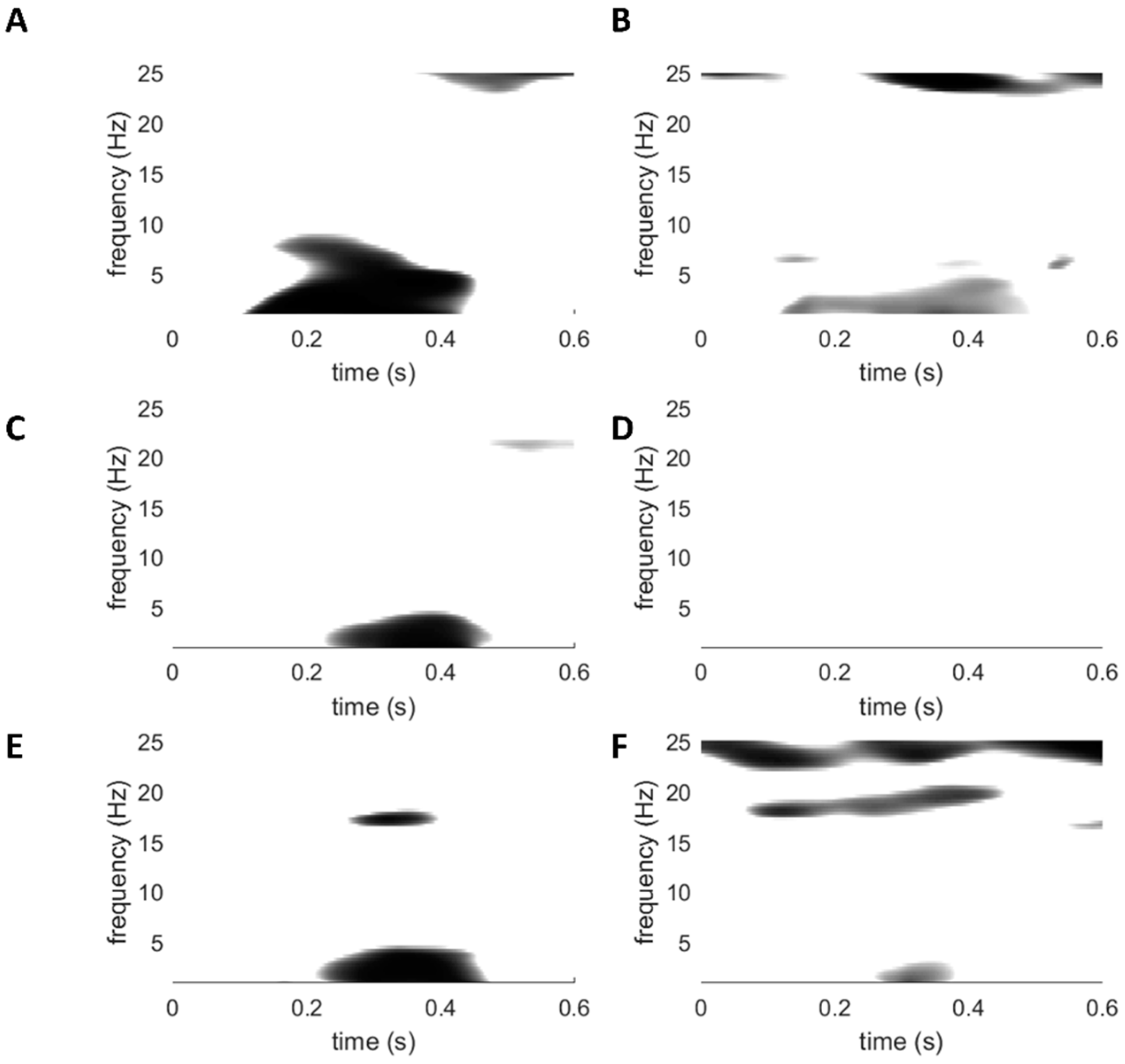
3.4. Laser-Evoked Brain Potentials: Time-Frequency Analysis
4. Discussion
4.1. No Reduced Motor-Induced Inhibition of Nociception in Patients Compared to Healthy Controls
4.2. Influence of Movement Preparation on LEP Components
4.3. Influence of Distraction on LEP Components
4.4. Time-Frequency Analyses
4.5. Motor Cortex Activation And Pain Relief
4.6. Clinical Implications
4.7. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nijs, J.; Daenen, L.; Cras, P.; Struyf, F.; Roussel, N.; Oostendorp, R.A. Nociception affects motor output: A review on sensory-motor interaction with focus on clinical implications. Clin. J. Pain 2012, 28, 175–181. [Google Scholar] [CrossRef]
- Bandholm, T.; Rasmussen, L.; Aagaard, P.; Diederichsen, L.; Jensen, B.R. Effects of experimental muscle pain on shoulder-abduction force steadiness and muscle activity in healthy subjects. Eur. J. Appl. Physiol. 2008, 102, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Mista, C.A.; Christensen, S.W.; Graven-Nielsen, T. Modulation of motor variability related to experimental muscle pain during elbow-flexion contractions. Hum. Mov. Sci. 2015, 39, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Drouot, X.; Keravel, Y.; Nguyen, J.P. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport 2001, 12, 2963–2965. [Google Scholar] [CrossRef] [PubMed]
- Galhardoni, R.; Correia, G.S.; Araujo, H.; Yeng, L.T.; Fernandes, D.T.; Kaziyama, H.H.; Marcolin, M.A.; Bouhassira, D.; Teixeira, M.J.; de Andrade, D.C. Repetitive transcranial magnetic stimulation in chronic pain: A review of the literature. Arch. Phys. Med. Rehabil. 2015, 96, S156–S172. [Google Scholar] [CrossRef]
- Koltyn, K.F. Analgesia following exercise: A review. Sports medicine 2000, 29, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kakigi, R.; Matsuda, Y.; Kuroda, Y. Effects of movement-related cortical activities on pain-related somatosensory evoked potentials following CO2 laser stimulation in normal subjects. Acta Neurol. Scand. 1993, 88, 376–380. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef] [Green Version]
- Babiloni, C.; Carducci, F.; Cincotti, F.; Rossini, P.M.; Neuper, C.; Pfurtscheller, G.; Babiloni, F. Human movement-related potentials vs desynchronization of EEG alpha rhythm: A high-resolution EEG study. Neuroimage 1999, 10, 658–665. [Google Scholar] [CrossRef]
- Walter, W.G.; Cooper, R.; Aldridge, V.J.; McCallum, W.C.; Winter, A.L. Contingent Negative Variation: An Electric Sign of Sensori-Motor Association and Expectancy in the Human Brain. Nature 1964, 203, 380. [Google Scholar] [CrossRef]
- Gomez, C.M.; Marco, J.; Grau, C. Preparatory visuo-motor cortical network of the contingent negative variation estimated by current density. Neuroimage 2003, 20, 216–224. [Google Scholar] [CrossRef]
