Mechanosynthesis and Thermoelectric Properties of Fe, Zn, and Cd-Doped P-Type Tetrahedrite: Cu12-xMxSb4S13
Abstract
:1. Introduction
2. Materials and Methods
Characterization
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
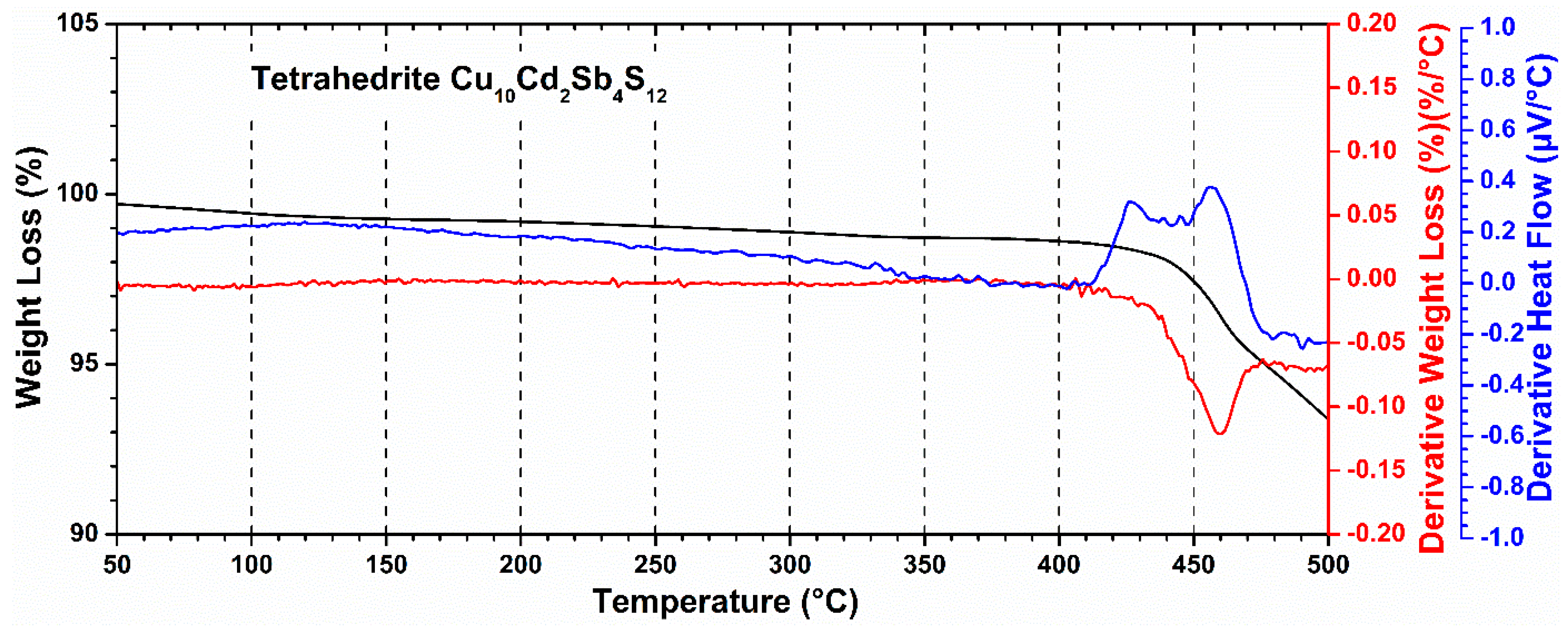
3.2. Thermal Analysis
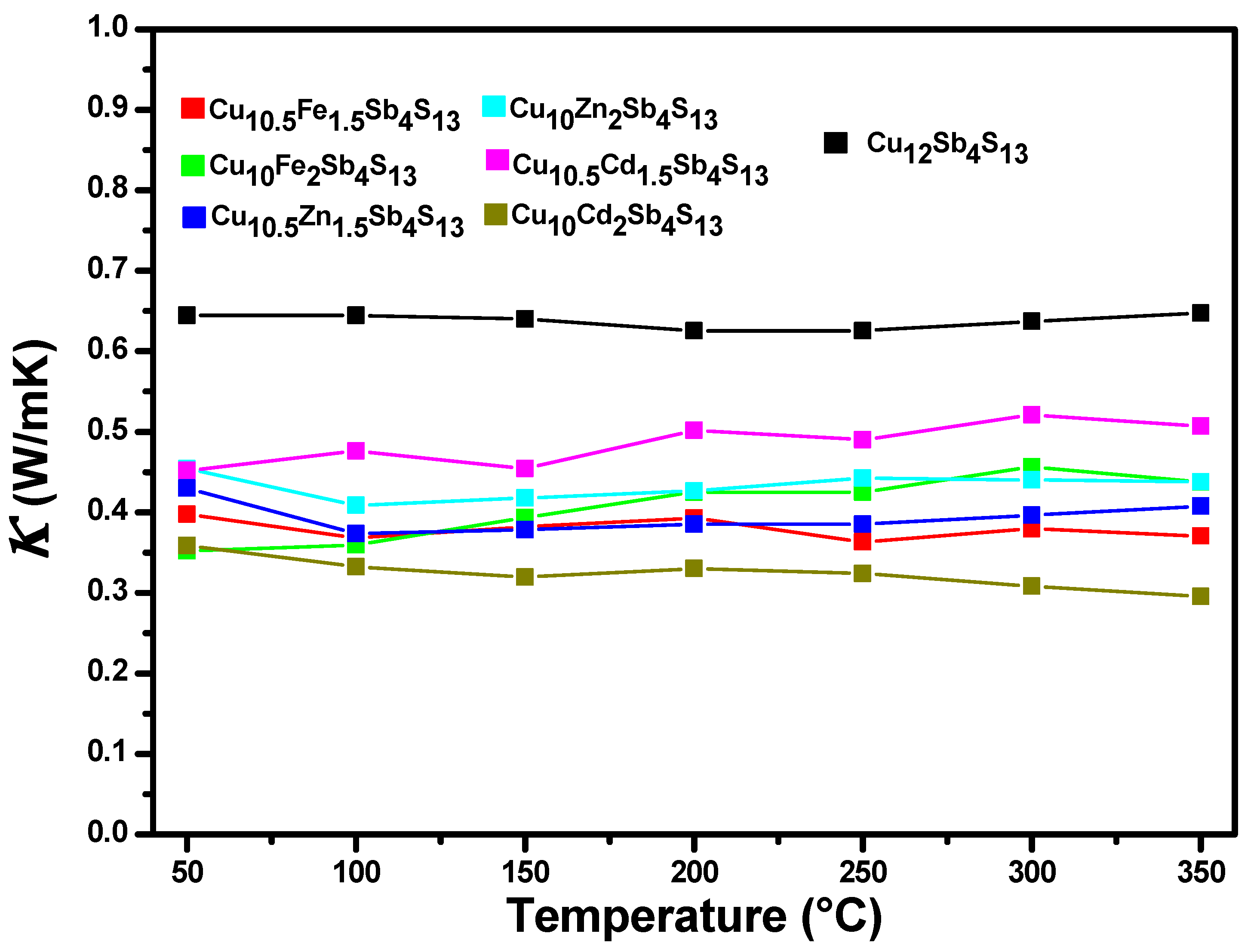
3.3. Thermal Conductivity
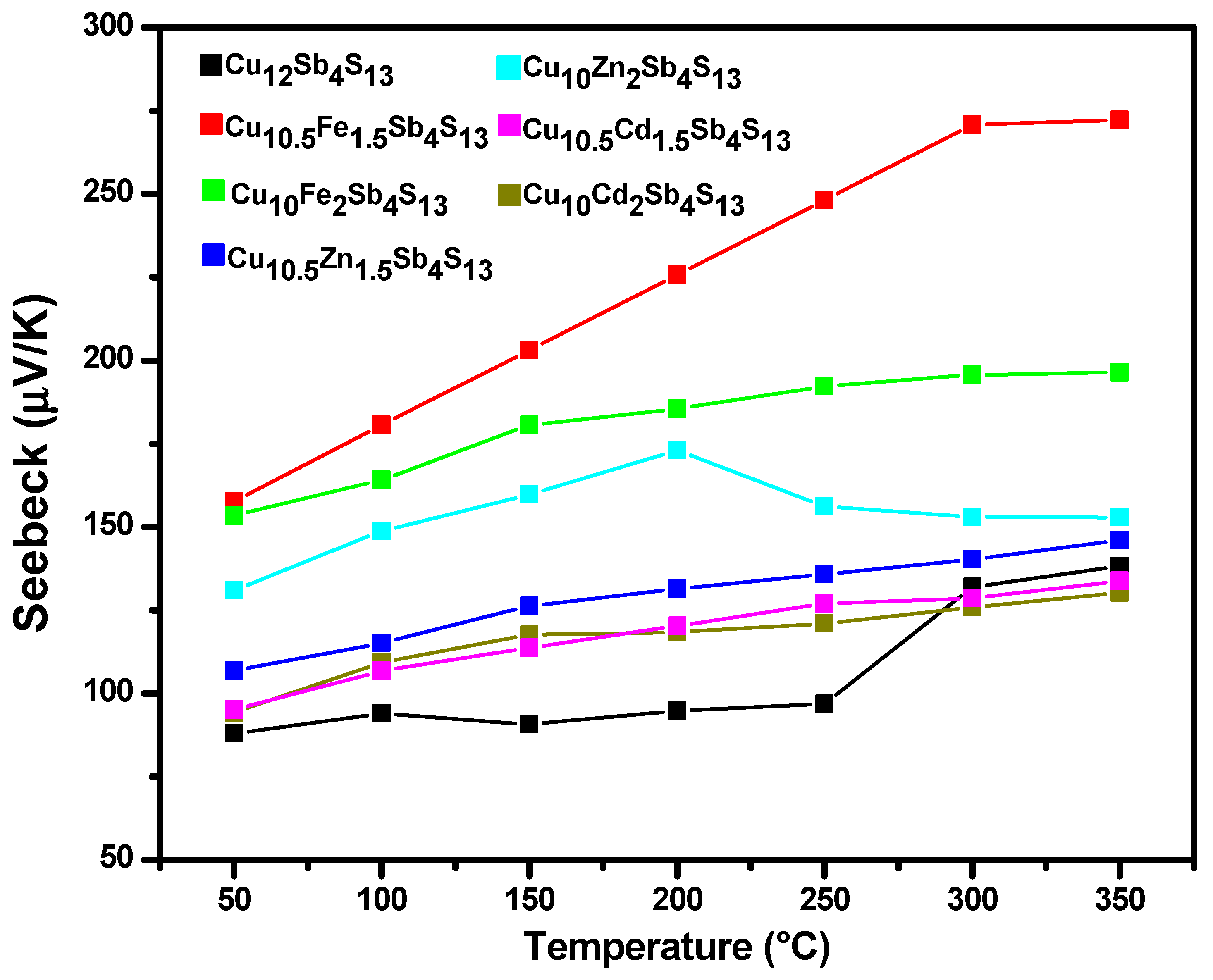
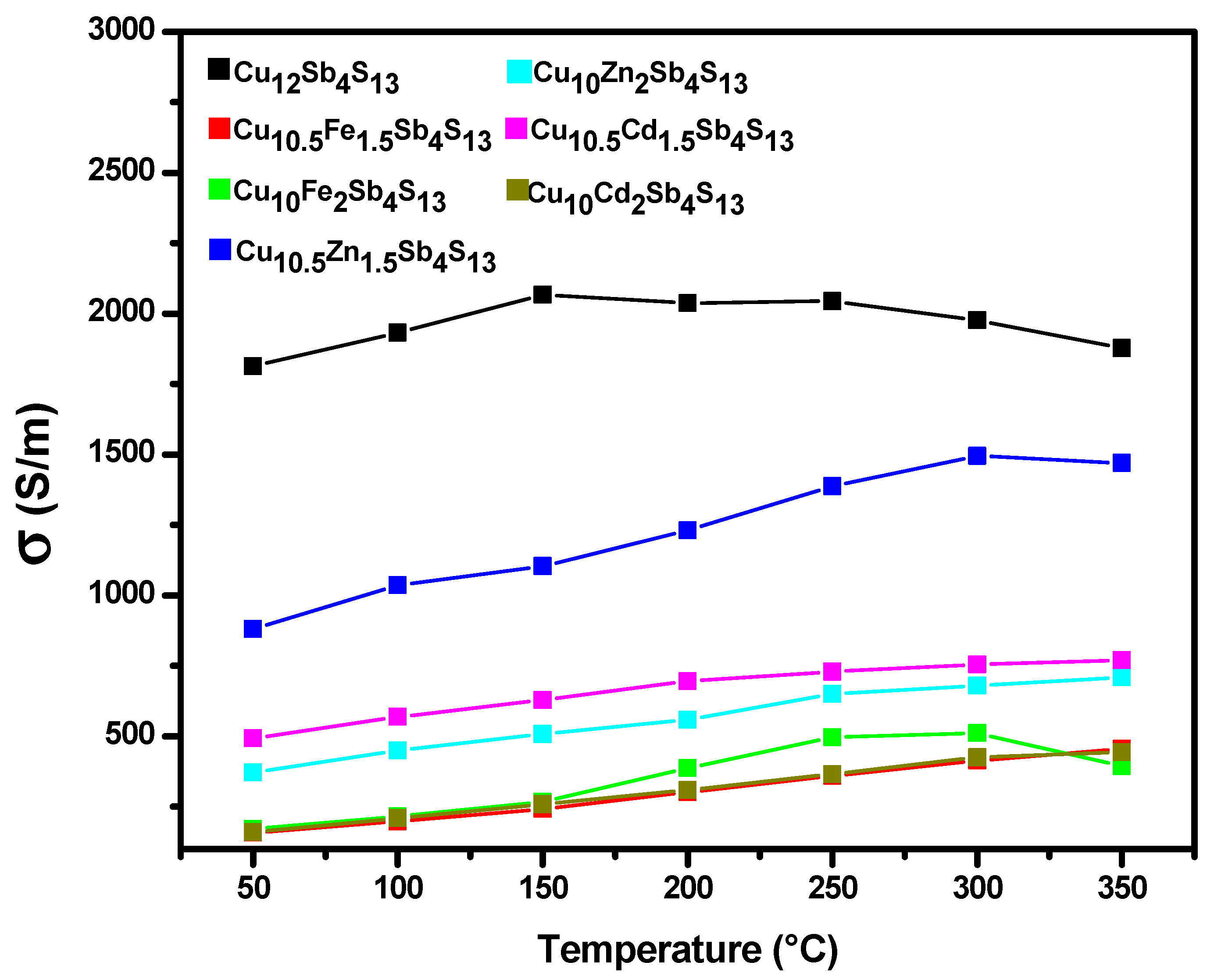
3.4. Power Factor
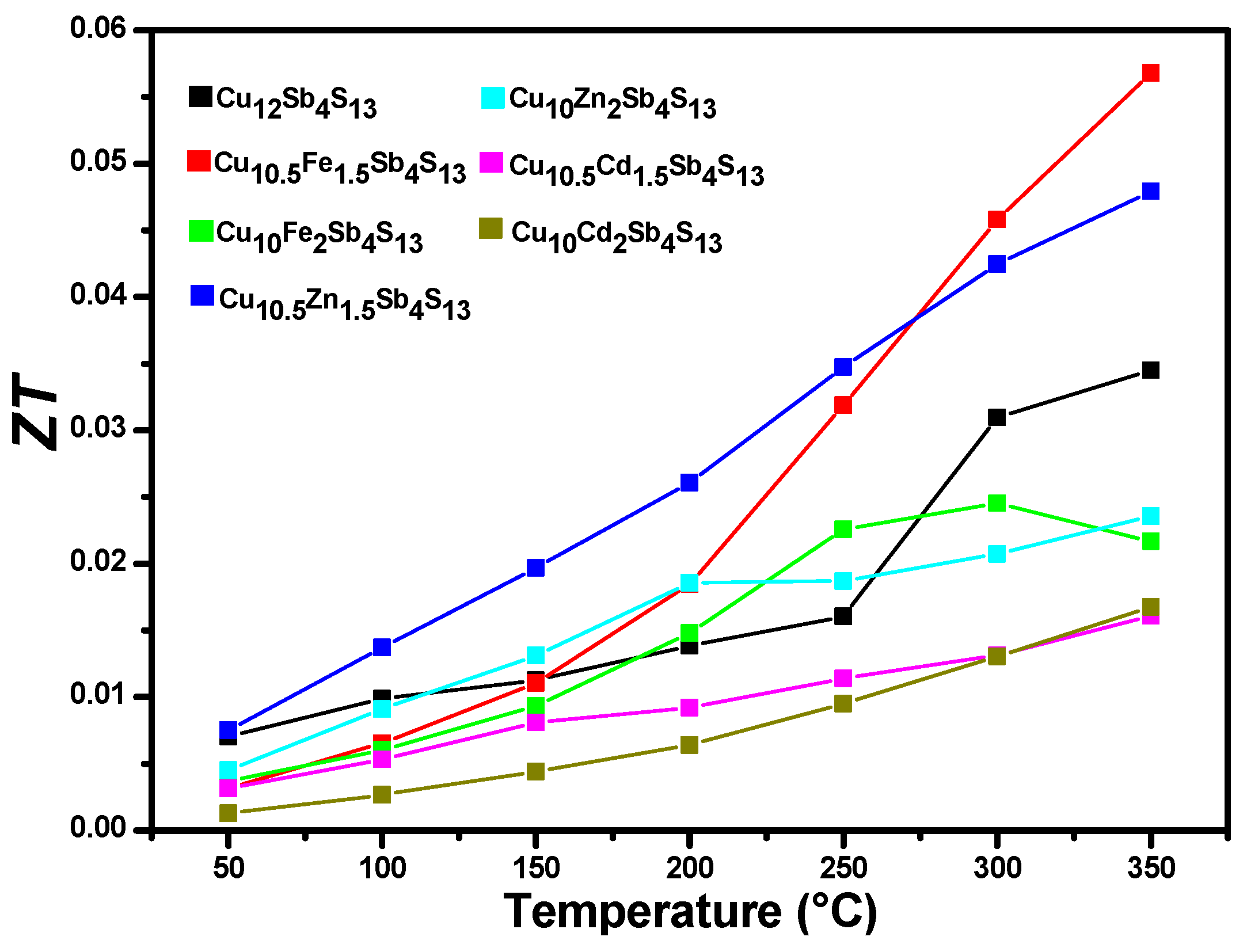
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, A.S.; Langari, R. Temperature measurement. In Measurement and Instrumentation: Theory and Applications, Chapter 14; Academy Press: San Diego, CA, USA, 2012; pp. 347–392. ISBN 978-0-12-381960-4. [Google Scholar]
- Janak, L.; Ancik, Z.; Vestika, J.; Hadas, Z. Thermoelectric Generator Based on MEMS Module as an Electric Powe Backup in Aerospace Applications. Mater. Today Proc. 2015, 2, 865–870. [Google Scholar] [CrossRef]
- Mahan, G.D.; Sofo, J.O. The best thermoelectric. Proc. Natl. Acad. Sci. USA 1996, 93, 7436–7439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishan, K.Y.; Bisht, N.; Hiragond, C.; Dey, A.; Khanna, P.K.; More, P.V. Room temperature thermoelectric performance of Methyl Ammonium Lead Iodide Perovskite and their MWCNT-PANI composites. Mater. Today Chem. 2020, 17, 100275. [Google Scholar] [CrossRef]
