Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation
Abstract
:1. Introduction
- (i)
- The mobility of potentially toxic metals in homogeneous (model) clays devoid of organic matter, or in bentonites, was evaluated. Studies on source clays will facilitate the interpretation of the processes responsible for the mobility of potentially toxic metal ions so that the results can be more easily related to natural conditions. In addition, it may not be possible to identify the mechanisms governing sorption in systems with many metals in the clay [46];
- (ii)
- The relationships between the mobility of potentially toxic metals and the physicochemical properties of soils can be interpreted. The authors emphasized that this is especially important in clay soils, where a significant portion of the metals may be bound to the clay surface. Li et al. [2] emphasized that the interactions between potentially toxic metal ions and clay minerals require further research. Ma et al. [19] emphasized that understanding the mechanisms and suitability of different types of clay minerals in immobilizing toxic metals is crucial to achieving remediation goals;
- (iii)
- The mobility of toxic metals, in the context of their physicochemical properties at subzero temperatures, was evaluated. Understanding the interactions of these processes under freezing conditions is important because the concentrations of mineral salts and other substances are highest in very-cold winters [45]. Because water can be a carrier for the migration of potentially toxic metals in cold regions, it is important to know the relationship between these factors to take appropriate steps to minimize the risk. It is particularly important to understand the relationships between the chemical fractions of metals that determine their mobility and changes in unfrozen water content. To date, no such studies have been conducted.
2. Materials and Methods
2.1. Materials
- (i)
- CH3COOH 0.11 mol/dm3 (CAS No. 64-19-7);
- (ii)
- NH2OH·HCl 0.5 mol/dm3 (CAS No. 5470-11-1);
- (iii)
- H2O2/CH3COONH4 8.8 mol/dm3 (CAS Nos. 7722-84-1 and 631-61-8);
- (iv)
- HNO3 and HCl 1 mol/dm3 (CAS Nos. 7697-37-2 and 7647-01-0).
Preparation of Homoionic Forms
2.2. Methods
2.2.1. Determination of the Content of Potentially Toxic Metals
2.2.2. Determination of the Physicochemical Properties
2.2.3. Determination of the Changes in Unfrozen Water Content
2.2.4. Data Quality Control
2.3. Statistical Treatments
3. Results and Discussion
3.1. The Properties of Bentonites
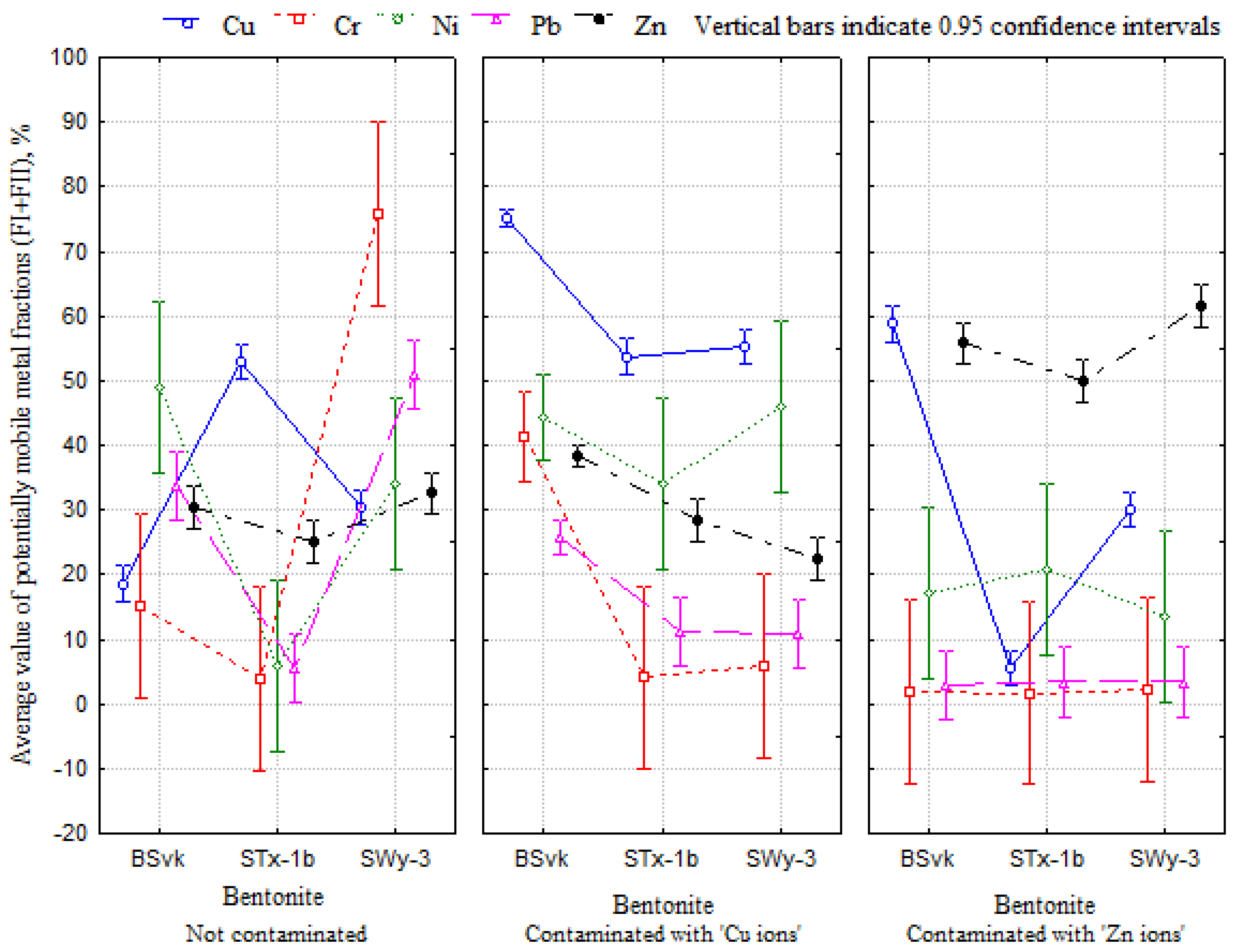
3.2. Effect of Bentonite Origin and Main Exchangeable Cation Cu or Zn on Mobility of Potentially Toxic Metals
3.3. The Effect of the Total Concentration of Zn or Cu on the Proportion of These Metals in Mobile and Non-Mobile Fractions
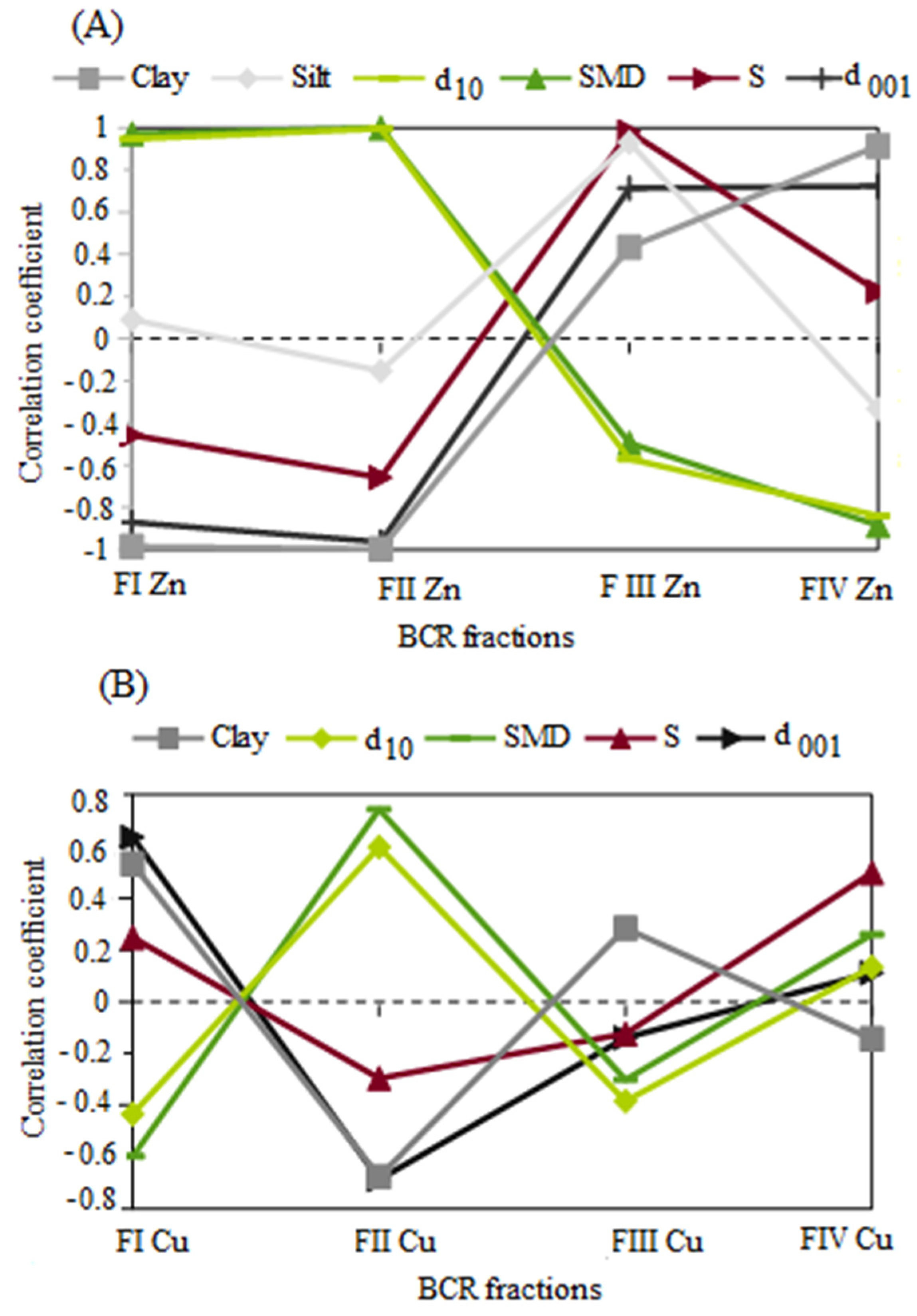
3.4. The Relationship between the Physicochemical Properties of Bentonites and the Mobility of Potentially Toxic Metals
The Impact of the Predominant Exchangeable Cation in Bentonites, Whether Zinc or Copper
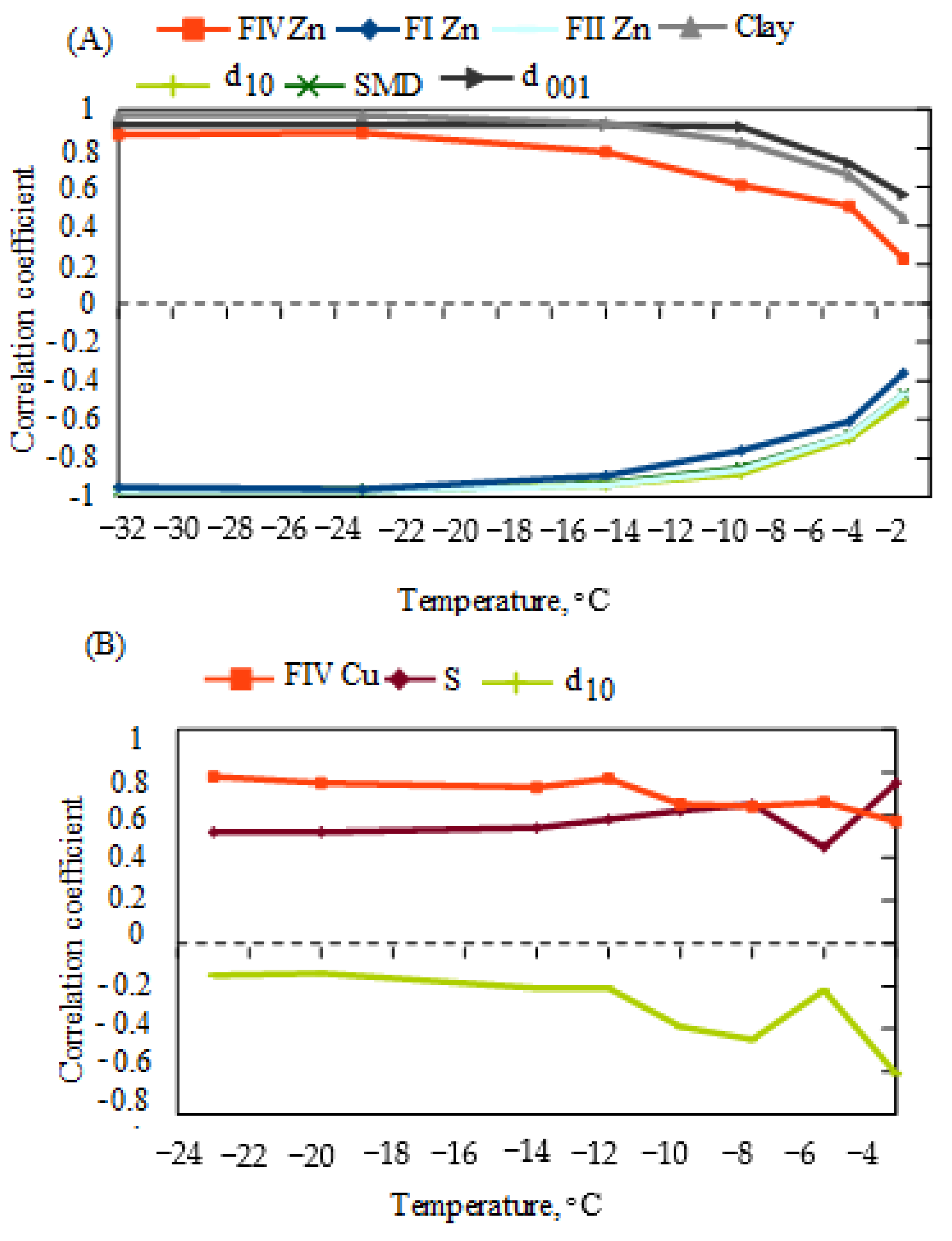
3.5. Mobility of Zn or Cu at Freezing Temperatures
4. Conclusions
- The mobility of potentially toxic metals such as Cu, Zn, Cr, Ni, and Pb in bentonites is primarily determined by the type of main exchangeable cation, whether Zn or Cu, its total concentration, and, to a lesser extent, the origin of the bentonite. Further studies on bentonites containing Cr, Ni, and Pb as the main exchange cations are necessary to assess their mobility;
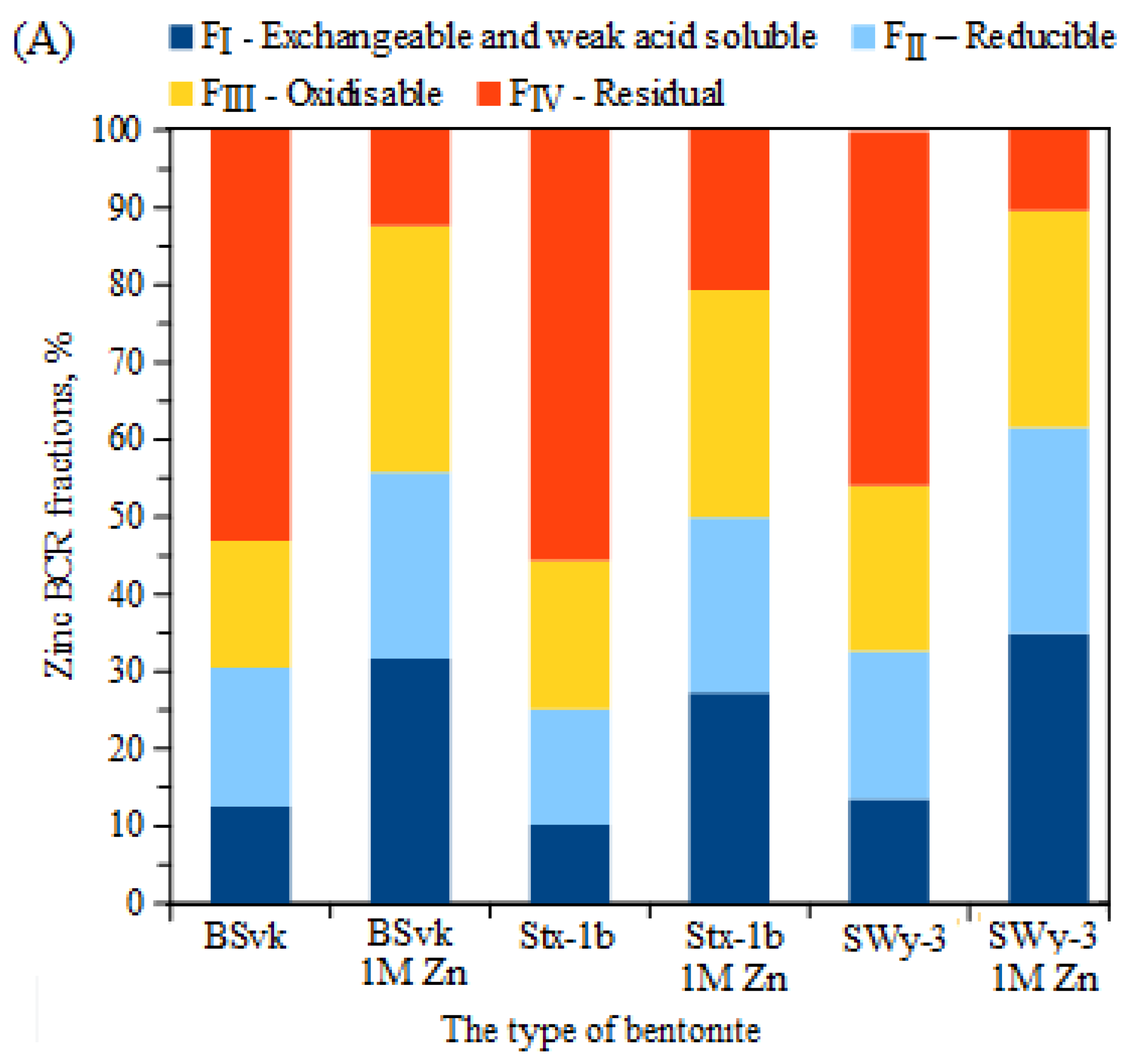
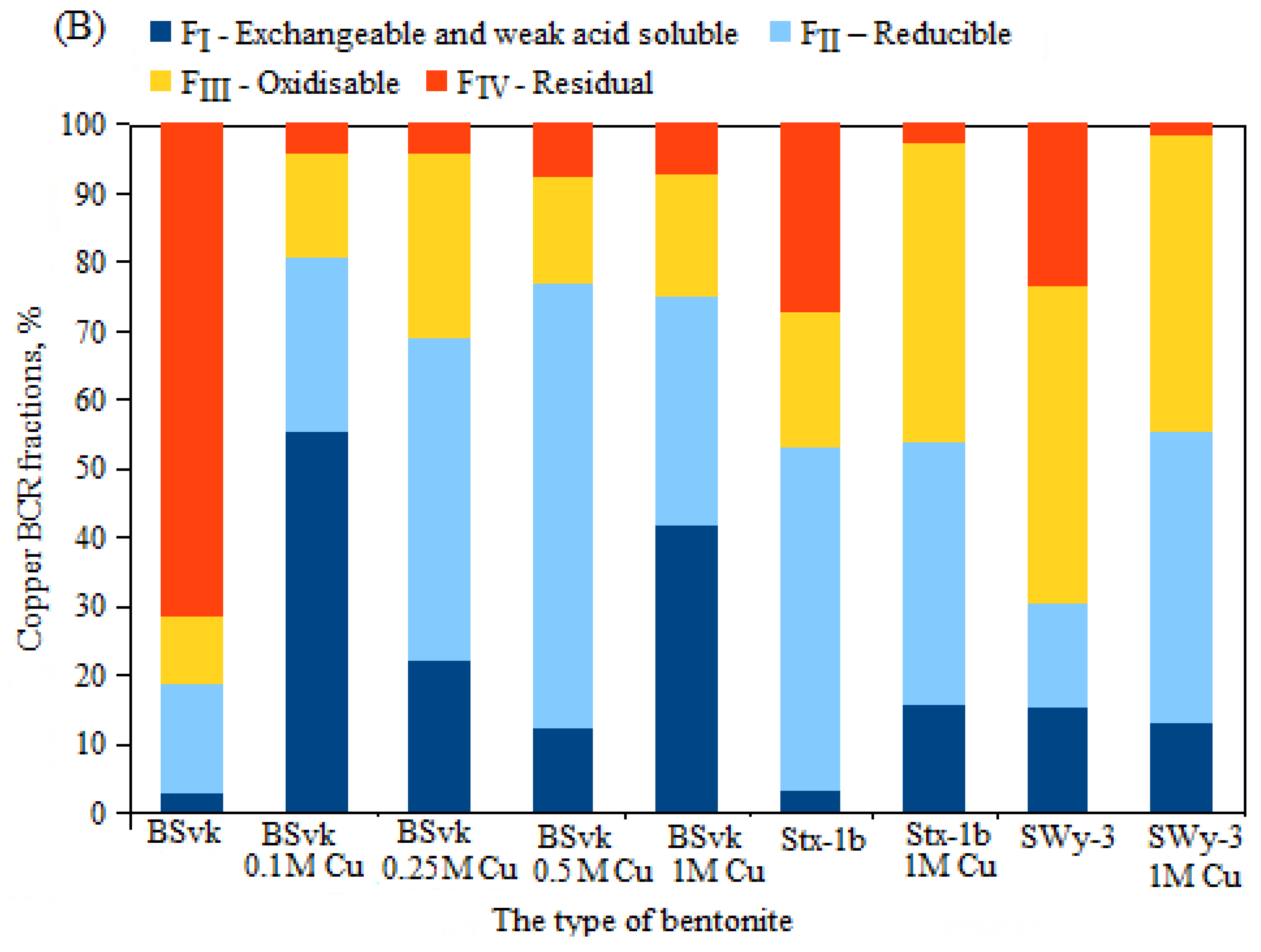
- High total concentrations of Zn ions in Zn bentonites led to increased mobility of Zn ions while simultaneously decreasing the proportion of stable fractions. Conversely, in Cu bentonites, increasing Cu concentrations resulted in decreased Cu mobility in stage 1 of the BCR method, likely due to the binding of Cu ions to the mineral phase of atacamite;
- There is a statistically significant relationship (p < 0.05) between Zn or Cu mobility and the structural and physicochemical properties of bentonites. The proportion of Zn mobile fractions (FIZn) increased with increasing effective diameter, decreasing clay fraction content, and interpacket distances, while the proportion of Cu mobile fractions (FICu) increased with decreasing effective diameter and increasing clay fraction content and interpacket distances. Zn ions likely accumulate in larger soil pores, while Cu ions are immobilized in the sorption complex of bentonite;
- The stability of potentially toxic Zn or Cu metals in bentonites increases with increasing clay fraction content or, respectively, with increasing specific surface area;