- Le Pera, D.; Brancucci, A.; De Armas, L.; Del Percio, C.; Miliucci, R.; Babiloni, C.; Restuccia, D.; Rossini, P.M.; Valeriani, M. Inhibitory effect of voluntary movement preparation on cutaneous heat pain and laser-evoked potentials. Eur. J. Neurosci. 2007, 25, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.M.; Vicenzino, B.; Lim, E.C.; Sterling, M. Measures of central hyperexcitability in chronic whiplash associated disorder--a systematic review and meta-analysis. Man Ther 2013, 18, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Don, S.; De Kooning, M.; Voogt, L.; Ickmans, K.; Daenen, L.; Nijs, J. The Effect of Visual Feedback of the Neck during Movement in People with Chronic Whiplash-Associated Disorders: An Experimental Study. J. Orthop. Sports Phys. Ther. 2017, 47, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Malfliet, A.; Pas, R.; Brouns, R.; De Win, J.; Hatem, S.M.; Meeus, M.; Ickmans, K.; van Hooff, R.J.; Nijs, J. Cerebral Blood Flow and Heart Rate Variability in Chronic Fatigue Syndrome: A Randomized Cross-Over Study. Pain Physician 2018, 21, E13–E24. [Google Scholar] [CrossRef]
- Gerwyn, M.; Maes, M. Mechanisms Explaining Muscle Fatigue and Muscle Pain in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Review of Recent Findings. Curr. Rheumatol. Rep. 2017, 19, 1. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; Shivapurkar, N. Exercise—Induced changes in cerebrospinal fluid miRNAs in Gulf War Illness, Chronic Fatigue Syndrome and sedentary control subjects. Sci. Rep. 2017, 7, 15338. [Google Scholar] [CrossRef] [Green Version]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Van Loo, M.; Paul, L. Lack of endogenous pain inhibition during exercise in people with chronic whiplash associated disorders: An experimental study. J. Pain 2012, 13, 242–254. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- De Pauw, R.; Coppieters, I.; Caeyenbergh, K.; Kregel, J.; Aerts, H.; Lenoir, D.; Cagnie, B. Associations between brain morphology and motor performance in chronic neck pain: A whole-brain surface-based morphometry approach. Hum. Brain Mapp. 2019, 40, 4266–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starr, A.; Scalise, A.; Gordon, R.; Michalewski, H.J.; Caramia, M.D. Motor cortex excitability in chronic fatigue syndrome. Clin. Neurophysiol. 2000, 111, 2025–2031. [Google Scholar] [CrossRef] [Green Version]
- Davey, N.J.; Puri, B.K.; Catley, M.; Main, J.; Nowicky, A.V.; Zaman, R. Deficit in motor performance correlates with changed corticospinal excitability in patients with chronic fatigue syndrome. Int. J. Clin. Pract. 2003, 57, 262–264. [Google Scholar] [PubMed]
- Naro, A.; Russo, M.; Leo, A.; Rifici, C.; Pollicino, P.; Bramanti, P.; Calabro, R.S. Cortical Responsiveness to Nociceptive Stimuli in Patients with Chronic Disorders of Consciousness: Do C-Fiber Laser Evoked Potentials Have a Role? PLoS ONE 2015, 10, e0144713. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Biasiotta, A.; La Cesa, S.; Di Stefano, G.; Galeotti, F.; Petrucci, M.T.; Inghilleri, M.; Cartoni, C.; Pergolini, M.; Cruccu, G. Mechanisms of pain in distal symmetric polyneuropathy: A combined clinical and neurophysiological study. Pain 2010, 150, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, M.; Pazzaglia, C.; Cruccu, G.; Truini, A. Clinical usefulness of laser evoked potentials. Neurophysiol. Clin. 2012, 42, 345–353. [Google Scholar] [CrossRef]
- De Tommaso, M.; Federici, A.; Santostasi, R.; Calabrese, R.; Vecchio, E.; Lapadula, G.; Iannone, F.; Lamberti, P.; Livrea, P. Laser-evoked potentials habituation in fibromyalgia. J. Pain 2011, 12, 116–124. [Google Scholar] [CrossRef]