- Harizanova, S.G.; Zhecheva, E.N.; Valchev, V.D.; Khristov, M.G.; Stoyanova, R.K. Improving the thermoelectric efficiency of Co based ceramics. Mater. Today Proc. 2015, 2, 4256–4261. [Google Scholar] [CrossRef]
- Ramasamy, K.; Gupta, R.K.; Palchoudhury, S.; Ivanov, S.; Gupta, A. Layer-Structured Copper Antimony Chalcogenides (CuSbSexS2-x): Stable Electrode Materials for Supercapacitors. Chem. Mater. 2015, 27, 379–386. [Google Scholar] [CrossRef]
- Wu, Y.; Finefrock, S.W.; Yang, H. Nanostructured thermoelectric: Opportunities and challenges. Nano Energy 2012, 1, 651–653. [Google Scholar] [CrossRef]
- Zhao, D.; Tan, G. A review of thermoelectric cooling: Materials, modeling and applications. Appl. Therm. Eng. 2014, 66, 15–24. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Agawane, G.L.; Shin, S.W.; Yang, H.S.; Patil, P.S.; Kim, J.Y.; Kim, J.H. Non-vacuum mechanochemical route to the synthesis of Cu2SnS3 nano-ink for solar cell applications. Acta Mater. 2015, 85, 314–321. [Google Scholar] [CrossRef]
- Su, H.; Xie, Y.; Wan, S.; Bin, B.; Qian, Y. A novel one-step solvothermal route to nanocrystalline CuSbS2 and Ag3SbS3. Solid State Ion. 1999, 123, 319–324. [Google Scholar] [CrossRef]