- It is possible that, at subzero temperatures, unfrozen water forms a concentrated solution of Zn or Cu, which may pose a threat to the groundwater environment, especially in cold regions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Metal | Total [mg/kg] Dry of Soil * | FI | FII | FIII | FIV | Comments | Potentially Mobile Factions [%] | Non Mobile Factions [%] | Mf | Ir |
|---|---|---|---|---|---|---|---|---|---|---|
| [%] | ||||||||||
| BSvk | ||||||||||
| Cu | 6.28 ± 0.06 | 2.8 | 15.8 | 9.7 | 71.7 | FIV > FII > FIII > FI | 18.6 | 81.4 | 2.8 | 0.81 |
| Cr | 10.09 ± 0.11 | 1.9 | 13.3 | 21.9 | 62.9 | FIV > FIII > FII > FI | 15.2 | 84.8 | 1.9 | 0.79 |
| Ni | 7.22 ± 0.21 | 3.6 | 45.4 | 36.9 | 14.1 | FII > FIII > FIV > FI | 49 | 51 | 3.6 | 0.46 |
| Pb | 17.02 ± 0.01 | 9.2 | 24.5 | 27.1 | 39.2 | FIV > FIII > FII > FI | 33.7 | 66.3 | 9.2 | 0.61 |
| Zn | 64.54 ± 0.69 | 12.5 | 18.0 | 16.4 | 53.1 | FIV > FII > FIII > FI | 30.5 | 69.5 | 12.5 | 0.68 |
| STx-1b | ||||||||||
| Cu | 8.97 ± 0.13 | 3.3 | 49.7 | 19.7 | 27.3 | FII > FIV > FIII > FI | 53 | 47 | 3.3 | 0.51 |
| Cr | 13.95 ± 0.34 | 0.1 | 3.7 | 52.3 | 42.9 | FIII > FIV > FII > FI | 3.8 | 96.2 | 0.1 | 0.73 |
| Ni | 7.57 ± 0.14 | 2.9 | 2.9 | 79.9 | 14.3 | FIII > FIV > FII > FI | 5.8 | 94.2 | 2.9 | 0.6 |
| Pb | 2.59 ± 0.04 | 0.2 | 5.3 | 37.9 | 56.6 | FIV > FIII > FII > FI | 5.5 | 94.5 | 0.2 | 0.79 |
| Zn | 73.68 ± 0.27 | 10.2 | 14.9 | 19.3 | 55.6 | FIV > FIII > FII > FI | 25.1 | 74.9 | 10.2 | 0.71 |
| SWy-3 | ||||||||||
| Cu | 12.80 ± 0.15 | 15.1 | 15.3 | 45.9 | 23.7 | FIII > FIV > FII > FI | 30.4 | 69.6 | 15.1 | 0.54 |
| Cr | 11.24 ± 0.13 | 75.7 | 1 | 13 | 10.3 | FI > FIV > FIII > FII | 75.8 | 24.2 | 75.7 | 0.23 |
| Ni | 7.27 ± 0.10 | 29 | 5.1 | 54.3 | 11.6 | FIII > FI > FIV > FII | 34.1 | 65.9 | 29 | 0.45 |
| Pb | 26.01 ± 0.05 | 37.2 | 13.7 | 39 | 10.1 | FIII ≥ FI > FII > FIV | 50.9 | 49.1 | 37.2 | 0.38 |
| Zn | 163.66 ± 1.50 | 13.5 | 19.1 | 21.4 | 45.9 | FIV > FIII > FII > FI | 32.6 | 67.4 | 13.5 | 0.64 |
| Metal | Total [mg/kg] Dry of Soil * | FI | FII | FIII | FIV | Comments | Potentially/Mobile Factions | Non Mobile Factions | Mf | Ir |
|---|---|---|---|---|---|---|---|---|---|---|
| [%] | ||||||||||
| BSvk after saturation of 1 M ZnCl2 & (BSvk 1 M Zn) | ||||||||||
| Cu | 39.55 ± 0.25 | 8 | 50.8 | 22.7 | 18.5 | FII > FIII > FIV > FI | 58.8 | 41.2 | 8 | 0.44 |
| Cr | 87.25 ± 0.73 | 1.7 | 0.2 | 77.8 | 20.3 | FIII > FIV > FI > FII | 1.9 | 89.1 | 1.7 | 0.64 |
| Ni | 24.20 ± 0.21 | 10.6 | 6.5 | 54.3 | 28.6 | FIII > FIV > FI > FII | 17.1 | 86.9 | 10.6 | 0.61 |
| Pb | 17.50 ± 0.17 | 1.2 | 1.8 | 22.2 | 74.8 | FIV > FIII > FII > FI | 3 | 97 | 1.2 | 0.88 |
| Zn | 17,857 ± 89 | 31.7 | 24.1 | 31.8 | 12.4 | FIII ≥ FI > FII > FIV | 55.8 | 44.2 | 31.7 | 0.38 |
| STx-1b after saturation of 1 M ZnCl2 & (STx-1b 1 M Zn) | ||||||||||
| Cu | 52.30 ± 0.53 | 3.2 | 2.4 | 8.3 | 86.1 | FIV > FIII > FI > FII | 5.6 | 94.4 | 3.2 | 0.92 |
| Cr | 82.40 ± 0.90 | 1.5 | 0.2 | 86.3 | 12 | FIII > FIV > FI > FII | 1.7 | 98.3 | 1.5 | 0.61 |
| Ni | 15.30 ± 0.11 | 13.4 | 7.5 | 58.9 | 0.2 | FIII > FI > FII > FIV | 20.9 | 79.1 | 13.4 | 0.36 |
| Pb | 7.53 ± 0.07 | 1 | 2.4 | 10.6 | 86 | FIV > FIII > FII > FI | 3.4 | 96.6 | 1 | 0.93 |
| Zn | 16,153 ± 75 | 27.3 | 22.6 | 29.4 | 20.7 | FIII > FI > FII > FIV | 49.9 | 50.1 | 27.3 | 0.45 |
| SWy-3 after saturation of 1 M ZnCl2 & (SWy-3 1 M Zn) | ||||||||||
| Cu | 109.47 ± 1.34 | 26.9 | 3.2 | 9 | 60.9 | FIV > FI > FIII > FII | 30.1 | 69.9 | 26.9 | 0.68 |
| Cr | 114.85 ± 1.20 | 2.1 | 0.2 | 82.6 | 15.1 | FIII > FIV > FI > FII | 2.3 | 97.7 | 2.1 | 0.62 |
| Ni | 27.27 ± 0.23 | 8.7 | 4.7 | 45.7 | 40.9 | FIII > FIV > FI > FII | 13.4 | 86.6 | 8.7 | 0.68 |
| Pb | 14.61 ± 0.15 | 2.4 | 1 | 47.1 | 49.4 | FIV > FIII > FI > FII | 3.4 | 96.6 | 2.4 | 0.76 |
| Zn | 44,463 ± 124 | 34.9 | 26.7 | 28 | 10.4 | FI > FIII > FII > FIV | 61.6 | 38.4 | 34.9 | 0.35 |
| BSvk after saturation of 1 M CuCl2 & (BSvk 1 M Cu) | ||||||||||
| Cu | 7677 ± 70 | 41.5 | 33.4 | 17.9 | 7.2 | FI > FII > FIII > FIV | 74.9 | 25.1 | 41.5 | 0.28 |
| Cr | 22.88 ± 0.17 | 0.6 | 45.9 | 19.0 | 34.5 | FII > FIV > FIII > FI | 46.5 | 53.5 | 0.6 | 0.57 |
| Ni | 22.02 ± 0.09 | 6 | 68.2 | 18.6 | 4.2 | FII > FIII > FI > FIV | 74.2 | 22.8 | 6 | 0.32 |
| Pb | 17.93 ± 0.18 | 1.6 | 25.7 | 14.9 | 57.8 | FIV > FII > FIII > FI | 27.3 | 72.7 | 1.6 | 0.73 |
| Zn | 95.61 ± 0.72 | 11.7 | 30.4 | 25.2 | 32.7 | FIV > FII > FIII > FI | 42.1 | 57.9 | 11.7 | 0.55 |
| BSvk after saturation of 0.1 M CuCl2 & (BSvk 0.1 M Cu) | ||||||||||
| Cu | 23,284 ± 121 | 55.4 | 25.2 | 15.2 | 4.2 | FI > FII > FIII > FIV | 80.6 | 19.4 | 55.4 | 0.23 |
| Cr | 13.97 ± 0.11 | 1.4 | 49.5 | 20 | 29.1 | FII > FIV > FIII > FI | 50.9 | 49.1 | 1.4 | 0.53 |
