Interactions of Analgesics with Cisplatin: Modulation of Anticancer Efficacy and Potential Organ Toxicity
Abstract
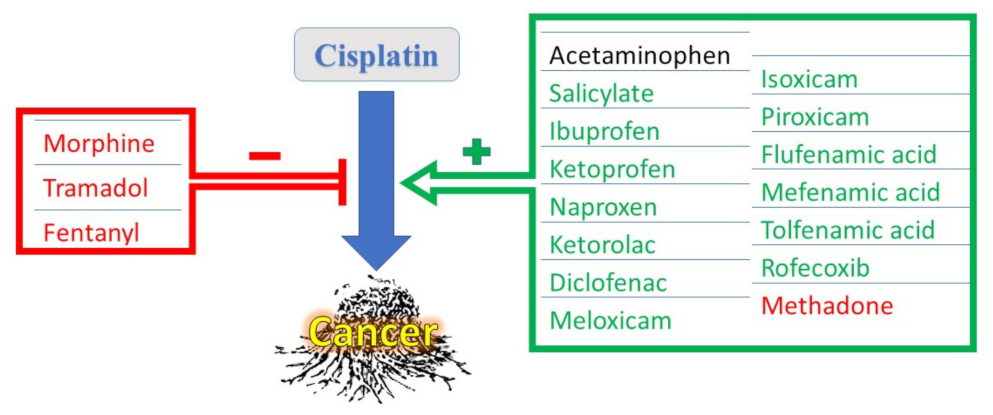
:1. Introduction
2. CDDP Efficacy and Toxicity
3. Interactions of Acetaminophen with CDDP
4. Interactions of NSAIDs with CDDP
4.1. Interactions of Salicylates with CDDP
4.2. Interaction of Propionic Acid-Derived NSAIDs (Profens) with CDDP
4.3. Interaction of Acetic Acid-Derived NSAIDS with CDDP
4.4. Interaction of Enolic Acid Derivatives of NSAIDs (Oxicams) with CDDP
4.5. Interaction of Anthranilic Acid and Naphthylalanine Derivatives of NSAIDs (Fenamates) with CDDP
4.6. Interaction of COX-II Selective NSAIDS (Coxibs) with CDDP
5. Interaction of Narcotic Analgesics with CDDP
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.; Gadanec, L.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Lv, Y.; Luo, C.; Liu, X.; Huang, C.; Xiu, Y.; Tang, L. Research Progress on the Functions and Mechanism of circRNA in Cisplatin Resistance in Tumors. Front. Pharmacol. 2021, 12, 709324. [Google Scholar] [CrossRef] [PubMed]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Dasari, S.; Noubissi, F.K.; Ray, P.; Kumar, S. Advances in Our Understanding of the Molecular Mechanisms of Action of Cisplatin in Cancer Therapy. J. Exp. Pharmacol. 2021, 13, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Hushmandi, K.; Zabolian, A.; Saleki, H.; Torabi, S.; Ranjbar, A.; SeyedSaleh, S.; Sharifzadeh, S.; Khan, H.; Ashrafizadeh, M.; et al. Elucidating Role of Reactive Oxygen Species (ROS) in Cisplatin Chemotherapy: A Focus on Molecular Pathways and Possible Therapeutic Strategies. Molecules 2021, 26, 2382. [Google Scholar] [CrossRef]
- Loren, P.; Saavedra, N.; Saavedra, K.; Zambrano, T.; Moriel, P.; Salazar, L. Epigenetic Mechanisms Involved in Cisplatin-Induced Nephrotoxicity: An Update. Pharmaceuticals 2021, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Rashid, N.A.; Halim, S.A.S.A.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Ridzuan, N.R.A.; Jalil, N.A.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A. P-Glycoprotein/ABCB1 Might Contribute to Morphine/Cisplatin-Induced Hepatotoxicity in Rats. Sci. Pharm. 2020, 88, 14. [Google Scholar] [CrossRef] [Green Version]
- Dugbartey, G.J.; Peppone, L.J.; de Graaf, I.A. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016, 371, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, F.; Farooqui, Z.; Khan, F. Cisplatin-induced gastrointestinal toxicity: An update on possible mechanisms and on available gastroprotective strategies. Eur. J. Pharmacol. 2018, 827, 49–57. [Google Scholar] [CrossRef]
- Gentilin, E.; Simoni, E.; Candito, M.; Cazzador, D.; Astolfi, L. Cisplatin-Induced Ototoxicity: Updates on Molecular Targets. Trends Mol. Med. 2019, 25, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, J.S.K.; Selakovic, D.; Mihailovic, V.; Rosic, G. Antioxidant Supplementation in the Treatment of Neurotoxicity Induced by Platinum-Based Chemotherapeutics—A Review. Int. J. Mol. Sci. 2020, 21, 7753. [Google Scholar] [CrossRef]