- Spitzer, W.; Skovron, M.; Salmi, L.; Cassidy, J.; Duranceau, J.; Suissa, S.; Zeiss, E. Scientific monograph of Quebec Task Force on whiplash associated disorders: Redefining ‘Whiplash’ and its management. Spine 1995, 20, 1S–73S. [Google Scholar]
- Suresh, K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]
- Bender, S.; Resch, F.; Weisbrod, M.; Oelkers-Ax, R. Specific task anticipation versus unspecific orienting reaction during early contingent negative variation. Clin. Neurophysiol. 2004, 115, 1836–1845. [Google Scholar] [CrossRef]
- Plaghki, L.; Delisle, D.; Godfraind, J.M. Heterotopic nociceptive conditioning stimuli and mental task modulate differently the perception and physiological correlates of short CO2 laser stimuli. Pain 1994, 57, 181–192. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Sullivan, M.J.L. The Pain Catstrophizing Scale: User Manual; McGill University: Montreal, QC, Canada, 2009; pp. 1–36. [Google Scholar]
- Osman, A.; Barrios, F.X.; Kopper, B.A.; Hauptmann, W.; Jones, J.; O’Neill, E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J. Behav. Med. 1997, 20, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Lame, I.E.; Peters, M.L.; Kessels, A.G.; Van Kleef, M.; Patijn, J. Test--retest stability of the Pain Catastrophizing Scale and the Tampa Scale for Kinesiophobia in chronic pain over a longer period of time. J. Health Psychol. 2008, 13, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.R.; Edgley, K.; Fisher, R.; Sullivan, M.J.L. Screening for depression in chronic low back pain with the Beck Depression Inventory. Can. J. Rehabil. 1993, 7, 143–148. [Google Scholar]
- Soer, R.; Reneman, M.F.; Vroomen, P.C.; Stegeman, P.; Coppes, M.H. Responsiveness and minimal clinically important change of the Pain Disability Index in patients with chronic back pain. Spine 2012, 37, 711–715. [Google Scholar] [CrossRef]
- Soer, R.; Koke, A.J.; Vroomen, P.C.; Stegeman, P.; Smeets, R.J.; Coppes, M.H.; Reneman, M.F. Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine 2013, 38, E562–E568. [Google Scholar] [CrossRef]
- Schwarz, S.; Greffrath, W.; Busselberg, D.; Treede, R.D. Inactivation and tachyphylaxis of heat-evoked inward currents in nociceptive primary sensory neurones of rats. J. Physiol. 2000, 528, 539–549. [Google Scholar] [CrossRef]
- Hatem, S.M.; Attal, N.; Ducreux, D.; Gautron, M.; Parker, F.; Plaghki, L.; Bouhassira, D. Clinical, functional and structural determinants of central pain in syringomyelia. Brain 2010, 133, 3409–3422. [Google Scholar] [CrossRef] [Green Version]
- Plaghki, L.; Decruynaere, C.; Van Dooren, P.; Le Bars, D. The fine tuning of pain thresholds: A sophisticated double alarm system. PLoS ONE 2010, 5, e10269. [Google Scholar] [CrossRef] [Green Version]
- Hatem, S.M.; Hu, L.; Rage, M.; Gierasimowicz, A.; Plaghki, L.; Bouhassira, D.; Attal, N.; Iannetti, G.D.; Mouraux, A. Automated single-trial assessment of laser-evoked potentials as an objective functional diagnostic tool for the nociceptive system. Clin. Neurophysiol. 2012, 123, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aminoff, M.J.; Curio, G.; Guerit, J.M.; Kakigi, R.; Mauguiere, F.; Rossini, P.M.; Treede, R.D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeke, E.N.; Lambert, J.; Huang, G.; Mouraux, A. Central Sensitization of Mechanical Nociceptive Pathways Is Associated with a Long-Lasting Increase of Pinprick-Evoked Brain Potentials. Front. Hum. Neurosci. 