| Ni | 3.47 ± 0.13 | 5.1 | 39.9 | 35.4 | 19.6 | FII > FIII > FIV > FI | 45 | 55 | 5.1 | 0.5 |
| Pb | 22.90 ± 0.29 | 5.1 | 33.2 | 15.7 | 46 | FIV > FII > FIII > FI | 38.3 | 61.7 | 5.1 | 0.63 |
| Zn | 71.36 ± 2.13 | 10.1 | 24.1 | 10.1 | 55.7 | FIV > FII > FIII > FI | 34.2 | 65.8 | 10.1 | 0.68 |
| BSvk after saturation of 0.25 M CuCl2 & (BSvk 0.25 M Cu) | ||||||||||
| Cu | 27,571 ± 90 | 21.9 | 46.9 | 26.7 | 4.5 | FII > FIII > FI > FIV | 68.8 | 31.2 | 21.9 | 0.33 |
| Cr | 35.73 ± 0.57 | 2.3 | 60.9 | 15.3 | 21.5 | FII > FIV > FIII > FI | 63.2 | 36.8 | 2.3 | 0.45 |
| Ni | 2.41 ± 0.65 | 3.2 | 38.2 | 32.4 | 26.1 | FII > FIII > FIV > FI | 41.4 | 58.5 | 3.2 | 0.54 |
| Pb | 14.53 ± 0.67 | 5.5 | 16.9 | 18.5 | 59.1 | FIV > FIII > FII > FI | 22.4 | 77.6 | 5.5 | 0.74 |
| Zn | 78.72 ± 0.86 | 15.8 | 28.9 | 16.3 | 39 | FIV > FII > FIII > FI | 44.7 | 55.3 | 15.8 | 0.56 |
| BSvk after saturation of 0.5 M CuCl2 & (BSvk 0.5 M Cu) | ||||||||||
| Cu | 28,773 ± 162 | 12.4 | 64.3 | 15.4 | 7.9 | FII > FIII > FI > FIV | 76.7 | 23.3 | 12.4 | 0.33 |
| Cr | 58.09 ± 0.85 | 1 | 3.9 | 80 | 15.1 | FIII > FIV > FII > FI | 4.9 | 95.1 | 1 | 0.61 |
| Ni | 6.44 ± 0.40 | 6.3 | 10.1 | 61.3 | 22.3 | FIII > FIV > FII > FI | 16.4 | 83.6 | 6.3 | 0.6 |
| Pb | 13.31 ± 0.84 | 5.2 | 10.5 | 30 | 54.3 | FIV > FIII > FII > FI | 15.7 | 84.3 | 5.2 | 0.74 |
| Zn | 80.75 ± 2.03 | 10.5 | 22.5 | 17.8 | 49.2 | FIV > FII > FIII > FI | 33 | 67 | 10.5 | 0.65 |
| STx-1b after saturation of 1 M CuCl2 & (STx-1b 1 M Cu) | ||||||||||
| Cu | 5427 ± 43 | 15.7 | 38 | 43.4 | 2.9 | FIII > FII > FI > FIV | 53.7 | 46.3 | 15.7 | 0.38 |
| Cr | 17.50 ± 0.30 | 0.3 | 3.8 | 87.4 | 8.5 | FIII > FIV > FII > FI | 4.1 | 95.9 | 0.3 | 0.59 |
| Ni | 16.27 ± 0.08 | 6.2 | 28 | 60.3 | 5.5 | FIII > FII > FI > FIV | 34.2 | 65.8 | 6.2 | 0.47 |
| Pb | 6.07 ± 0.10 | 2.5 | 8.6 | 39.9 | 49.1 | FIV > FIII > FII > FI | 11.1 | 89 | 2.5 | 0.74 |
| Zn | 92.96 ± 2.90 | 11.3 | 17.1 | 15.9 | 55.7 | FIV > FII > FIII > FI | 28.4 | 71.6 | 11.3 | 0.7 |
| SWy-3 after saturation of 1 M CuCl2 & (SWy-3 1 M Cu) | ||||||||||
| Cu | 11,221 ± 61 | 13 | 42.3 | 43.1 | 1.6 | FII > FIII > FI > FIV | 55.3 | 44.7 | 13 | 0.37 |
| Cr | 38.88 ± 0.69 | 0.2 | 5.6 | 83 | 11.2 | FIII > FIV > FII > FI | 5.8 | 44.2 | 0.2 | 0.59 |
| Ni | 33.53 ± 0.52 | 6.1 | 38.9 | 51.8 | 3.2 | FIII > FII > FI > FIV | 46 | 54 | 6.1 | 0.42 |
| Pb | 9.50 ± 0.12 | 1.5 | 9.3 | 26.1 | 63.1 | FIV > FIII > FII > FI | 10.8 | 89.2 | 1.5 | 0.8 |
| Zn | 83.20 ± 1.30 | 8.3 | 14.2 | 10.2 | 67.3 | FIV > FII > FIII > FI | 22.5 | 77.5 | 8.3 | 0.77 |
| Soil | The average of the unfrozen water content [%] at a given temperature [°C] determined by 1H NMR method [26] | ||||||||
| −32 | −23 | −14 | −9 | −5 | −3 | ||||
| BSvk 1 M Zn | 12.97 ± 0.43 | 15.15 ± 0.54 | 18.74 ± 0.29 | 24.29 ± 1.60 | 27.89 ± 1.21 | 33.55 ± 0.47 | |||
| STx-1b 1 M Zn | 15.17 ± 0.64 | 17.60 ± 0.54 | 20.38 ± 1.00 | 24.57 ± 0.10 | 28.11 ± 1.31 | 32.96 ± 1.37 | |||
| SWy-3 1 M Zn | 9.74 ± 0.62 | 11.69 ± 0.61 | 14.60 ± 1.12 | 17.73 ± 1.96 | 22.69 ± 1.93 | 30.79 ± 2.33 | |||
| Soil | The average of the unfrozen water content [%] at a given temperature [°C] determined by DSC method [47] | ||||||||
| −23 | −20 | −14 | −12 | −10 | −8 | −6 | −4 | −2 | |
| BSvk 1 M Cu | 22.74 ± 1.65 | 22.98 ± 1.96 | 24.78 ± 2.05 | 25.91 ± 2.14 | 27.59 ± 2.08 | 30.43 ± 2.03 | 32.42 ± 2.21 | 37.31 ± 1.32 | 41.40 ± 4.07 |
| BSvk 0.5 M Cu | 22.47 ± 1.44 | 22.61 ± 1.65 | 24.16 ± 1.60 | 25.21 ± 1.33 | 26.21 ± 1.94 | 28.15 ± 1.51 | 31.86 ± 1.44 | 33.24 ± 1.13 | 41.79 ± 8.82 |
| BSvk 0.25 M Cu | 20.27 ± 1.18 | 20.27 ± 1.18 | 22.21 ± 0.67 | 22.99 ± 0.61 | 24.92 ± 0.47 | 27.68 ± 1.50 | 30.68 ± 2.01 | 33.95 ± 1.41 | 41.05 ± 6.61 |
| BSvk 0.1 M Cu | 17.51 ± 1.38 | 18.94 ± 1.50 | 20.03 ± 1.77 | 21.47 ± 1.41 | 23.61 ± 1.12 | 23.61 ± 1.12 | 24.65 ± 2.34 | 28.91 ± 0.73 | 32.26 ± 5.24 |
References
- Nkwaju, R.Y.; Nouping, J.N.F.; Bachirou, S.; Abo, T.M.; Deutou, J.G.N.; Djobo, J.N.Y. Effective Stabilization of Cadmium and Copper in Iron-Rich Laterite-Based Geopolymers and Influence on Physical Properties. Materials 2023, 16, 7605. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, K.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Jiang, X.; Liu, C.; McCall, W.; Lu, J. Stabilization of heavy metals in soil using two organo-bentonites. Chemosphere 2017, 184, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, Z.; Záleská, M.; Pavlíková, M.; Pivák, A.; Nábělková, J.; Jankovský, O.; Jiříčková, A.; Chmel, O.; Průša, F. Simultaneous Immobilization of Heavy Metals in MKPC-Based Mortar—Experimental Assessment. Materials 2023, 16, 7525. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gómez, F.; Escribano-García, R. Biosorption of Cu(II) ions as a method for the effective use of activated carbon from grape stalk waste: RMS optimization and kinetic studies. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 4706–4726. [Google Scholar] [CrossRef]
