Approach to Neurological Channelopathies and Neurometabolic Disorders in Newborns
Abstract
:1. Introduction
2. Neurological Disease in Newborns with Heterogenous Etiologies
2.1. Neurometabolic Disorders in Newborns
2.2. Mitochondrial Disease
2.3. Risks for the Patient When a Misdiagnosis Is Made between Channelopathy and a Metabolic Disorder
3. Common Appearance of Channelopathies and Metabolic Disease
4. Differences between Neurological Channelopathies and Neurometabolic Disorders in Newborns
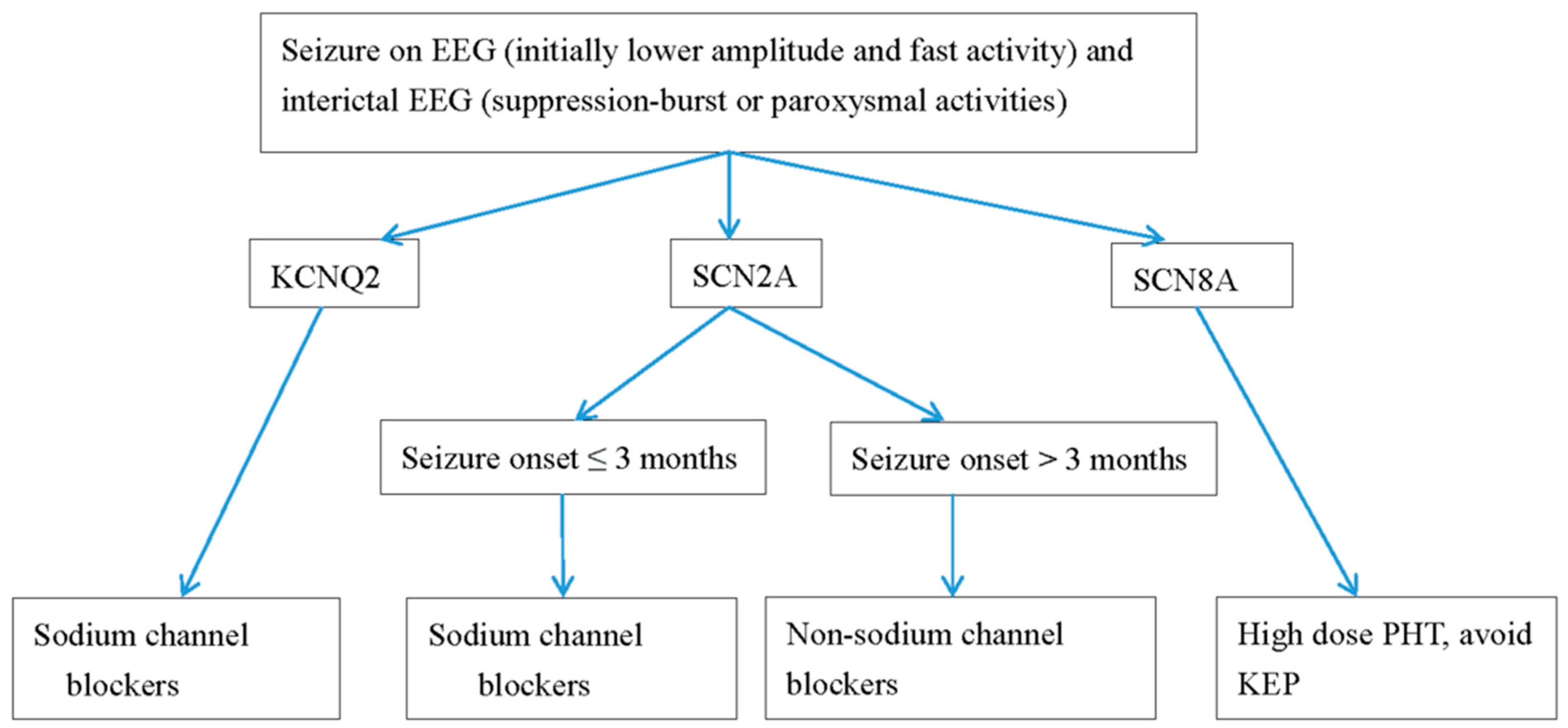
4.1. Seizure Type
4.2. Changes in Consciousness in Neurological Channelopathies and Neurometabolic Disorders in Newborns
4.3. Amplitude-Integrated EEG (aEEG) and EEG Monitoring
4.4. Brain MRI
5. Syndrome Diagnosis
5.1. Developmental Epileptic Encephalopathies
5.2. Early Infantile Epileptic Encephalopathy
5.3. Early Myoclonic Encephalopathy (EME)
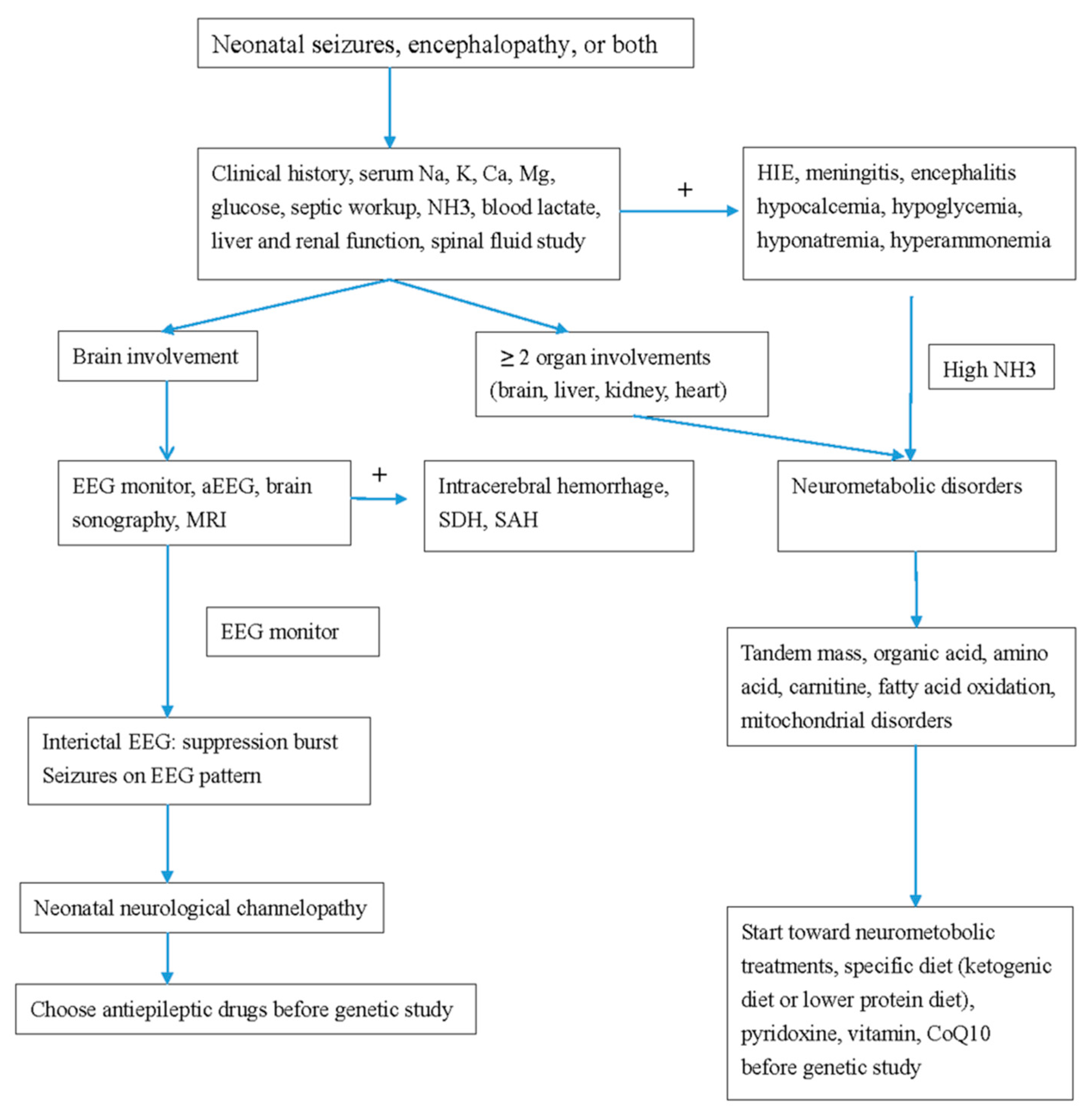
6. Study Supporting the Diagnosis
6.1. Lumbar Puncture
6.2. Brain MRI and/or CT Scan
6.3. Electroencephalography, aEEG and EEG Monitoring
7. Drugs Treatment
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surtees, R. Inherited ion channel disorders. Eur. J. Pediatrics 2000, 159 (Suppl. 3), S199–S203. [Google Scholar] [CrossRef]
- Gürsoy, S.; Erçal, D. Diagnostic Approach to Genetic Causes of Early-Onset Epileptic Encephalopathy. J. Child. Neurol. 2015, 31, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Gardella, E.; Marini, C.; Trivisano, M.; Fitzgerald, M.P.; Alber, M.; Howell, K.B.; Darra, F.; Siliquini, S.; Bölsterli, B.K.; Masnada, S.; et al. The phenotype of SCN8A developmental and epileptic encephalopathy. Neurology 2018, 91, e1112–e1124. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yang, D.; Kim, S.H.; Kim, B.; Kim, H.; Lee, J.; Choi, J.; Lee, S.-T.; Kang, H.-C. The phenotype and treatment of SCN2A-related developmental and epileptic encephalopathy. Epileptic Disord. 2020, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kothur, K.; Holman, K.; Farnsworth, E.; Ho, G.; Lorentzos, M.; Troedson, C.; Gupta, S.; Webster, R.; Procopis, P.G.; Menezes, M.P.; et al. Diagnostic yield of targeted massively parallel sequencing in children with epileptic encephalopathy. Seizure 2018, 59, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Malhotra, M.; Mankad, K.; Carney, O.; D’Arco, F.; Muthusamy, K.; Sudhakar, S.V. Clinico-radiological phenotyping and diagnostic pathways in childhood neurometabolic disorders-a practical introductory guide. Transl. Pediatrics 2021, 10, 1201–1230. [Google Scholar] [CrossRef] [PubMed]