2016, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Van den Broeke, E.N.; de Vries, B.; Lambert, J.; Torta, D.M.; Mouraux, A. Phase-locked and non-phase-locked EEG responses to pinprick stimulation before and after experimentally-induced secondary hyperalgesia. Clin. Neurophysiol. 2017, 128, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeke, E.N.; Mouraux, A.; Groneberg, A.H.; Pfau, D.B.; Treede, R.D.; Klein, T. Characterizing pinprick-evoked brain potentials before and after experimentally induced secondary hyperalgesia. J. Neurophysiol. 2015, 114, 2672–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.G.; Hu, L.; Hung, Y.S.; Mouraux, A.; Iannetti, G.D. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J. Neurosci. 2012, 32, 7429–7438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torta, D.M.; Legrain, V.; Algoet, M.; Olivier, E.; Duque, J.; Mouraux, A. Theta burst stimulation applied over primary motor and somatosensory cortices produces analgesia unrelated to the changes in nociceptive event-related potentials. PLoS ONE 2013, 8, e73263. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, A.W. The late CNV wave: Preparation versus expectancy. Psychophysiology 1977, 14, 563–568. [Google Scholar] [CrossRef]
- Cui, R.Q.; Egkher, A.; Huter, D.; Lang, W.; Lindinger, G.; Deecke, L. High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clin. Neurophysiol. 2000, 111, 1847–1859. [Google Scholar] [CrossRef]
- Van Oosterwijck, J.; Marusic, U.; De Wandele, I.; Paul, L.; Meeus, M.; Moorkens, G.; Lambrecht, L.; Danneels, L.; Nijs, J. The Role of Autonomic Function in Exercise induced Endogenous Analgesia: A Case-control Study in Myalgic Encephalomyelitis⁄Chronic Fatigue Syndrome and Healthy People. Pain Physician 2017, 20, E389–E399. [Google Scholar]
- Dum, R.P.; Strick, P.L. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 1991, 11, 667–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valeriani, M.; Rambaud, L.; Mauguiere, F. Scalp topography and dipolar source modelling of potentials evoked by CO2 laser stimulation of the hand. Electroencephalogr. Clin. Neurophysiol. 1996, 100, 343–353. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Frot, M.; Valeriani, M. Brain generators of laser-evoked potentials: From dipoles to functional significance. Neurophysiol. Clin. 2003, 33, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Valentini, E.; Hu, L.; Chakrabarti, B.; Hu, Y.; Aglioti, S.M.; Iannetti, G.D. The primary somatosensory cortex largely contributes to the early part of the cortical response elicited by nociceptive stimuli. Neuroimage 2012, 59, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larrea, L.; Peyron, R.; Laurent, B.; Mauguiere, F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport 1997, 8, 3785–3789. [Google Scholar] [CrossRef]
- Yamasaki, H.; Kakigi, R.; Watanabe, S.; Naka, D. Effects of distraction on pain perception: Magneto- and electro-encephalographic studies. Cogn. Brain Res. 1999, 8, 73–76. [Google Scholar] [CrossRef]