- Ziejewska, C.; Grela, A.; Łach, M.; Marczyk, J.; Hordyńska, N.; Szechyńska-Hebda, M.; Hebda, M. Eco-friendly zeolites for innovative purification of water from cationic dye and heavy metal ions. J. Clean. Prod. 2023, 406, 136947. [Google Scholar] [CrossRef]
- Hazrat, A.; Ezzat, K.; Ikram, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Munir, N.; Jahangeer, M.; Bouyahya, A.; El Omari, N.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Ali Shah, S.M.; et al. Heavy Metal Contamination of Natural Foods Is a Serious Health Issue: A Review. Sustainability 2022, 14, 161. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Janaszek, A.; Kowalik, R. Analysis of Heavy Metal Contaminants and Mobility in Sewage sludge-soil Mixtures for Sustainable Agricultural Practices. Water 2023, 15, 3992. [Google Scholar] [CrossRef]
- Piri, M.; Sepehr, E.; Samadi, A.; Farhadi, K.H.; Alizadeh, M. Contaminated Soil Amendment by Diatomite: Chemical Fractions of Zinc, Lead, Copper and Cadmium. Int. J. Environ. Sci. Technol. 2021, 18, 1191–1200. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Yang, J.; Cui, J.; Zhou, W.; Guo, X. Study on mechanical and permeability characteristics of nickel-copper-contaminated soil solidified by CFG. Environ. Sci. Pollut. Res. 2020, 27, 18577–18591. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.; Tew, K. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Janaszek, A.; Silva, A.F.d.; Jurišević, N.; Kanuchova, M.; Kozáková, Ľ.; Kowalik, R. The Assessment of Sewage Sludge Utilization in Closed-Loop Economy from an Environmental Perspective. Water 2024, 16, 383. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Liao, X.Y.; Chen, T.B.; Wu, B.; Yan, X.L.; Nie, C.J.; Xie, H.; Zhai, L.M.; Xiao, X.Y. Mining urban soil pollution: Concentrations and patterns of heavy metals in the soils of Jinchang, China. Chin. J. Geogr. Res. 2006, 25, 843–852. [Google Scholar]
- Shang, W.; Tang, Q.; Zheng, L.; Cheng, H. Chemical forms of heavy metals in agricultural soils affected by coal mining in the Linhuan subsidence of Huaibei Coalfield, Anhui Province, China. Environ. Sci. Pollut. Res. 2016, 23, 23683–23693. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, X.; Li, J.; Xiao, Q.; Ma, L.; Gao, Q.; Wan, Y.; Huang, Y.; Liu, T.; Luo, X.; et al. Bioleaching and immobilizing of copper and zinc using endophytes coupled with biochar-hydroxyapatite: Bipolar remediation for heavy metals contaminated mining soils. Chemosphere 2023, 315, 137730. [Google Scholar] [CrossRef]
- Ma, C.; Ci, K.; Zhu, J.; Sun, Z.; Liu, Z.; Li, X.; Zhu, Y.; Tang, C.; Wang, P.; Liu, Z. Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health. Sci. Total Environ. 2021, 759, 143501. [Google Scholar] [CrossRef]
- Koda, E. Influence of vertical barrier surrounding old sanitary landfill on eliminating transport of pollutants on the basis of numerical modelling and monitoring results. Pol. J. Environ. Stud. 2012, 21, 929–935. [Google Scholar]
- Koda, E.; Osinski, P. Bentonite Cut Off Walls: Solution for Landfill Remedial Works. Environ. Geotech. 2017, 4, 223–232. [Google Scholar] [CrossRef]
- Koda, E.; Miszkowska, A.; Osinski, P.; Sieczka, A. Heavy metals contamination within restored landfill site in Poland. Environ. Geotech. 2020, 7, 512–521. [Google Scholar] [CrossRef]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef]
- Nartowska, E. The effects of potentially toxic metals (copper and zinc) on selected physical and physico-chemical properties of bentonites. Heliyon 2019, 5, e02563. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.M.; Darrow, M.M. Adsorbed cation effects on unfrozen water in fine-grained frozen soil measured using pulsed nuclear magnetic resonance. Cold Reg. Sci. Technol. 2017, 142, 42–54. [Google Scholar] [CrossRef]
- Nartowska, E.; Kozłowski, T.; Kolankowska, M. Exchangeable cations (Cu2+, Zn2+) effects on unfrozen water content in clay-water system using 1H NMR method. Cold Reg. Sci. Technol. 2021, 192, 103403. [Google Scholar] [CrossRef]