- Zou, Y.; Jiang, J. Colloidal synthesis of chalcostibite copper antimony sulfide nanocrystals. Mater. Lett. 2014, 123, 66–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, Z.; Chen, X. Microwave-Assisted Elemental-Direct-Reaction route to Nanocrystalline Copper sulfides Cu9S8 and Cu7S6. J. Solid Satate Chem. 2002, 253, 249–253. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Baláž, M.; Zorkovská, A.; Urakaev, F.; Baláž, P.; Briančin, J.; Bujňáková, Z.; Achimovičová, M.; Gock, E. Ultrafast mechanochemical synthesis of copper sulfides. RSC Adv. 2016, 6, 87836–87842. [Google Scholar] [CrossRef]
- Baláž, P.; Guilmeau, E.; Daneau, N.; Dobrozhan, O.; Baláž, M.; Hegedus, M.; Barbier, T.; Achimovicová, M.; Briancin, J. Tetrahedrites synthesized via scalable mechanochemical process and spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 1922–1930. [Google Scholar] [CrossRef]
- Barbier, T.; Rollin-Martinet, S.; Lemoine, P.; Gascoin, F.; Kaltzolou, A.; Vaqueiro, P.; Powell, A.V.; Guikmeau, E. Thermoelectric Material: A New Rapid Synthesis Process for Nontoxic and High-Performance Tetrahedrite Compound. J. Am. Ceram. Soc. 2015, 6, 36801. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–84. [Google Scholar]