- Weckhuysen, S.; Ivanovic, V.; Hendrickx, R.; Van Coster, R.; Hjalgrim, H.; Møller, R.S.; Grønborg, S.; Schoonjans, A.S.; Ceulemans, B.; Heavin, S.B.; et al. Extending the KCNQ2 encephalopathy spectrum: Clinical and neuroimaging findings in 17 patients. Neurology 2013, 81, 1697–1703. [Google Scholar] [CrossRef] [Green Version]
- Weckhuysen, S.; Mandelstam, S.; Suls, A.; Audenaert, D.; Deconinck, T.; Claes, L.R.; Deprez, L.; Smets, K.; Hristova, D.; Yordanova, I.; et al. KCNQ2 encephalopathy: Emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 2012, 71, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Weckhuysen, S.; Korff, C.M. Epilepsy: Old syndromes, new genes. Curr. Neurol. Neurosci. Rep. 2014, 14, 447. [Google Scholar] [CrossRef]
- Fang, Z.X.; Xie, L.L.; Yan, L.S.; Lin, H.; Pan, Y.N.; Liu, B.K.; Jiang, Y.; Cheng, M.; Li, X.J.; Jiang, L. Clinical and genetic characteristics of epilepsy of infancy with migrating focal seizures in Chinese children. Epilepsy Res. 2021, 174, 106669. [Google Scholar] [CrossRef]
- Liu, X.; Shen, Q.; Zheng, G.; Guo, H.; Lu, X.; Wang, X.; Yang, X.; Cao, Z.; Chen, J. Gene and Phenotype Expansion of Unexplained Early Infantile Epileptic Encephalopathy. Front. Neurol. 2021, 12, 633637. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Chen, Z.; Wang, L.; Tang, Z.; Zhang, J.; Miao, Q.; Zhai, Q. Mutations in the sodium channel genes SCN1A, SCN3A, and SCN9A in children with epilepsy with febrile seizures plus (EFS+). Seizure 2021, 88, 146–152. [Google Scholar] [CrossRef]
- Cornet, M.C.; Morabito, V.; Lederer, D.; Glass, H.C.; Ferrao Santos, S.; Numis, A.L.; Ferriero, D.M.; Sands, T.T.; Cilio, M.R. Neonatal presentation of genetic epilepsies: Early differentiation from acute provoked seizures. Epilepsia 2021, 62, 1907–1920. [Google Scholar] [CrossRef]
- Ibrahim, M.; Parmar, H.A.; Hoefling, N. Srinivasan A: Inborn errors of metabolism: Combining clinical and radiologic clues to solve the mystery. Am. J. Roentgenol. 2014, 203, W315–W327. [Google Scholar] [CrossRef]
- Falsaperla, R.; Vari, M.; Toldo, I.; Murgia, A.; Sartori, S.; Vecchi, M.; Suppiej, A.; Burlina, A.; Mastrangelo, M.; Leuzzi, V.; et al. Pyridoxine-dependent epilepsies: An observational study on clinical, diagnostic, therapeutic and prognostic features in a pediatric cohort. Metab. Brain Dis. 2018, 33, 261–269. [Google Scholar] [CrossRef]