- Nevidomskaya, D.G.; Minkina, T.M.; Soldatov, A.V.; Bauer, T.V.; Shuvaeva, V.A.; Zubavichus, Y.V.; Trigub, A.L.; Mandzhieva, S.S.; Dorovatovskii, P.V.; Popov, Y.V. Speciation of Zn and Cu in Technosol and evaluation of a sequential extraction procedure using XAS, XRD and SEM-EDX analyses. Environ. Geochem. 2021, 43, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.; Mateu, J.; Rigol, A.; Vidal, M.; López-Sánchez, J.F.; Rauret, G. Use of the modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environ. Pollut. 2008, 152, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.J.; Weindorf, D.C. Heavy Metal and Trace Metal Analysis in Soil by Sequential Extraction: A Review of Procedures. Int. J. Anal. Chem. 2010, 2010, 387803. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, G.; Dong, P.; Xiao, J.; Meng, Q.; Baumgartel, M.; Xu, B.; Hao, T. Enhanced electrokinetic remediation of lead- and cadmium- contaminated paddy soil by composite electrolyte of sodium chloride and citric acid. J. Soils Sediments 2018, 18, 1915–1924. [Google Scholar] [CrossRef]
- Kashem, A.; Singh, B.R.; Huq, S.M.I.; Kawai, S. Fractionation and mobility of cadmium, lead and zinc in some contaminated and non-contaminated soils of Japan. J. Soil Sci. Environ. Manag. 2011, 3, 241–249. [Google Scholar]
- Nartowska, E.; Kozłowski, T. The Effect of Freeze-Thaw Cycling and the Initial Mass of Water on the Unfrozen Water Content of Calcium Bentonites Modified by Copper Ions. Minerals 2022, 12, 66. [Google Scholar] [CrossRef]
- Sintorini, M.; Widyatmoko, H.; Sinaga, E.; Aliyah, N. Effect of pH on metal mobility in the soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 737, 012071. [Google Scholar] [CrossRef]
- Dutta, J.; Mishra, A.K.; Das, P. Combined Effect of Inorganic Salts and Heavy Metals on the Engineering Behaviour of Compacted Bentonites. Int. J. Geosynth. Ground Eng. 2018, 4, 17. [Google Scholar] [CrossRef]
- Nartowska, E.; Kozłowski, T.; Gawdzik, J. Assessment of the influence of copper and zinc on the microstructural parameters and hydraulic conductivity of bentonites on the basis of SEM tests. Heliyon 2019, 5, e02142. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; He, Y.; Hu, H.; Lou, W.; Zhang, Z.; Zhang, K.; Chen, Y.; Ye, W.; Sun, J. Laboratory investigation on the retention performance of a soil–bentonite mixture used as an engineered barrier: Insight into the effects of ionic strength and associated heavy metal ions. Environ. Sci. Pollut. Res. 2023, 30, 50162–50173. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, L.; Wang, D.; Ren, L.; Li, W. Effect of modified bentonite on copper migration via bank soils in the Jialing River, Southwest China. Soil Tillage Res. 2022, 218, 105322. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X. Effects of Bentonite Addition on the Speciation and Mobility of Cu and Ni in Soils from Old Mine Tailings. Sustainability 2022, 14, 10878. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2021, 73, e13203. [Google Scholar] [CrossRef]
- Xu, T.; Nan, F.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Effect of soil pH on the transport, fractionation, and oxidation of chromium(III). Ecotoxicol. Environ. Saf. 2020, 195, 110459. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.D.; Pudasainee, D.; Guota, R.; Kashiwabara, T.; Alessi, D.S.; Konhauser, K.O. Effect of acidic conditions on surface properties and metal binding capacity of clay minerals. ACS Earth Space Chem. 2019, 11, 2421–2429. [Google Scholar] [CrossRef]
- Finžgar, N.; Tlustoš, P.; Leštan, D. Relationship of soil properties to fractionation, bioavailability and mobility of lead and zinc in soil. Plant Soil Environ. 2007, 53, 225–238. [Google Scholar] [CrossRef]
- Namieśnik, J.; Rabajczyk, A. The speciation and physicochemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab. 2010, 22, 1–24. [Google Scholar] [CrossRef]
- Musso, T.B.; Parolo, M.E.; Pettinari, G. pH, Ionic Strength, and Ion Competition Effect on Cu(II) and Ni(II) Sorption by a Na-bentonite Used as Liner Material. Pol. J. Environ. Stud. 2019, 28, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Nartowska, E.; Kozłowski, T. The Effect of the Concentration of Copper Ions on the Unfrozen Water Content in Bentonites Measured with the Use of DSC Method. Minerals 2022, 12, 632. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. Baseline studies of the clay minerals society source clays: Powder x-ray diffraction analyses. Clays Clay Miner. 2001, 49, 398–409. [Google Scholar] [CrossRef]
- Górniak, K. Bentonite from the Central Slovakia Volcanic Field—A Prospective Raw Material for Polish Industry. Mineralogia 2017, 48, 23–38. [Google Scholar] [CrossRef]
- PN-ISO 9297:1994, Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method), Polish Committee for Standardization. Available online: https://www.intertekinform.com/en-gb/standards/pn-iso-9297-1994-941921_saig_pkn_pkn_2216839 (accessed on 1 January 2024).
- Einax, J.W.; Nischwitz, V. Inert sampling and sample preparation- the influence of oxygen on heavy metal mobility in river sediments. Fresenius J. Anal Chem. 2001, 371, 643–651. [Google Scholar] [CrossRef]