- Lu, X.; Morelli, D.T. Rapid synthesis of high-performance thermoelectric materials directly from natural mineral tetrahedrite. MRS Commun. 2013, 3, 129–133. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Le Bail, A. Whole powder pattern decomposition methods and applications: A retrospection. Powder Diffr. 2005, 20, 316–326. [Google Scholar] [CrossRef] [Green Version]
- NIST Inorganic Crystal Structure Database, 2012 Version; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Makovicky, E. Crystal structures of sulfides and other chalcogenides. Rev. Miner. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Chetty, R.; Bali, A.; Mallik, R.C. Tetrahedrites as thermoelectric materials: An overview. J. Mater. Chem. 2015, 3, 12364–12378. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.; Chen, W.; Chen, Q.; Zhou, P.; Liu, Y. A green synthesis route for the phase and size tunability of copper antimony sulfide. Nanoscale 2016, 8, 5146–5152. [Google Scholar] [CrossRef]
- Gonzales, A.P.; Lopes, E.B.; Villeroy, B.; Monnier, J.; Godart, C.; Lenoir, B. Effect of Ni, Bi and Se on the tetrahedrite formation. RSC Adv. 2016, 6, 102359–102367. [Google Scholar]
- Goto, Y.; Sakai, Y.; Kamihara, Y.; Matoba, M. Effect of Sn-Substitution on Thermoelectric Properties of Copper-Based sulfide, Famatinite Cu3SbS4. J. Phys. Soc. Jpn. 2015, 84, 2015. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.S.P.; Chetty, R.; Rogl, P.; Rogl, G.; Baer, E.; Malar, P.; Mallik, R.C. Thermoelectric properties of Cd doped tetrahedrite: Cu12-xCdxSb4S13. Intermetallics 2016, 78, 21–29. [Google Scholar] [CrossRef]
- Levinsky, P.; Vaney, J.B.; Candolfi, C.; Dauscher, A.; Lenoir, B.; Hejtmanek, J. Electrical, Thermal, and Magnetic Characterization of Natural Tetrahedrites-Tennantites of Different Origin. J. Electron. Mater. 2015, 45, 1351–1357. [Google Scholar] [CrossRef]
- Weller, D.P.; Morelli, D.T. Rapid synthesis of zinc and nickel co-doped tetrahedrite thermoelectrics by reactive spark plasma sintering and mechanical alloying. J. Alloys Compd. 2017, 710, 794–799. [Google Scholar] [CrossRef]
- Nasonova, D.; Verchenko VYu Tsirlin, A.A.; Shevelkov, A.V. Low temperature structure and thermoelectric properties of pristine tetrahedrite Cu12Sb4S13. Chem. Mater. 2016, 28, 6621–6627. [Google Scholar] [CrossRef]
- Wang, J.; Gu, M.; Bao, Y.; Li, X.; Chen, L. Quick fabrication and thermoelectric properties of tetrahedrite. J. Electron. Mater. 2016, 45, 2274–2277. [Google Scholar] [CrossRef]
- Sun, F.-H.; Wu, C.-F.; Li, Z.; Pan, Y.; Asfandiyar Dong, J.; Li, J.-F. Powder metallurgically synthesized tetrahedrites: Phase transition and high thermoelectricity. RSC Adv. 2017, 7, 18909–18916. [Google Scholar] [CrossRef] [Green Version]