- Hopkins, H.L.; Duggett, N.; Flatters, S.J. Chemotherapy-induced painful neuropathy. Curr. Opin. Support. Palliat. Care 2016, 10, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, N.; Erduran, E.; Reis, G.P.; Bahadır, A. Chemotherapy-related fever or infection fever? Support. Care Cancer 2021, 29, 1859–1862. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.J.; Wu, Y.J.; Knap, N.; Losin, M.; Neuwelt, E.A.; Pagel, M.A.; Warmann, S.; Fuchs, J.; Czauderna, P.; Woźniak, M. Using Acetaminophen’s Toxicity Mechanism to Enhance Cisplatin Efficacy in Hepatocarcinoma and Hepatoblastoma Cell Lines. Neoplasia 2009, 11, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Neuwelt, A.J.; Nguyen, T.; Wu, Y.J.; Donson, A.M.; Vibhakar, R.; Venkatamaran, S.; Amani, V.; Neuwelt, E.A.; Rapkin, L.B.; Foreman, N.K. Preclinical high-dose acetaminophen with N -acetylcysteine rescue enhances the efficacy of cisplatin chemotherapy in atypical teratoid rhabdoid tumors. Pediatr. Blood Cancer 2014, 61, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-J.J.; Neuwelt, A.J.; Muldoon, L.L.; Neuwelt, E.A. Acetaminophen enhances cisplatin- and paclitaxel-mediated cytotoxicity to SKOV3 human ovarian carcinoma. Anticancer Res. 2013, 33, 2391–2400. [Google Scholar] [PubMed]
- Sohail, N.; Hira, K.; Tariq, A.; Sultana, V.; Ehteshamul-Haque, S. Marine macro-algae attenuates nephrotoxicity and hepatotoxicity induced by cisplatin and acetaminophen in rats. Environ. Sci. Pollut. Res. 2019, 26, 25301–25311. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing: Treasure Island, FL, USA, 2021; pp. 1–11. [Google Scholar]
- Ibrahim, M.A.; Albahlol, I.A.; Wani, F.A.; Tammam, A.A.-E.; Kelleni, M.T.; Sayeed, M.U.; El-Fadeal, N.M.A.; Mohamed, A.A. Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem. Interact. 2021, 338, 109402. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yang, C.-X.; Zhao, D.; Shen, L.-J.; Zhou, T.-J.; Bi, Y.-Y.; Huang, Z.-J.; Wei, Q.; Li, L.; Li, F.; et al. A carrier-free anti-inflammatory platinum (II) self-delivered nanoprodrug for enhanced breast cancer therapy. J. Control. Release 2021, 331, 460–471. [Google Scholar] [CrossRef]
- Dehkordi, N.G.; Mirzaei, S.A.; Elahian, F. Pharmacodynamic mechanisms of anti-inflammatory drugs on the chemosensitization of multidrug-resistant cancers and the pharmacogenetics effectiveness. Inflammopharmacology 2021, 29, 49–74. [Google Scholar] [CrossRef]
- Ravera, M.; Zanellato, I.; Gabano, E.; Perin, E.; Rangone, B.; Coppola, M.; Osella, D. Antiproliferative Activity of Pt(IV) Conjugates Containing the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Ketoprofen and Naproxen. Int. J. Mol. Sci. 2019, 20, 3074. [Google Scholar] [CrossRef] [Green Version]
- Walker, C. Are All Oral COX-2 Selective Inhibitors the Same? A Consideration of Celecoxib, Etoricoxib, and Diclofenac. Int. J. Rheumatol. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Morsy, M.; El-Sheikh, A. Prevention of Gastric Ulcers. In Peptic Ulcer Disease; IntechOpen: London, UK, 2011; pp. 437–460. [Google Scholar]
- Drożdżal, S.; Lechowicz, K.; Szostak, B.; Rosik, J.; Kotfis, K.; Machoy-Mokrzyńska, A.; Białecka, M.; Ciechanowski, K.; Gawrońska-Szklarz, B. Kidney damage from nonsteroidal anti-inflammatory drugs—Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 2021, 9, e00817. [Google Scholar] [CrossRef]
- Arora, M.; Choudhary, S.; Singh, P.K.; Sapra, B.; Silakari, O. Structural investigation on the selective COX-2 inhibitors mediated cardiotoxicity: A review. Life Sci. 2020, 251, 117631. [Google Scholar] [CrossRef]