- Ure, A.; Quevaullier, P.H.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the CEC. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Fernández-Ondoño, E.; Bacchetta, G.; Lallena, A.; Navarro, F.; Ortiz, I.; Jiménez, N. Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia. J. Geochem. Explor. 2017, 172, 133–141. [Google Scholar] [CrossRef]
- ISO 13320:2020 Standard, Particle Size Analysis—Laser Diffraction Methods, Technical Committee: ISO/TC 24/SC 4, ICS: 19.120. Available online: https://www.iso.org/standard/69111.html (accessed on 1 January 2022).
- Sympatec Guidelines. Available online: https://www.tecnos.ro/brosuri/HELOS.pdf (accessed on 9 June 2024).
- Su, M.; Liu, C.; Wang, L.; Zheng, W. Prediction of saturated hydraulic conductivity of sandy soil using Sauter mean diameter of soil particles. Eur. J. Soil Sci. 2022, 73, e13229. [Google Scholar] [CrossRef]
- Nartowska, E.; Kozłowski, T.; Kolankowska, M. The changes in the microstructure of ion-exchanged clays. E3S Web Conf. 2017, 17, 00063. [Google Scholar] [CrossRef]
- Nartowska, E.; Kanuchova, M.; Kozáková, Ľ. Assessment of Unfrozen Water Content in Copper Bentonites Using the 1H NMR Technique: Optimization, the Method’s Limitation, and Comparative Analysis with DSC. Materials 2023, 16, 7577. [Google Scholar] [CrossRef]
- Hube, L. Understanding and Implementing ISO/IEC 17025. Available online: https://www.demarcheiso17025.com/document/Understanding%20and%20Implementing%20ISO17025.pdf (accessed on 3 June 2024).
- Rabiej, M. (Ed.) Statistical Analyses Using Statistica Program, 2nd ed.; Heliyon: Gliwice, Poland, 2018; (In Polish). ISBN 978-83-246-4110-9. [Google Scholar]
- Klik, B.; Holatko, J.; Jaskulska, I.; Gusiatin, M.Z.; Hammerschmiedt, T.; Brtnicky, M.; Liniauskienė, E.; Baltazar, T.; Jaskulski, D.; Kintl, A.; et al. Bentonite as a Functional Material Enhancing Phytostabilization of Post-Industrial Contaminated Soils with Heavy Metals. Materials 2022, 15, 8331. [Google Scholar] [CrossRef] [PubMed]
- Nath, H.; Kabir, M.H.; Kafy, A.; Zullyadini, A.; Rahaman, A.; Rahman, M.T. Geotechnical properties and applicability of bentonite-modified local soil as landfill and environmental sustainability liners. Environ. Sustain. Indic. 2023, 18, 100241. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, J.; Yang, X.; Fu, Q.; Hu, H.; Huang, Q. Mineralization of organic matter during the immobilization of heavy metals in polluted soil treated with minerals. Chemosphere 2022, 301, 134794. [Google Scholar] [CrossRef]
- Zhang, D.; Li, T.; Ding, A.; Wu, X. Effects of an additive (hydroxyapatite-bentonite-biochar) on Cd and Pb stabilization and microbial community composition in contaminated vegetable soil. RSC Adv. Mar. 2021, 26, 12200–12208. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Li, Y.B.; Tan, X.P.; He, W.X.; Xie, W.; Megharaj, M.; Wei, G.H. Effect of arsenate contamination on free, immobilized and soil alkaline phosphatases: Activity, kinetics and thermodynamics. Eur. J. Soil Sci. 2017, 68, 126–135. [Google Scholar] [CrossRef]
- Angelaki, A.; Dionysidis, A.; Sihag, P.; Golia, E.E. Assessment of Contamination Management Caused by Copper and Zinc Cations Leaching and Their Impact on the Hydraulic Properties of a Sandy and a Loamy Clay Soil. Land 2022, 11, 290. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Blackie Academic and Professional: London, UK, 2013; ISBN 9789400744691. [Google Scholar]
- Barker, A.J.; Douglas, T.A.; Jacobson, A.D.; McClelland, J.W.; Ilgen, A.G.; Khosh, M.S.; Lehn, G.O.; Trainor, T.P. Late season mobilization of trace metals in two small Alaskan arctic watersheds as a proxy for landscape scale permafrost active layer dynamics. Chem. Geol. 2014, 381, 180–193. [Google Scholar] [CrossRef]
- Rui, D.; Wu, W.; Zhang, H.; Li, G.; Wang, S.; Ito, Y. Optimization analysis of heavy metal pollutants removal from fine-grained soil by freeze-thaw and washing technology. Cold Reg. Sci. Technol. 2020, 173, 103025. [Google Scholar] [CrossRef]
- Rooney, E.C.; Bailey, V.L.; Patel, K.F.; Dragila, M.; Battu, A.K.; Buchko, A.C.; Gallo, A.C.; Hatten, J.; Possinger, A.R.; Qafoku, O.; et al. Soil pore network response to freeze-thaw ycles in permafrost aggregates. Geoderma 2022, 411, 115674. [Google Scholar] [CrossRef]
- Wu, T.; Rui, D.; Zhang, J.; Li, G.; Wang, S.; Ito, Y. Removal of heavy metal pollutant from clayey soil based on repeated ice-segregation: A laboratory evaluation. Cold Reg. Sci. Technol. 2022, 201, 103626. [Google Scholar] [CrossRef]
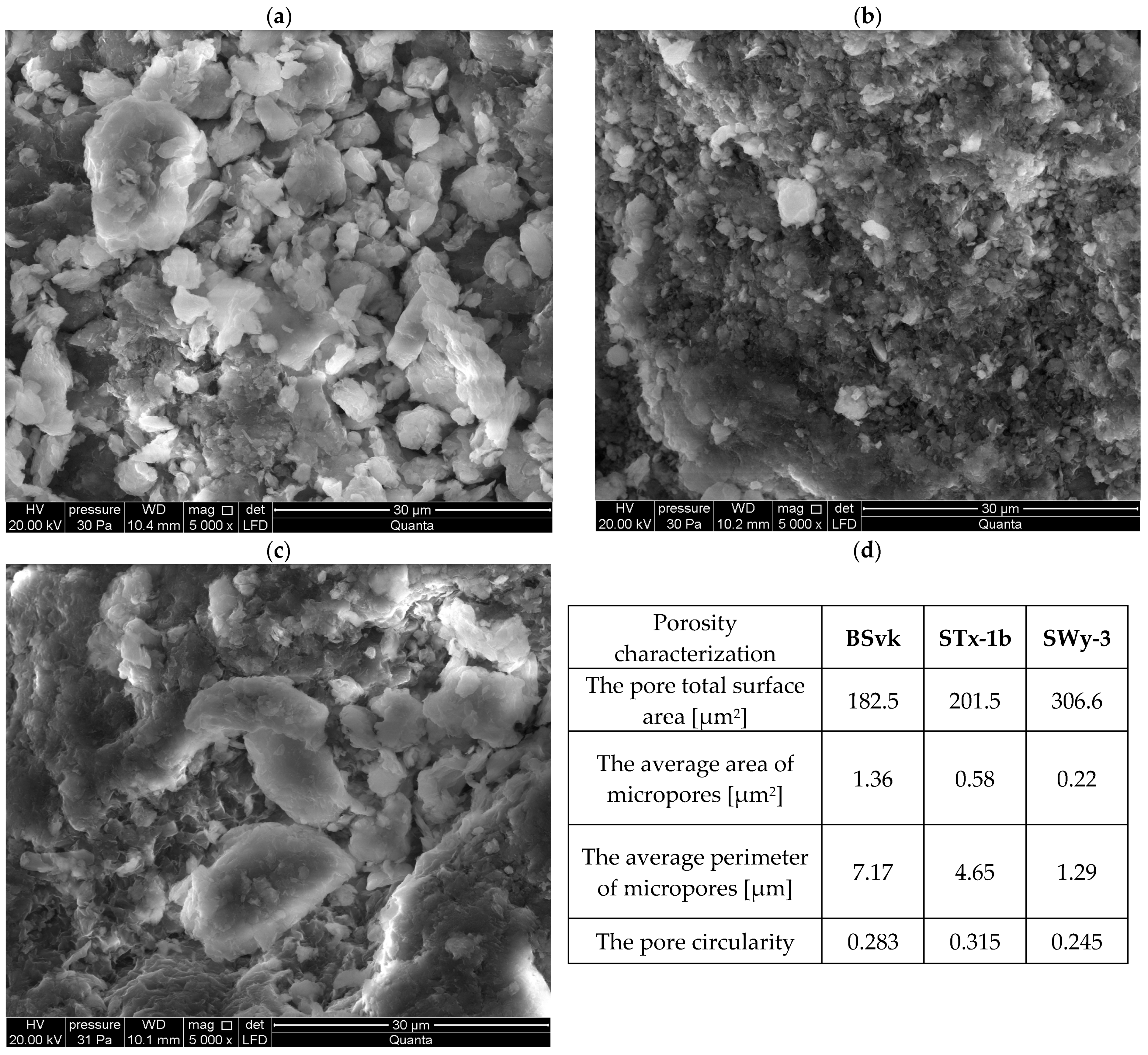



| Step No. | Procedure/Condition | Fraction |
|---|---|---|