- Suekuni, K.; Tsuruta, K.; Ariga, T.; Koyano, M. Thermoelectric properties of mineral tetrahedrites Cu10Tr2Sb4S13 with low thermal conductivity. Appl. Phys. Express 2012, 5, 051201. [Google Scholar] [CrossRef]
- Guler, A.; Ballikaya, S.; Boyraz, C.; Okay, C.; Shulgin, D.; Rameev, B. Thermoelectric properties and EPR analysis of Fe doped Cu12Sb4S13. J. Solid State Chem. 2019, 269, 547–552. [Google Scholar] [CrossRef]
- Lu, X.; Morelli, D.T.; Xia, Y.; Zhou, F.; Ozolins, V.; Chi, H.; Zhou, X.; Uher, C. High performance thermoelectricity in earth-abundant compounds based on natural mineral tetrahedrites. Adv. Energy Mater. 2013, 3, 342–348. [Google Scholar] [CrossRef]
- Harish, S.; Sivaprahasam, D.; Battabyal, M.; Gopalan, R. Phase stability and thermoelectric properties of Cu10.5Zn1.5Sb4S13 tetrahedrite. J. Alloys Compd. 2016, 667, 323–328. [Google Scholar] [CrossRef]

| Compound | Cu10.5Cd1.5Sb4S13 | Cu10Cd2Sb4S13 | Cu10.5Zn1.5Sb4S13 | Cu10Zn2Sb4S13 | Cu10.5Fe1.5Sb4S13 | Cu10Fe2Sb4S13 |
|---|---|---|---|---|---|---|
| ICSD | 84571 | |||||
| Unit Cell | ||||||
| Space group | I-43m | I-43m | I-43m | I-43m | I-43m | I-43m |
| Cell parameters (Å) | a = 10.4627(10) | a = 10.4870(9) | a = 10.3540(9) | a = 10.3667(9) | a = 10.3562(13) | a = 10.3662(12) |