- Yamakawa, N.; Suemasu, S.; Kimoto, A.; Arai, Y.; Ishihara, T.; Yokomizo, K.; Okamoto, Y.; Otsuka, M.; Tanaka, K.-I.; Mizushima, T. Low Direct Cytotoxicity of Loxoprofen on Gastric Mucosal Cells. Biol. Pharm. Bull. 2010, 33, 398–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suemasu, S.; Yamakawa, N.; Ishihara, T.; Asano, T.; Tahara, K.; Tanaka, K.-I.; Matsui, H.; Okamoto, Y.; Otsuka, M.; Takeuchi, K.; et al. Identification of a unique nsaid, fluoro-loxoprofen with gastroprotective activity. Biochem. Pharmacol. 2012, 84, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Tripp, M.; Howell, T.; Darland, G.; Bland, J.; Babish, J. Gastric mucosal cell model for estimating relative gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 9–17. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.J.; Villani, L.A.; Broadfield, L.A.; Houde, V.P.; Galic, S.; Blandino, G.; Kemp, B.E.; Tsakiridis, T.; Muti, P.; Steinberg, G.R. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem. J. 2015, 469, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Broadfield, L.A.; Marcinko, K.; Tsakiridis, E.; Zacharidis, P.G.; Villani, L.; Lally, J.S.V.; Menjolian, G.; Maharaj, D.; Mathurin, T.; Smoke, M.; et al. Salicylate enhances the response of prostate cancer to radiotherapy. Prostate 2019, 79, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Karalis, T.T.; Chatzopoulos, A.; Kondyli, A.; Aletras, A.J.; Karamanos, N.K.; Heldin, P.; Skandalis, S.S. Salicylate suppresses the oncogenic hyaluronan network in metastatic breast cancer cells. Matrix Biol. Plus 2020, 6, 100031. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.M. Priming effect of aspirin for tumor cells to augment cytotoxic action of cisplatin against tumor cells: Implication of altered constitution of tumor microenvironment, expression of cell cycle, apoptosis, and survival regulatory molecules. Mol. Cell. Biochem. 2012, 371, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Zhong, L.; Duan, T.; Zhang, R.-H.; Wang, X.; Wang, G.; Hu, K.; Lv, X.; Kang, T. Aspirin Suppresses the Growth and Metastasis of Osteosarcoma through the NF-κB Pathway. Clin. Cancer Res. 2015, 21, 5349–5359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, P.; Bhattacharya, A.; Sengupta, D.; Banerjee, S.; Adhikary, A.; Das, T. Aspirin enhances cisplatin sensitivity of resistant non-small cell lung carcinoma stem-like cells by targeting mTOR-Akt axis to repress migration. Sci. Rep. 2019, 9, 16913. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, Y.; Chen, M.; Luo, G.; Hao, J.; Pan, J.; Hu, S.; Guo, P.; Li, W.; Wang, R.; et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. Aging 2020, 12, 611–627. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, T.; Hui, Z. Aspirin overcomes cisplatin resistance in lung cancer by inhibiting cancer cell stemness. Thorac. Cancer 2020, 11, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, Y.; Yu, L.; Li, Y.; Guo, J.; Cai, J.; Liu, L.; Wang, Z. Aspirin inhibits tumor progression and enhances cisplatin sensitivity in epithelial ovarian cancer. PeerJ 2021, 9, e11591. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zheng, W.; Fan, H.; Deng, G.; Lu, S.-H.; Jiang, W.; Yu, X. Aspirin enhances the therapeutic efficacy of cisplatin in oesophageal squamous cell carcinoma by inhibition of putative cancer stem cells. Br. J. Cancer 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, H.; Wang, H.; Min, Y.; Wang, J.; Liu, Y. The ligation of aspirin to cisplatin demonstrates significant synergistic effects on tumor cells. Chem. Commun. 2014, 50, 7427–7430. [Google Scholar] [CrossRef]