| 1 | Weigh 2 g of dry mass of each bentonite and transfer to a 100 cm3 rotary tube. Add 40 cm3 of 0.11 mol/dm3 CH3COOH. Shake for 16 h (T = 22 °C). Separate extract via centrifugation. | FI—Exchangeable and/or carbonate-bound metals |
| 2 | Flood clay samples with 40 cm3 of 0.5 mol/dm3 NH2OH∙HCl, pH = 2. Use HNO3 to correct reaction. Perform procedure as in step one. | FII—Metals combined with amorphous Fe and Mn oxides |
| 3 | Add 10 cm3 of 8.8 mol/dm3 H2O2 to remaining clay samples after step 2. Place in water bath at 85 °C until solution evaporates. Pour cooled samples into 50 cm3 ammonium acetate solution (1 mol/dm3, pH = 2 after HNO3 correction). Shake for 16 h and centrifuge to separate clay precipitate from extract. | FIII—Metals bound to organic matter and sulfides |
| 4 | Dry remaining sediment at 105–110 °C. Place 1 g of clay in a 300 cm3 conical volumetric flask. Add 7.5 cm3 concentrated HCl and 2.5 cm3 concentrated HNO3. Heat until the solution evaporates. Add 25 cm3 of HCl (1 + 5) to the cooled flasks, transfer the dissolved precipitate to a volumetric flask and fill up to 50 cm3 with distilled water. Filter the solution and determine the metals in fraction IV. | FIV—Metals bound to primary and secondary minerals |
| Properties | BSvk | BSvk Cu | BSvk 1 M Zn | Stx-1b | Stx-1b 1M Cu | Stx-1b 1 M Zn | SWy-3 | SWy-3 1 M Cu | SWy-3 1 M Zn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 M | 0.1 M | 0.25 M | 0.5 M | |||||||||
| Cu & (mg/kg) | 6.28 ± 0.06 | 7677 ± 70 | 23,284 ± 121 | 27,571 ± 90 | 28,773 ± 162 | 39.55 ± 0.25 | 8.97 ± 0.13 | 5427 ± 43 | 52.30 ± 0.53 | 12.80 ± 0.15 | 11,221 ± 61 | 109.47 ± 1.34 |
| Zn & (mg/kg) | 64.54 ± 0.69 | 95.61 ± 0.72 | 71.36 ± 2.13 | 78.72 ± 0.86 | 80.75 ± 2.03 | 17,857 ± 89 | 73.68 ± 0.27 | 92.96 ± 2.91 | 16,153 ± 75 | 163.66 ± 1.50 | 83.20 ± 1.30 | 44,463 ± 124 |
| Na & (mg/kg) | 1151 ± 9 | 413 ± 5 | 168 ± 1 | 195 ± 2 | 133 ± 2 | 1204 ± 22 | 1970 ± 18 | 363 ± 7 | 885 ± 16 | 10,086 ± 81 | 405 ± 6 | 995 ± 9 |
| Ca & (mg/kg) | 11,945 ± 140 | 1598 ± 28 | 676 ± 23 | 328 ± 6 | 385 ± 5 | 2778 ± 31 | 11,802 ± 101 | 1491 ± 19 | 2985 ± 23 | 8282 ± 57 | 2028 ± 21 | 4526 ± 34 |
| clay * (%) | 19.8 | 20.5 | 18 | 18.5 | 16 | 8.9 | 18.5 | 23.5 | 10.7 | 42.6 | 13.2 | 6.55 |
| silt * (%) | 80.2 | 79.5 | 82 | 81.5 | 84 | 90.2 | 81.5 | 76.5 | 87 | 57.4 | 86.8 | 87 |
| d10 * (μm) | 1.33 | 1.31 | 1.44 | 1.39 | 1.52 | 2.18 | 1.39 | 1.26 | 1.91 | 0.96 | 1.71 | 2.83 |
| d32 * (μm) | 3.16 | 3.01 | 3.26 | 3.22 | 3.56 | 5.22 | 3.44 | 2.85 | 4.57 | 1.88 | 4.07 | 6.31 |
| S # (m2/g) | 671 | 460 | 183 | 189 | 203 | 557 | 568 | 414 | 538 | 307 | 355 | 516 |
| d001 + (Å) | 14.88 | 12.48 | 12.38 | 12.34 | 12.31 | 14.17 | 14.88 | 12.48 | 14.29 | 11.44 | 12.32 | 13.21 |
| Parameter | Wilks—Test Value | F-Test Value | Degrees of Freedom (df) | df Error | p-Value | Significance |
|---|---|---|---|---|---|---|
| Potentially mobile fraction (FI + FII) of Cu, Zn, Cr, Ni, Pb | ||||||
| Intercept | 0.003 | 3796.845 | 3 | 37 | 0.000 | |
| Bentonite (B) | 0.084 | 30.143 | 6 | 74 | 0.000 | *** |
| Contamination (C) | 0.017 | 82.705 | 6 | 74 | 0.000 | *** |
| B × C | 0.007 | 45.217 | 12 | 98.184 | 0.000 | *** |
| The Standardized Beta (ß) | Std. Error ß | The Unstandardized Beta (B) | Std. Error B | t Test Value | p-Value | Significance | |
|---|---|---|---|---|---|---|---|
| Dependent variable: FI Zn/Cu | |||||||
| R = 0.847 R2 = 0.717 adj. R2 = 0.689 Std. error of estimate: 1.81 | |||||||
| Intercept | 25.976 | 1.180 | 22.011 | 0.000 | |||
| Zn total | 0.847 | 0.168 | 0.000 | 0.000 | 5.035 | 0.000 | *** |
| R = 0.522 R2 = 0.273 adj. R2 = 0.221 Std. error of estimate: 15.26 | |||||||
| Intercept | 55.465 | 10.603 | 5.231 | 0.000 | |||
| Cu total | −0.522 | 0.228 | −0.001 | 0.000 | −2.291 | 0.038 | * |
| Dependent variable: FII Zn/Cu | |||||||
| R = 0.950 R2 = 0.903 adj. R2 = 0.894 Std. error of estimate: 0.58 | |||||||
| Intercept | 21.218 | 0.375 | 56.561 | 0.000 | |||
| Zn total | 0.950 | 0.098 | 0.000 | 0.000 | 9.664 | 0.000 | *** |
| R = 0.562 R2 = 0.316 adj. R2 = 0.267 Std. error of estimate: 13.09 | |||||||
| Intercept | 20.880 | 9.092 | 2.296 | 0.038 | |||
| Cu total | 0.562 | 0.221 | 0.000 | 0.000 | 2.543 | 0.023 | * |
| Dependent variable: FIII Zn/Cu | |||||||
| R = 0.746 R2 = 0.557 adj. R2 = 0.513 Std. error of estimate: 1.14 | |||||||
| Intercept | 32.097 | 0.744 | 43.162 | 0.000 | |||
| Zn total | −0.746 | 0.210 | −0.000 | 0.000 | −3.547 | 0.005 | ** |
| R = 0.185 R2 = 0.034 adj. R2 = 0.004 Std. error of estimate: 4.92 | |||||||
| Intercept | 16.549 | 3.417 | 4.842 | 0.000 | |||
| Cu total | 0.185 | 0.262 | 0.000 | 0.000 | 0.706 | 0.492 | NS |
| Dependent variable: FIV Zn/Cu | |||||||
| R = 0.690 R2 = 0.476 adj. R2 = 0.424 Std. error of estimate: 3.54 | |||||||
| Intercept | 20.709 | 2.299 | 9.008 | 0.000 | |||