- Saudubray, J.M.; Nassogne, M.C.; De Lonlay, P.; Touati, G. Clinical approach to inherited metabolic disorders in neonates: An overview. Semin. Neonatol. 2002, 7, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreedhara, M.; Balakrishnan, U.; Amboiram, P.; Chandrasekeran, A.; Thangaraj, A.; Shaik, M.S.; Devi, U.; Kumar, T. 3-Methylglutaconic aciduria type VIII in an Indian neonate. Birth Defects Res. 2020, 112, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Honzik, T.; Tesarova, M.; Magner, M.; Mayr, J.; Jesina, P.; Vesela, K.; Wenchich, L.; Szentivanyi, K.; Hansikova, H.; Sperl, W.; et al. Neonatal onset of mitochondrial disorders in 129 patients: Clinical and laboratory characteristics and a new approach to diagnosis. J. Inherit. Metab. Dis. 2012, 35, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.; Halliday, J.L.; Kirby, D.M.; Yaplito-Lee, J.; Thorburn, D.R.; Boneh, A. Mitochondrial oxidative phosphorylation disorders presenting in neonates: Clinical manifestations and enzymatic and molecular diagnoses. Pediatrics 2008, 122, 1003–1008. [Google Scholar] [CrossRef]
- Pronicka, E.; Piekutowska-Abramczuk, D.; Pronicki, M. Mitochondrial diseases in children including Leigh syndrome--biochemical and molecular background. Postepy Biochemii 2008, 54, 161–168. [Google Scholar] [PubMed]
- Fan, H.-C.; Lee, H.-F.; Chi, C.-S. SCN8A Encephalopathy: Case Report and Literature Review. Neurol. Int. 2021, 13, 143–150. [Google Scholar] [CrossRef]
- Lee, H.-F.; Chi, C.S.; Tsai, C.R. Diagnostic yield and treatment impact of whole-genome sequencing in paediatric neurological disorders. Dev. Med. Child Neurol. 2020, 63, 934–938. [Google Scholar] [CrossRef]
- Lee, I.C.; Hong, S.Y.; Weng, Y.H.; Chen, Y.T. Amplitude Integrated Electroencephalography and Continuous Electroencephalography Monitoring Is Crucial in High-Risk Infants and Their Findings Correlate with Neurodevelopmental Outcomes. Front. Pediatrics 2021, 9, 691764. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Yamagata, T.; Kubota, M.; Arai, H.; Yamashita, S.; Nakagawa, T.; Fujii, T.; Sugai, K.; Imai, K.; Uster, T.; et al. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia 2013, 54, 1282–1287. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Kwon, S. Early-onset epileptic encephalopathies and the diagnostic approach to underlying causes. Korean J. Pediatr. 2015, 58, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Hellström-Westas, L. Continuous electroencephalography monitoring of the preterm infant. Clin. Perinatol. 2006, 33, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Westas, L. Amplitude-integrated electroencephalography for seizure detection in newborn infants. Semin. Fetal Neonatal Med. 2018, 23, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Worden, L.T.; Chinappen, D.M.; Stoyell, S.M.; Gold, J.; Paixao, L.; Krishnamoorthy, K.; Kramer, M.A.; Westover, M.B.; Chu, C.J. The probability of seizures during continuous EEG monitoring in high-risk neonates. Epilepsia 2019, 60, 2508–2518. [Google Scholar] [CrossRef]