- Pathak, R.K.; Marrache, S.; Choi, J.H.; Berding, T.B.; Dhar, S. The Prodrug Platin-A: Simultaneous Release of Cisplatin and Aspirin. Angew. Chem. Int. Ed. 2014, 53, 1963–1967. [Google Scholar] [CrossRef]
- Ulubaş, B.; Cimen, M.Y.B.; Apa, D.D.; Saritaş, E.; Muslu, N.; Cimen, Ö.B. The Protective Effects of Acetylsalicylic Acid on Free Radical Production in Cisplatin Induced Nephrotoxicity: An Experimental Rat Model. Drug Chem. Toxicol. 2003, 26, 259–270. [Google Scholar] [CrossRef]
- Yıldırım, M.; Inançlı, H.M.; Samancı, B.; Oktay, M.F.; Enöz, M.; Topçu, I. Preventing cisplatin induced ototoxicity by N-acetylcysteine and salicylate. Kulak Burun Bogaz Ihtis Derg 2010, 20, 173–183. [Google Scholar]
- Cetin, D.; Hacımuftuoglu, A.; Tatar, A.; Turkez, H.; Togar, B. The in vitro protective effect of salicylic acid against paclitaxel and cisplatin-induced neurotoxicity. Cytotechnology 2016, 68, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Kłobucki, M.; Urbaniak, A.; Grudniewska, A.; Kocbach, B.; Maciejewska, G.; Kiełbowicz, G.; Ugorski, M.; Wawrzeńczyk, C. Syntheses and cytotoxicity of phosphatidylcholines containing ibuprofen or naproxen moieties. Sci. Rep. 2019, 9, 220. [Google Scholar] [CrossRef]
- Alves, S.R.; Colquhoun, A.; Wu, X.Y.; Silva, D.D.O. Synthesis of terpolymer-lipid encapsulated diruthenium(II, III)-anti-inflammatory metallodrug nanoparticles to enhance activity against glioblastoma cancer cells. J. Inorg. Biochem. 2020, 205, 110984. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Yano, M.; Okumura, Y.; Kido, H. Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 2014, 5, e1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruzzella, E.; Sirota, R.; Solazzo, I.; Gandin, V.; Gibson, D. Triple action Pt(iv) derivatives of cisplatin: A new class of potent anticancer agents that overcome resistance. Chem. Sci. 2018, 9, 4299–4307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, C.-C.; Tsai, S.-T.; Lin, C.-Y.; Chang, L.-C.; Yang, J.-C.; Chen, G.; Sher, Y.-P.; Wang, S.-C.; Hsiao, M.; Chang, W. EFHD2 contributes to non-small cell lung cancer cisplatin resistance by the activation of NOX4-ROS-ABCC1 axis. Redox Biol. 2020, 34, 101571. [Google Scholar] [CrossRef]
- Gunjal, P.M.; Schneider, G.; Ismail, A.A.; Kakar, S.S.; Kucia, M.; Ratajczak, M.Z. Evidence for induction of a tumor metastasis-receptive microenvironment for ovarian cancer cells in bone marrow and other organs as an unwanted and underestimated side effect of chemotherapy/radiotherapy. J. Ovarian Res. 2015, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Awad, D.S.; Ali, R.M.; Mhaidat, N.M.; Shotar, A.M. Zizyphus jujuba protects against ibuprofen-induced nephrotoxicity in rats. Pharm. Biol. 2013, 52, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, E.J.; Lim, K.-M. Ibuprofen Increases the Hepatotoxicity of Ethanol through Potentiating Oxidative Stress. Biomol. Ther. 2021, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Song, X.-Q.; Hu, J.-J.; Wang, D.-B.; Ding, X.-J.; Liu, R.-P.; Dai, M.-L.; Meng, F.-Y.; Xu, J.-Y. Ketoplatin in triple-negative breast cancer cells MDA-MB-231: High efficacy and low toxicity, and positive impact on inflammatory microenvironment. Biochem. Pharmacol. 2021, 188, 114523. [Google Scholar] [CrossRef]
- Yasuyuki, S.; Yoshihiko, S.; Yoshio, T.; Sadao, H. Protection against cisplatin-induced nephrotoxicity in the rat by inducers and an inhibitor of glutathione S-transferase. Biochem. Pharmacol. 1994, 48, 453–459. [Google Scholar] [CrossRef]