| V(Å3) | 1145.33(2) | 1153.33(2) | 1110.01(2) | 1114.09(2) | 1110.71(2) | 1113.93(2) |
| Z | 2 | 2 | 2 | 2 | 2 | 2 |
| Refinement | ||||||
| Number of reflections | 51 | 51 | 51 | 51 | 51 | 51 |
| Number of refined parameters | ||||||
| Structural | 9 | 9 | 9 | 9 | 9 | 9 |
| Profile | 8 | 8 | 8 | 8 | 8 | 8 |
| Rexp | 1.85 | 1.87 | 1.88 | 1.9 | 1.86 | 1.96 |
| Rwp | 3.98 | 3.81 | 3.84 | 3.92 | 3.68 | 3.7 |
| RB | 2.33 | 2.26 | 2.22 | 2.23 | 2.1 | 2.13 |
| S | 2.15 | 2.04 | 2.04 | 2.06 | 1.97 | 1.89 |
| Tetrahedrites | Cp J/kg K | |
|---|---|---|
| Pristine | Cu12Sb4S13 | 515.99 |
| Mono-substituted | Cu10.5Fe1.5Sb4S13 | 524.29 |
| Cu10Fe2Sb4S13 | 567.043 | |
| Cu10.5Zn1.5Sb4S13 | 548.63 | |
| Cu10Zn2Sb4S13 | 522.67 | |
| Cu10.5Cd1.5Sb4S13 | 559.22 | |
| Cu10Cd2Sb4S13 | 495.12 |
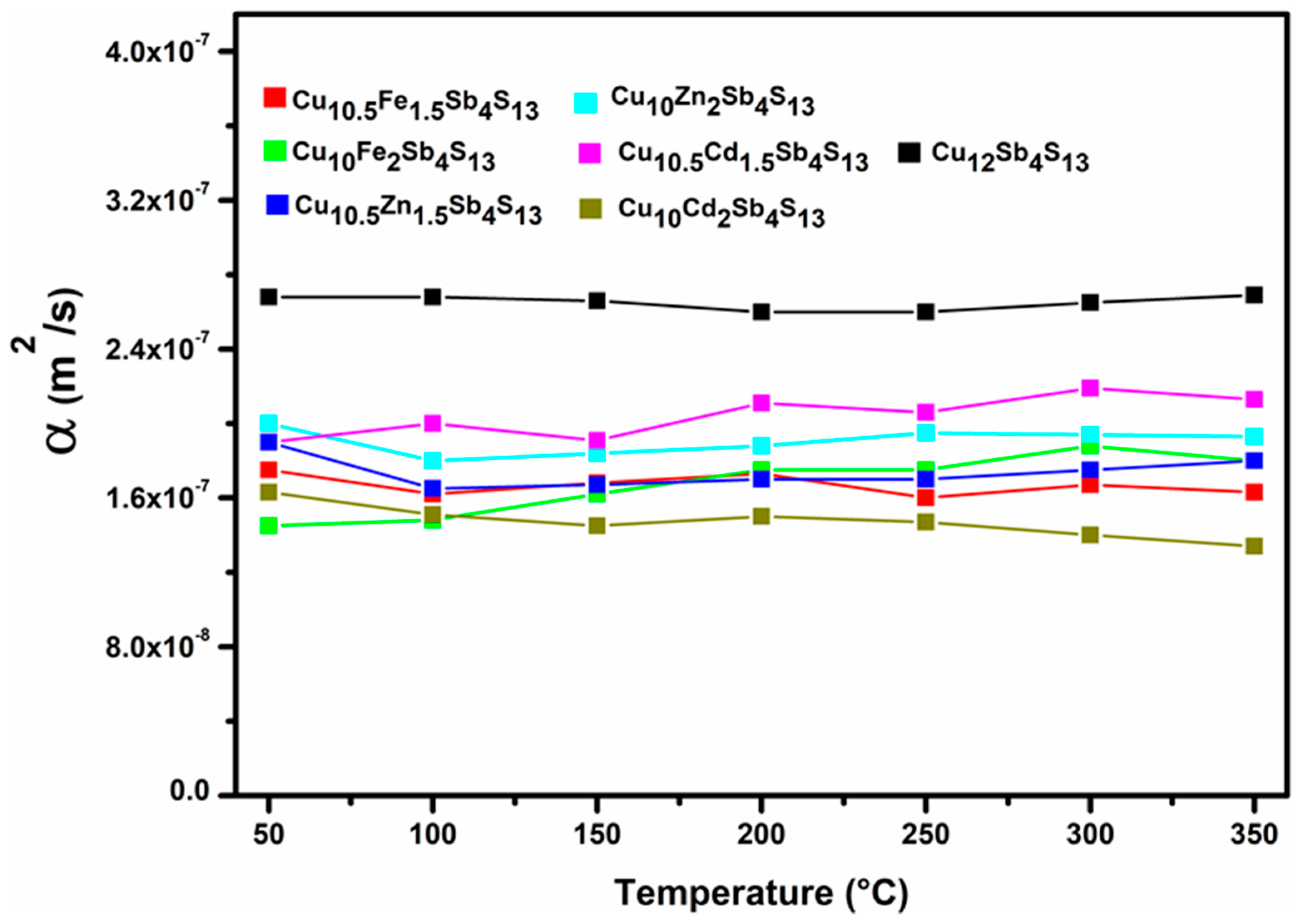
| Tetrahedrites | ρ (kg/m3) | α (m2/s) | κ (W/m K) | S (µV/K) | σ (S) | zT | |
|---|---|---|---|---|---|---|---|
| Pristine | Cu12Sb4S13 | 4662.162 | 2.69 × 10−7 | 0.647 | 1.91 × 10−8 | 1876.89 | 0.035 |
| Mono-substituted | Cu10Fe1.5Cu0.5Sb4S13 | 4333.333 | 1.63 × 10−7 | 0.370 | 7.41 × 10−8 | 455.52 | 0.057 |
| Cu10Fe2Sb4S13 | 4283.582 | 1.80 × 10−7 | 0.437 | 3.86 × 10−8 | 394.32 | 0.022 | |