- Numis, A.L.; Angriman, M.; Sullivan, J.E.; Lewis, A.J.; Striano, P.; Nabbout, R.; Cilio, M.R. KCNQ2 encephalopathy: Delineation of the electroclinical phenotype and treatment response. Neurology 2014, 82, 368–370. [Google Scholar] [CrossRef] [Green Version]
- Ergene, M.; Yarar, N.; Öncel, E.P.; Sezer, T.; Çavdarlı, B.; Ecevit İ, Z.; Aydın, H. Severe isolated sulfide oxidase deficiency with a novel mutation. Turk. J. Pediatrics 2021, 63, 716–720. [Google Scholar] [CrossRef]
- Ohashi, T.; Akasaka, N.; Kobayashi, Y.; Magara, S.; Kawashima, H.; Matsumoto, N.; Saitsu, H.; Tohyama, J. Infantile epileptic encephalopathy with a hyperkinetic movement disorder and hand stereotypies associated with a novel SCN1A mutation. Epileptic Disord. Int. Epilepsy J. Videotape 2014, 16, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.Y.; Yang, J.J.; Li, S.Y.; Lee, I.C. A Wide Spectrum of Genetic Disorders Causing Severe Childhood Epilepsy in Taiwan: A Case Series of Ultrarare Genetic Cause and Novel Mutation Analysis in a Pilot Study. J. Pers. Med. 2020, 10, 281. [Google Scholar] [CrossRef]
- Engel, J., Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001, 42, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.B.; Freeman, J.L.; Mackay, M.T.; Fahey, M.C.; Archer, J.; Berkovic, S.F.; Chan, E.; Dabscheck, G.; Eggers, S.; Hayman, M.; et al. The severe epilepsy syndromes of infancy: A population-based study. Epilepsia 2021, 62, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Ohtahara, S.; Yamatogi, Y.; Ohtsuka, Y. Prognosis of the Lennox syndrome-long-term clinical and electroencephalographic follow-up study, especially with special reference to relationship with the West syndrome. Folia Psychiatr. Neurol. Jpn. 1976, 30, 275–287. [Google Scholar] [CrossRef]
- Thömke, F.; Brand, A.; Weilemann, S.L. The temporal dynamics of postanoxic burst-suppression EEG. J. Clin. Neurophysiol. 2002, 19, 24–31. [Google Scholar] [CrossRef]
- Nordli, D.R., Jr. Epileptic encephalopathies in infants and children. J. Clin. Neurophysiol. 2012, 29, 420–424. [Google Scholar] [CrossRef]
- Ohtahara, S.; Yamatogi, Y. Epileptic encephalopathies in early infancy with suppression-burst. J. Clin. Neurophysiol. 2003, 20, 398–407. [Google Scholar] [CrossRef]