| Zn total | −0.690 | 0.229 | −0.000 | 0.000 | −3.014 | 0.013 | ** |
| R = 0.275 R2 = 0.075 adj. R2 = 0.009 Std. error of estimate: 1.668 | |||||||
| Intercept | 7.106 | 1.158 | 6.134 | 0.000 | |||
| Cu total | −0.275 | 0.257 | −0.000 | 0.000 | −1.070 | 0.303 | NS |
| The Standardized Beta (ß) | Std. Error ß | The Unstandardized Beta (B) | Std. Error B | t-Test Value | p-Value | Significance | |
|---|---|---|---|---|---|---|---|
| Dependent variable: FI Zn | |||||||
| R = 0.949 R2 = 0.902 adj. R2 = 0.888 Std. error of estimate: 0.819 | |||||||
| Intercept | 34.592 | 2.755 | 12.555 | 0.000 | |||
| Un $ (T = −32 °C) | −0.949 | 0.118 | −0.702 | 0.087 | −8.011 | 0.000 | *** |
| R = 0.956 R2 = 0.915 adj. R2 = 0.903 Std. error of estimate: 1.031 | |||||||
| Intercept | 38.767 | 2.775 | 13.969 | 0.000 | |||
| Un $ (T = −23 °C) | −0.956 | 0.110 | −0.765 | 0.088 | −8.673 | 0.000 | *** |
| R = 0.887 R2 = 0.787 adj. R2 = 0.757 Std. error of estimate: 1.352 | |||||||
| Intercept | 40.960 | 4.551 | 8.999 | 0.000 | |||
| Un $ (T = −14 °C) | −0.887 | 0.174 | −0.736 | 0.145 | −5.089 | 0.001 | *** |
| Dependent variable: FII Zn | |||||||
| R = 0.968 R2 = 0.937 adj. R2 = 0.928 Std. error of estimate: 0.656 | |||||||
| Intercept | 44.820 | 3.166 | 14.156 | 0.000 | |||
| Un $ (T = −32 °C) | −0.968 | 0.095 | −1.316 | 0.129 | −10.193 | 0.000 | *** |
| R = 0.972 R2 = 0.946 adj. R2 = 0.938 Std. error of estimate: 0.659 | |||||||
| Intercept | 49.829 | 3.180 | 15.669 | 0.000 | |||
| Un $ (T = −23 °C) | −0.972 | 0.088 | −1.432 | 0.129 | −11.036 | 0.000 | *** |
| R = 0.936 R2 = 0.876 adj. R2 = 0.858 Std. error of estimate: 1.034 | |||||||
| Intercept | 52.863 | 4.992 | 10.590 | 0.000 | |||
| Un $ (T = −14 °C) | −0.936 | 0.133 | −1.429 | 0.203 | −7.019 | 0.000 | *** |
| R = 0.857 R2 = 0.734 adj. R2 = 0.668 Std. error of estimate: 2.196 | |||||||
| Intercept | 65.323 | 12.984 | 5.031 | 0.007 | |||
| Un $ (T = −9 °C) | −0.857 | 0.257 | −1.763 | 0.529 | −3.329 | 0.029 | * |
| R = 0.683 R2 = 0.466 adj. R2 = 0.390 Std. error of estimate: 1.806 | |||||||
| Intercept | 48.418 | 8.720 | 5.552 | 0.001 | |||
| Un $ (T = −5 °C) | −0.683 | 0.276 | −0.879 | 0.355 | −2.474 | 0.042 | * |
| Dependent variable: FIV Zn | |||||||
| R = 0.873 R2 = 0.762 adj. R2 = 0.728 Std. error of estimate: 1.273 | |||||||
| Intercept | 6.091 | 1.443 | 4.218 | 0.004 | |||
| Un $ (T = −32 °C) | 0.873 | 0.184 | 0.450 | 0.095 | 4.736 | 0.002 | ** |
| R = 0.882 R2 = 0.778 adj. R2 = 0.746 Std. error of estimate: 1.332 | |||||||
| Intercept | 7.668 | 1.510 | 5.076 | 0.001 | |||
| Un $ (T = −23 °C) | 0.882 | 0.178 | 0.493 | 0.099 | 4.951 | 0.002 | ** |
| R = 0.784 R2 = 0.615 adj. R2 =0.559 Std. error of estimate: 1.820 | |||||||
| Intercept | 11.316 | 2.064 | 5.482 | 0.000 | |||
| Un $ (T = −14 °C) | 0.784 | 0.235 | 0.455 | 0.136 | 3.341 | 0.012 | * |
| Dependent variable: FIV Cu | |||||||
| R = 0.895 R2 = 0.802 adj. R2 = 0.782 Std. error of estimate: 1.024 | |||||||
| Intercept | 13.850 | 1.123 | 12.328 | 0.000 | |||
| Un & (T = −23 °C) | 0.895 | 0.141 | 1.159 | 0.182 | 6.364 | 0.000 | *** |
| R = 0.899 R2 = 0.807 adj. R2 = 0.788 Std. error of estimate: 0.892 | |||||||
| Intercept | 16.675 | 0.979 | 17.037 | 0.000 | |||
| Un & (T = −14 °C) | 0.899 | 0.139 | 1.028 | 0.159 | 6.480 | 0.000 | *** |
| R = 0.848 R2 = 0.720 adj. R2 = 0.692 Std. error of estimate: 0.858 | |||||||
| Intercept | 20.980 | 0.941 | 22.290 | 0.000 | |||
| Un & (T = −10 °C) | 0.848 | 0.167 | 0.773 | 0.153 | 5.069 | 0.000 | *** |
| R = 0.599 R2 = 0.359 adj. R2 = 0.295 Std. error of estimate: 2.622 | |||||||
| Intercept | 26.778 | 2.877 | 9.308 | 0.000 | |||
| Un & (T = −4 °C) | 0.599 | 0.253 | 1.105 | 0.466 | 2.369 | 0.039 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartowska, E.; Podlasek, A.; Vaverková, M.D.; Koda, E.; Jakimiuk, A.; Kowalik, R.; Kozłowski, T. Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation. Materials 2024, 17, 2957. https://doi.org/10.3390/ma17122957
Nartowska E, Podlasek A, Vaverková MD, Koda E, Jakimiuk A, Kowalik R, Kozłowski T. Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation. Materials. 2024; 17(12):2957. https://doi.org/10.3390/ma17122957
Chicago/Turabian StyleNartowska, Edyta, Anna Podlasek, Magdalena Daria Vaverková, Eugeniusz Koda, Aleksandra Jakimiuk, Robert Kowalik, and Tomasz Kozłowski. 2024. "Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation" Materials 17, no. 12: 2957. https://doi.org/10.3390/ma17122957
APA StyleNartowska, E., Podlasek, A., Vaverková, M. D., Koda, E., Jakimiuk, A., Kowalik, R., & Kozłowski, T. (2024). Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation. Materials, 17(12), 2957. https://doi.org/10.3390/ma17122957