- Ploner, M.; Sorg, C.; Gross, J. Brain Rhythms of Pain. Trends Cogn. Sci. 2017, 21, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Hauck, M.; Domnick, C.; Lorenz, J.; Gerloff, C.; Engel, A.K. Top-down and bottom-up modulation of pain-induced oscillations. Front. Hum. Neurosci. 2015, 9, 375. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Gentile, E.; Brunetti, A.; Ricci, K.; Delussi, M.; Bevilacqua, V.; de Tommaso, M. Mutual interaction between motor cortex activation and pain in fibromyalgia: EEG-fNIRS study. PLoS ONE 2020, 15, e0228158. [Google Scholar] [CrossRef]
- Lima, M.C.; Fregni, F. Motor cortex stimulation for chronic pain: Systematic review and meta-analysis of the literature. Neurology 2008, 70, 2329–2337. [Google Scholar] [CrossRef]
- Mercier, C.; Léonard, G. Interactions between Pain and the Motor Cortex: Insights from Research on Phantom Limb Pain and Complex Regional Pain Syndrome. Physiother. Can. 2011, 63, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Sterling, M.; Jull, G.; Vicenzino, B.; Kenardy, J.; Darnell, R. Development of motor system dysfunction following whiplash injury. Pain 2003, 103, 65–73. [Google Scholar] [CrossRef]
- Jull, G.; Kristjansson, E.; Dall’Alba, P. Impairment in the cervical flexors: A comparison of whiplash and insidious onset neck pain patients. Man. Ther. 2004, 9, 89–94. [Google Scholar] [CrossRef]
- Woodhouse, A.; Vasseljen, O. Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet. Disord. 2008, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Sterling, M.; Jull, G.; Kenardy, J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain 2006, 122, 102–108. [Google Scholar] [CrossRef]
- Daenen, L.; Nijs, J.; Roussel, N.; Wouters, K.; Van Loo, M.; Cras, P. Dysfunctional pain inhibition in patients with chronic whiplash-associated disorders: An experimental study. Clin. Rheumatol. 2013, 32, 23–31. [Google Scholar] [CrossRef]
- Sterlin, M.; Jull, G.; Vicenzino, B.; Kenardy, J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 2003, 104, 509–517. [Google Scholar] [CrossRef]
- Nijs, J.; Van Oosterwijck, J.; De Hertogh, W. Rehabilitation of chronic whiplash: Treatment of cervical dysfunctions or chronic pain syndrome? Clin. Rheumatol 2009, 28, 243–251. [Google Scholar] [CrossRef]
- Meeus, M.; Hermans, L.; Ickmans, K.; Struyf, F.; Van Cauwenbergh, D.; Bronckaerts, L.; De Clerck, L.S.; Moorken, G.; Hans, G.; Grosemans, S.; et al. Endogenous pain modulation in response to exercise in patients with rheumatoid arthritis, patients with chronic fatigue syndrome and comorbid fibromyalgia, and healthy controls: A double-blind randomized controlled trial. Pain Pract. 2015, 15, 98–106. [Google Scholar] [CrossRef]
- Dannaway, J.; New, C.C.; New, C.H.; Maher, C.G. Exercise therapy is a beneficial intervention for chronic fatigue syndrome (PEDro synthesis). Br. J. Sports Med. 2018, 52, 542–543. [Google Scholar] [CrossRef]
- Larun, L.; Brurberg, K.G.; Odgaard-Jensen, J.; Price, J.R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2017, 4, Cd003200. [Google Scholar] [CrossRef]
- Teasell, R.W.; McClure, J.A.; Walton, D.; Pretty, J.; Salter, K.; Meyer, M.; Sequeira, K.; Death, B. A research synthesis of therapeutic interventions for whiplash-associated disorder (WAD): Part 4—noninvasive interventions for chronic WAD. Pain Res. Manag. 2010, 15, 313–322. [Google Scholar] [CrossRef]
- Anderson, C.; Yeung, E.; Toong, T.; Tong, T.; Reed, N. A narrative review on cervical interventions in adults with chronic whiplash-associated disorder. BMJ Open Sport Exerc. Med. 2018, 4, e000299. [Google Scholar] [CrossRef] [Green Version]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef] [Green Version]