- Beal, J.C.; Cherian, K.; Moshe, S.L. Early-onset epileptic encephalopathies: Ohtahara syndrome and early myoclonic encephalopathy. Pediatric Neurol. 2012, 47, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtahara, S.; Ohtsuka, Y.; Yamatogi, Y.; Oka, E. The early-infantile epileptic encephalopathy with suppression-burst: Developmental aspects. Brain Dev. 1987, 9, 371–376. [Google Scholar] [CrossRef]
- Reid, E.S.; Williams, H.; Stabej Ple, Q.; James, C.; Ocaka, L.; Bacchelli, C.; Footitt, E.J.; Boyd, S.; Cleary, M.A.; Mills, P.B.; et al. Seizures Due to a KCNQ2 Mutation: Treatment with Vitamin B6. JIMD Rep. 2016, 27, 79–84. [Google Scholar] [PubMed] [Green Version]
- Liu, Y.-H.; Cheng, Y.-T.; Tsai, M.-H.; Chou, I.J.; Hung, P.-C.; Hsieh, M.-Y.; Wang, Y.-S.; Chen, Y.-J.; Kuo, C.-Y.; Lin, J.-J.; et al. Genetics and clinical correlation of Dravet syndrome and its mimics–experience of a tertiary center in Taiwan. Pediatrics Neonatol. 2021, 62, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Trollmann, R. Neuromonitoring in Neonatal-Onset Epileptic Encephalopathies. Front. Neurol. 2021, 12, 623625. [Google Scholar] [CrossRef]
- Tao, J.D.; Mathur, A.M. Using amplitude-integrated EEG in neonatal intensive care. J. Perinatol. 2010, 30, S73–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, S.L.; Jensen, F.E.; Abend, N.S. Electroencephalographic monitoring for seizure identification and prognosis in term neonates. Semin. Fetal Neonatal Med. 2018, 23, 168–174. [Google Scholar] [CrossRef]
- Bustamante-Hervás, C.; Valverde, E.; Vega-Del-Val, C.; Schuffelmann, S.; Arnaez, J. Inter-observer reliability for amplitude-integrated EEG in the newborn with perinatal asphyxia. Anales de Pediatria 2021. [Google Scholar]
- De Vries, L.S.; Toet, M.C. How to assess the aEEG background. J. Pediatrics 2009, 154, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Shellhaas, R.A.; Gallagher, P.R.; Clancy, R.R. Assessment of neonatal electroencephalography (EEG) background by conventional and two amplitude-integrated EEG classification systems. J. Pediatrics 2008, 153, 369–374. [Google Scholar] [CrossRef]
- Al Naqeeb, N.; Edwards, A.D.; Cowan, F.M.; Azzopardi, D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics 1999, 103, 1263–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boerma, R.S.; Braun, K.P.; Van den Broek, M.P.; Van Berkestijn, F.M.; Swinkels, M.E.; Hagebeuk, E.O.; Lindhout, D.; Van Kempen, M.; Boon, M.; Nicolai, J.; et al. Remarkable Phenytoin Sensitivity in 4 Children with SCN8A-related Epilepsy: A Molecular Neuropharmacological Approach. Neurotherapeutics 2016, 13, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Møller, R.S.; Johannesen, K.M. Precision Medicine: SCN8A Encephalopathy Treated with Sodium Channel Blockers. Neurotherapeutics 2016, 13, 190–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melikishvili, G.; Dulac, O.; Gataullina, S. Neonatal SCN2A encephalopathy: A peculiar recognizable electroclinical sequence. Epilepsy Behav. 2020, 111, 107187. [Google Scholar] [CrossRef] [PubMed]