- Fazzio, L.; Raggio, S.; Romero, J.; Membrebe, J.; Minervino, A. Safety Study on Ketoprofen in Pigs: Evaluating the Effects of Different Dosing and Treatment Scheme on Hematological, Hepatic, and Renal Parameters. Vet. Sci. 2021, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Zhao, Y.; Zhang, J.; Liu, M.; Wang, Z.; Li, D.; Han, J. Naproxen platinum(iv) hybrids inhibiting cycloxygenases and matrix metalloproteinases and causing DNA damage: Synthesis and biological evaluation as antitumor agents in vitro and in vivo. Dalton Trans. 2020, 49, 5192–5204. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, X.; Gao, J.; Ren, C.; Guo, Q.; Fan, H.; Liu, J.; Zhang, W.; Liu, J. A coassembled peptide hydrogel boosts the radiosensitization of cisplatin. Chem. Commun. 2020, 56, 13017–13020. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Hua, X.; Han, J.; Chang, C.; Wang, Z.; Li, D. Albumin-encapsulated Nanoparticles of Naproxen Platinum(IV) Complexes with Inflammation Inhibitory Competence Displaying Effective Antitumor Activities in vitro and in vivo. Int. J. Nanomed. 2021, 16, 5513–5529. [Google Scholar] [CrossRef]
- Jin, S.; Muhammad, N.; Sun, Y.; Tan, Y.; Yuan, H.; Song, D.; Guo, Z.; Wang, X. Multispecific Platinum(IV) Complex Deters Breast Cancer via Interposing Inflammation and Immunosuppression as an Inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed. 2020, 59, 23313–23321. [Google Scholar] [CrossRef] [PubMed]
- Poradowski, D.; Obmińska-Mrukowicz, B. Effect of selected nonsteroidal anti-inflammatory drugs on the viability of canine osteosarcoma cells of the D-17 line: In vitro studies. J. Vet. Res. 2019, 63, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, Ö.; Marinelli, L.; Cacciatore, I.; Ciulla, M.; Emsen, B.; Di Stefano, A.; Mardinoglu, A.; Turkez, H. Anticancer effects of novel NSAIDs derivatives on cultured human glioblastoma cells. Zeitschrift für Naturforschung C 2021, 76, 329–335. [Google Scholar] [CrossRef]
- Aytaç, P.; Sahin, I.D.; Atalay, R.Ç.; Tozkoparan, B. Design, Synthesis, and Biological Evaluation of Novel Triazolothiadiazoles Derived from NSAIDs as Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, Q.; Han, W.; Laster, K.; Hu, Y.; Ma, F.; Chen, H.; Tian, X.; Qiao, Y.; Liu, H.; et al. Targeting integrin αvβ3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin. Transl. Med. 2021, 11, e548. [Google Scholar] [CrossRef]
- Kozlowska, A.K.; Topchyan, P.; Kaur, K.; Tseng, H.-C.; Teruel, A.; Hiraga, T.; Jewett, A. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J. Cancer 2017, 8, 537–554. [Google Scholar] [CrossRef]
- Zhao, H.; Yi, B.; Liang, Z.; Phillips, C.; Lin, H.-Y.; Riker, A.I.; Xi, Y. Cyclin G2, a novel target of sulindac to inhibit cell cycle progression in colorectal cancer. Genes Dis. 2021, 8, 320–330. [Google Scholar] [CrossRef]
- Machkalyan, G.; Hèbert, T.E.; Miller, G.J. PPIP5K1 Suppresses Etoposide-triggered Apoptosis. J. Mol. Signal. 2016, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shriwas, O.; Priyadarshini, M.; Samal, S.K.; Rath, R.; Panda, S.; Das Majumdar, S.K.; Muduly, D.K.; Botlagunta, M.; Dash, R. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m6A-demethylation of FOXM1 and NANOG. Apoptosis 2020, 25, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ueda, H.; Saito, Y.; Narumi, K.; Furugen, A.; Kobayashi, M. Diclofenac potentiates the antitumor effect of cisplatin in a xenograft mouse model transplanted with cisplatin-resistant cells without enhancing cisplatin-induced nephrotoxicity. Drug Metab. Pharmacokinet. 2021, 41, 100417. [Google Scholar] [CrossRef]