- Truini, A.; Galeotti, F.; Romaniello, A.; Virtuoso, M.; Iannetti, G.D.; Cruccu, G. Laser-evoked potentials: Normative values. Clin. Neurophysiol. 2005, 116, 821–826. [Google Scholar] [CrossRef]
- Staikou, C.; Kokotis, P.; Kyrozis, A.; Rallis, D.; Makrydakis, G.; Manoli, D.; Karandreas, N.; Stamboulis, E.; Moschovos, C.; Fassoulaki, A. Differences in Pain Perception Between Men and Women of Reproductive Age: A Laser-Evoked Potentials Study. Pain Med. 2017, 18, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Truini, A.; Panuccio, G.; Galeotti, F.; Maluccio, M.R.; Sartucci, F.; Avoli, M.; Cruccu, G. Laser-evoked potentials as a tool for assessing the efficacy of antinociceptive drugs. Eur. J. Pain 2010, 14, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, J.; Beck, H.; Bromm, B. Differential changes of laser evoked potentials, late auditory evoked potentials and P300 under morphine in chronic pain patients. Electroencephalogr. Clin. Neurophysiol. 1997, 104, 514–521. [Google Scholar] [CrossRef]
- Petersen-Felix, S.; Arendt-Nielsen, L.; Bak, P.; Fischer, M.; Zbinden, A.M. Psychophysical and electrophysiological responses to experimental pain may be influenced by sedation: Comparison of the effects of a hypnotic (propofol) and an analgesic (alfentanil). Br. J. Anaesth. 1996, 77, 165–171. [Google Scholar] [CrossRef]
- De Tommaso, M.; Losito, L.; Libro, G.; Guido, M.; Di Fruscolo, O.; Sardaro, M.; Sciruicchio, V.; Lamberti, P.; Livrea, P. Effects of symptomatic treatments on cutaneous hyperalgesia and laser evoked potentials during migraine attack. Cephalalgia 2005, 25, 359–368. [Google Scholar] [CrossRef]



| HC (n = 18) | CFS (n = 20) | cWAD (n = 21) | Test Statistic | p-Value | Post-Hoc | |
|---|---|---|---|---|---|---|
| Age (years) | 46.8 (27.7–51.2) | 43.8 (35.9–48.4) | 45.8 (40.4–51.1) | χ2(2) = 1.38 | a 0.501 | |
| Sex | M: 7 (38.9%) F: 11 (61.1%) | M: 2 (10%) F: 18 (90%) | M: 11 (52.4%) F: 10 (47.6%) | χ2(2) = 8.50 | b 0.014 | CFS vs. cWAD: 0.009 |
| VAS current pain (mm) (0–100) | 49 (19–54.5) | 28 (20–53) | W = 213 | c 0.507 | ||
| VAS last 7 days (mm) (0–100) | 50 (31.25–61) | 51 (35–62) | W = 179 | c 0.79 | ||
| PCS (0–52) | 17.95 (11.73) | 18.1 (10.69) | t(34.6) = 0.133 | d 0.895 | ||
| BDI (0–63) | 16 (11–22) | 11 (10–20.5) | W = 131 | c 0.332 | ||
| PDI (0–70) | 38.9 (11.46) | 35 (14.34) | t(36.9) = −0.721 | d 0.475 | ||
| Time since accident (years) | 4.7 (4.34) |
| Resting Condition | Movement Preparation Condition | Calculation Condition | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hand | Foot | Hand | Foot | Hand | Foot | |||||||||||||
| HC (n = 18) | CFS (n = 19) | cWAD (n = 21) | HC (n = 18) | CFS (n = 19) | cWAD (n = 21) | HC (n = 18) | CFS (n = 19) | cWAD (n = 21) | HC (n = 18) | CFS (n = 19) | cWAD (n = 21) | HC (n = 18) | CFS (n = 19) | cWAD (n = 21) | HC (n = 18) | CFS (n = 19) | cWAD (n = 21) | |
| N1 amplitude (µV) | −4.47 (2.37) | −3.32 (2.61) | −4.67 (2.81) | −3.17 (2.83) | −3.81 (2.07) | −3.09 (3.35) | −2.98 (1.46) | −2.28 (1.77) | −2.68 (2.24) | −1.81 (1.23) | −1.75 (1.48) | −1.78 (1.55) | −3.71 (3.53) | −2.42 (1.77) | −4.73 (6.09) | −4.04 (5.81) | −3.03 (2.19) | −3.03 (1.41) |