| Cu10Zn1.5Cu0.5Sb4S13 | 4127.45 | 1.80 × 10−7 | 0.408 | 2.13 × 10−8 | 1469.59 | 0.048 | |
| Cu10Zn2Sb4S13 | 4342.857 | 1.93 × 10−7 | 0.438 | 2.34 × 10−8 | 708.66 | 0.024 | |
| Cu10Cd1.5Cu0.5Sb4S13 | 4255.319 | 2.13 × 10−7 | 0.507 | 1.70 × 10−8 | 769.99 | 0.016 | |
| Cu10Cd2Sb4S13 | 4448.275 | 1.34 × 10−7 | 0.295 | 1.79 × 10−8 | 443.26 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Cota, F.A.; Díaz-Guillén, J.A.; Juan Dura, O.; López de la Torre, M.A.; Rodríguez-Hernández, J.; Fernández Fuentes, A. Mechanosynthesis and Thermoelectric Properties of Fe, Zn, and Cd-Doped P-Type Tetrahedrite: Cu12-xMxSb4S13. Materials 2021, 14, 3448. https://doi.org/10.3390/ma14133448
López Cota FA, Díaz-Guillén JA, Juan Dura O, López de la Torre MA, Rodríguez-Hernández J, Fernández Fuentes A. Mechanosynthesis and Thermoelectric Properties of Fe, Zn, and Cd-Doped P-Type Tetrahedrite: Cu12-xMxSb4S13. Materials. 2021; 14(13):3448. https://doi.org/10.3390/ma14133448
Chicago/Turabian StyleLópez Cota, Francisco Arturo, José Alonso Díaz-Guillén, Oscar Juan Dura, Marco Antonio López de la Torre, Joelis Rodríguez-Hernández, and Antonio Fernández Fuentes. 2021. "Mechanosynthesis and Thermoelectric Properties of Fe, Zn, and Cd-Doped P-Type Tetrahedrite: Cu12-xMxSb4S13" Materials 14, no. 13: 3448. https://doi.org/10.3390/ma14133448