- Kuersten, M.; Tacke, M.; Gerstl, L.; Hoelz, H.; Stülpnagel, C.V.; Borggraefe, I. Antiepileptic therapy approaches in KCNQ2 related epilepsy: A systematic review. Eur. J. Med. Genet. 2020, 63, 103628. [Google Scholar] [CrossRef] [PubMed]
| Channelopathy | Neurometabolic Disorder | |
|---|---|---|
| Age of seizure onset | Newborn (1–2 weeks after birth) | Newborn (1–2 weeks after birth) |
| Brain involvement | Positive | Positive |
| Seizure frequencies | + to +++ | + to +++ |
| EEG | Unremarkable to severe suppression-burst | Unremarkable to severe suppression-burst |
| MRI at age of seizure onset | Unremarkable | Unremarkable |
| MRI at follow up after first seizure | Brain atrophy (if not treated) | Brain atrophy (if not treated) |
| Channelopathy | Neurometabolic Disorder | |
|---|---|---|
| Inheritance pattern | AD, de novo | AR or maternal inheritance |
| Organ involvement | Brain | Multiple organs |
| aEEG and EEG | ||
| Background | Better | Worse |
| Seizure on EEG | Initially fast activity, followed up with deta-theta spikes | Delta-theta waves or spikes |
| Seizure type | ||
| Tonic (general or focal) | +++ | + |
| Myoclonic | + | +++ |
| Initial MRI at first seizure | Unremarkable or mild ventriculomegaly | Varied |
| MRI at follow up | Brain atrophy | Variable depending on etiology |
| Treatment | ||
| Antiepileptic drugs | Na channel blockers (PHT, OXC) | Pyridoxine, Na channel blockers, non-Na channel blockers |
| Avoid drug | KEP (SCN8A) | VPA (mitochondrial) |
| Diet | Normal to ketogenic diet if uncontrolled seizures | Variable depending on etiology (ketogenic diet, lower protein diet, pyridoxine) |
| Outcomes in neurodevelopment | Unremarkable to severe | Moderate to severe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-C. Approach to Neurological Channelopathies and Neurometabolic Disorders in Newborns. Life 2021, 11, 1244. https://doi.org/10.3390/life11111244
Lee I-C. Approach to Neurological Channelopathies and Neurometabolic Disorders in Newborns. Life. 2021; 11(11):1244. https://doi.org/10.3390/life11111244
Chicago/Turabian StyleLee, Inn-Chi. 2021. "Approach to Neurological Channelopathies and Neurometabolic Disorders in Newborns" Life 11, no. 11: 1244. https://doi.org/10.3390/life11111244