| N2 amplitude (µV) | −3.41 (3.25) | −2.73 (2.67) | −2.54 (1.70) | −4.31 (4.77) | −2.68 (1.98) | −2.92 (2.08) | −2.77 (1.64) | −2.35 (1.50) | −1.89 (1.22) | −2.63 (1.75) | −2.44 (1.40) | −2.35 (1.91) | −1.60 (2.40) | −1.87 (1.42) | −1.91 (1.81) | −3.41 (3.50) | −2.65 (2.73) | −2.35 (2.40) |
| P2 amplitude (µV) | 5.56 (2.83) | 4.48 (3.02) | 4.27 (3.11) | 4.72 (4.29) | 3.71 (2.82) | 3.65 (1.80) | 2.17 (1.82) | 3.18 (3.50) | 2.52 (2.01) | 2.03 (2.44) | 2.93 (2.23) | 2.34 (2.12) | 3.41 (3.12) | 2.18 (1.23) | 3.06 (2.68) | 2.53 (3.81) | 2.62 (1.81) | 3.08 (1.86) |
| N2P2 amplitude (µV) | 8.97 (5.29) | 7.21 (3.29) | 6.81 (4.32) | 9.03 (8.57) | 6.39 (3.31) | 6.57 (3.09) | 4.94 (2.78) | 5.54 (3.41) | 4.41 (2.31) | 4.66 (2.79) | 5.37 (3.05) | 4.69 (3.40) | 4.68 (3.21) | 4.05 (1.82) | 4.96 (2.84) | 5.94 (6.25) | 5.28 (2.87) | 5.44 (3.27) |
| N1 latency (ms) | 252 (56) | 216 (54) | 225 (65) | 235 (46) | 214 (45) | 227 (40) | 214 (52) | 204 (46) | 208 (54) | 226 (51) | 218 (59) | 210 (54) | 200 (45) | 209 (45) | 222 (42) | 231 (47) | 220 (61) | 225 (62) |
| N2 latency (ms) | 229 (54) | 241 (46) | 225 (51) | 227 (59) | 252 (45) | 232 (64) | 217 (57) | 243 (54) | 175 (24) | 227 (52) | 222 (51) | 212 (50) | 191 (46) | 207 (53) | 214 (41) | 228 (50) | 231 (65) | 208 (60) |
| P2 latency (ms) | 374 (59) | 388 (55) | 388 (68) | 404 (53) | 414 (53) | 414 (51) | 359 (44) | 415 (73) | 435 (18) | 406 (62) | 412 (63) | 383 (63) | 371 (68) | 395 (81) | 396 (66) | 402 (58) | 389 (90) | 405 (56) |
| VAS | 38 (19.3) | 42 (21.7) | 43 (17.6) | 39 (22.9) | 43 (21.7) | 44 (25.5) | 37 (21.1) | 43 (20.1) | 41 (16.8) | 34 (24.1) | 41 (20.5) | 40 (23.3) | 32 (22.8) | 37 (20.7) | 39 (16.8) | 35 (24.7) | 43 (20.6) | 40 (24.1) |
| Peak | Interaction Effect (Condition x Population) | Effect of Condition | Effect of Population | Condition Post-Hoc Testing (mean (95% CI) | Post-Hoc Rest vs. Counting (Mean Difference (95% CI)) | Post-Hoc Rest vs. Movement (Mean Difference (95% CI)) | Post-Hoc Counting vs. Movement (Mean Difference (95% CI)) |
|---|---|---|---|---|---|---|---|
| HAND | |||||||
| N1 amp | F = 0.568, p = 0.635 | F = 3.925, p = 0.035 * | F = 2.545, p = 0.088 | Resting: −4.1 (−4.84; −3.46) Counting: −3.6 (−4.75; −2.49) Movement: −2.6 ( −3.14; −2.16) | −0.53 ( −1.91 to 0.84) p = 0.442 | −1.50 ( −2.39 to −0.62) p = 0.001 * | −0.97 (−1.92 to −0.02) p = 0.044 * |
| N1 lat | F = 1.418, p = 0.235 | F = 3.581, p = 0.033 * | F = 0.761, p = 0.472 | Resting: 231 (215;246) Counting: 210 (199;222) Movement:209 (195;222) | 21 (0 to 41) p = 0.049 * | 22 (4 to 41) p = 0.017 * | 2 (−15 to 19) p = 0.839 |
| N2 amp | F = 0.862, p = 0.470 | F = 6.211, p = 0.005 * | F = 0.501, p = 0.609 | Resting: −2.9 ( −3.57; −2.21) Counting: −1.8 ( −2.30; −1.29) Movement: −2.3 ( −2.72; −1.96) | −1.10 ( −1.86 to −0.33) p = 0.006 * | −0.55 ( −1.11 to −0.00) p = 0.049 * | 0.54 (0.01 to 1.08) p = 0.045 * |
| N2 lat | F = 1.017, p = 0.381 | F = 1.344, p = 0.259 | F = 1.025, p = 0.365 | ||||
| P2 amp | F = 2.668, p = 0.037 * | F = 23.574, p < 0.001 * | F = 0.232, p = 0.794 | Resting: 4.8 (3.98;5.56) Counting: 2.9 (2.23;3.54) Movement: 2.6 (1.95;3.30) | 1.89 (1.18 to 2.6) p < 0.001 * | 2.15 (1.51 to 2.79) p < 0.001 * | 0.26 (−0.44 to 0.96) p = 0.461 |
| P2 lat | F = 0.812, p = 0.485 | F = 0.935, p = 0.368 | F = 2.499, p = 0.091 | ||||
| N2P2 amp | F = 2.080, p = 0.091 | F = 26.724, p < 0.001 * | F = 0.447, p = 0.642 | Resting: 7.7 (6.52;8.81) Counting: 4.6 (3.86;5.27) Movement: 5.0 (4.21;5.72) | 3.10 (2.09 to 4.10) p < 0.001 * | 2.70 (1.78 to 3.62) p < 0.001 * | −0.40 (−1.24 to 0.44) p = 0.348 |
| FOOT | |||||||
| N1 amp | F = 0.597, p = 0.616 | F = 7.964, p = 0.002 * | F = 0.201, p = 0.819 | Resting: −3.6 ( −4.10; −2.61) Counting: −3.4 ( −4.31; −2.43) Movement −1.8 ( −2.16; −1.40) | 0.01 ( −1.13 to 1.15) p = 0.986 | −1.58 ( −2.25 to −0.91) p < 0.001 * | −1.59 (−2.47 to −0.71) p = 0.001 * |
| N1 lat | F = 0.226, p = 0.914 | F = 0.415, p = 0.647 | F = 0.853, p = 0.432 | ||||
| N2 amp | F = 0.805, p = 0.523 | F = 2.848, p = 0.063 | F = 1.037, p = 0.361 | ||||
| N2 lat | F = 0.663, p = 0.609 | F = 2.144, p = 0.126 | F = 0.902, p = 0.412 | ||||
| P2 amp | F = 1.473, p = 0.217 | F = 9.835, p < 0.001 * | F = 0.007, p = 0.993 | Resting: 4.0 (3.22;4.84) Counting: 2.7 (2.06;3.44) Movement: 2.4 (1.83;3.03) | 1.28 (0.45 to 2.11) p = 0.003 * | 1.60 (0.87 to 2.33) p < 0.001 * | 0.32 (−1.04 to 0.41) p = 0.386 |
| P2 lat | F = 1.086, p = 0.366 | F = 0.804, p = 0.444 | F = 0.06, p = 0.942 | ||||
| N2P2 amp | F = 1.931, p = 0.118 | F = 11.905, p < 0.001 * | F = 0.375, p = 0.689 | Resting: 7.3 (5.89;8.77) Counting: 5.6 (4.41;6.69) Movement: 4.9 (4.08;5.72) | 1.78 (0.79 to 2.77) p = 0.001 * | 2.43 (1.24 to 3.61) p < 0.001 * | 0.65 (−0.25 to 1.55) p = 0.154 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goudman, L.; Mouraux, A.; Daenen, L.; Nijs, J.; Cras, P.; Roussel, N.; Moens, M.; Lenoir, D.; Coppieters, I.; Huysmans, E.; et al. Does Motor Cortex Engagement During Movement Preparation Differentially Inhibit Nociceptive Processing in Patients with Chronic Whiplash Associated Disorders, Chronic Fatigue Syndrome and Healthy Controls? An Experimental Study. J. Clin. Med. 2020, 9, 1520. https://doi.org/10.3390/jcm9051520
Goudman L, Mouraux A, Daenen L, Nijs J, Cras P, Roussel N, Moens M, Lenoir D, Coppieters I, Huysmans E, et al. Does Motor Cortex Engagement During Movement Preparation Differentially Inhibit Nociceptive Processing in Patients with Chronic Whiplash Associated Disorders, Chronic Fatigue Syndrome and Healthy Controls? An Experimental Study. Journal of Clinical Medicine. 2020; 9(5):1520. https://doi.org/10.3390/jcm9051520
Chicago/Turabian StyleGoudman, Lisa, André Mouraux, Liesbeth Daenen, Jo Nijs, Patrick Cras, Nathalie Roussel, Maarten Moens, Dorine Lenoir, Iris Coppieters, Eva Huysmans, and et al. 2020. "Does Motor Cortex Engagement During Movement Preparation Differentially Inhibit Nociceptive Processing in Patients with Chronic Whiplash Associated Disorders, Chronic Fatigue Syndrome and Healthy Controls? An Experimental Study" Journal of Clinical Medicine 9, no. 5: 1520. https://doi.org/10.3390/jcm9051520
APA StyleGoudman, L., Mouraux, A., Daenen, L., Nijs, J., Cras, P., Roussel, N., Moens, M., Lenoir, D., Coppieters, I., Huysmans, E., & De Kooning, M. (2020). Does Motor Cortex Engagement During Movement Preparation Differentially Inhibit Nociceptive Processing in Patients with Chronic Whiplash Associated Disorders, Chronic Fatigue Syndrome and Healthy Controls? An Experimental Study. Journal of Clinical Medicine, 9(5), 1520. https://doi.org/10.3390/jcm9051520