- Okamoto, K.; Saito, Y.; Narumi, K.; Furugen, A.; Iseki, K.; Kobayashi, M. Anticancer effects of non-steroidal anti-inflammatory drugs against cancer cells and cancer stem cells. Toxicol. In Vitro 2021, 74, 105155. [Google Scholar] [CrossRef]
- Heidarian, E.; Nouri, A. Hepatoprotective effects of silymarin against diclofenac-induced liver toxicity in male rats based on biochemical parameters and histological study. Arch. Physiol. Biochem. 2021, 127, 112–118. [Google Scholar] [CrossRef]
- Naruse, T.; Nishida, Y.; Ishiguro, N. Synergistic effects of meloxicam and conventional cytotoxic drugs in human MG-63 osteosarcoma cells. Biomed. Pharmacother. 2007, 61, 338–346. [Google Scholar] [CrossRef]
- Honma, S.; Takahashi, N.; Shinohara, M.; Nakamura, K.; Mitazaki, S.; Abe, S.; Yoshida, M. Amelioration of cisplatin-induced mouse renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Eur. J. Pharmacol. 2013, 715, 181–188. [Google Scholar] [CrossRef]
- Tamasi, G.; Casolaro, M.; Magnani, A.; Sega, A.; Chiasserini, L.; Messori, L.; Gabbiani, C.; Valiahdi, S.M.; Jakupec, M.A.; Keppler, B.; et al. New platinum–oxicam complexes as anti-cancer drugs. Synthesis, characterization, release studies from smart hydrogels, evaluation of reactivity with selected proteins and cytotoxic activity in vitro. J. Inorg. Biochem. 2010, 104, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Menale, C.; Piccolo, M.T.; Favicchia, I.; Aruta, M.G.; Baldi, A.; Nicolucci, C.; Barba, V.; Mita, D.G.; Crispi, S.; Diano, N. Efficacy of Piroxicam Plus Cisplatin-Loaded PLGA Nanoparticles in Inducing Apoptosis in Mesothelioma Cells. Pharm. Res. 2014, 32, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.N.; Ramos-Vara, J.A.; Craig, B.A.; Hooser, S.B.; Anderson, C.; Fourez, L.M.; Johnson, B.M.; Stewart, J.C.; Knapp, D.W. Effects of cyclooxygenase inhibitor treatment on the renal toxicity of cisplatin in rats. Cancer Chemother. Pharmacol. 2010, 65, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, O.G.; Aasarod, K.; Wideroe, T.-E.; Guentert, T.W. Single- and multiple-dose pharmacokinetics, kidney tolerability and plasma protein binding of tenoxicam in renally impaired patients and healthy volunteers. Pharmacol. Toxicol. 2001, 89, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Karatopuk, D.U.; Gokcimen, A. Effect of tenoxicam on rat liver tissue. Turk. J. Gastroenterol. 2010, 21, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, R.; Tsuda, M.; Yoshida, K.; Tanino, M.; Kimura, T.; Nishihara, H.; Abe, T.; Shinohara, N.; Nonomura, K.; Tanaka, S. Aldo-keto reductase 1C1 induced by interleukin-1β mediates the invasive potential and drug resistance of metastatic bladder cancer cells. Sci. Rep. 2016, 6, 34625. [Google Scholar] [CrossRef] [Green Version]
- Shiiba, M.; Yamagami, H.; Yamamoto, A.; Minakawa, Y.; Okamoto, A.; Kasamatsu, A.; Sakamoto, Y.; Uzawa, K.; Takiguchi, Y.; Tanzawa, H. Mefenamic acid enhances anticancer drug sensitivity via inhibition of aldo-keto reductase 1C enzyme activity. Oncol. Rep. 2017, 37, 2025–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano-Hernandez, A.D.; Madrigal-Pérez, D.; Galvan-Salazar, H.R.; Martinez-Fierro, M.L.; Valdez, L.; Gomez, F.E.; Vazquez-Vuelvas, O.F.; Olmedo-Buenrostro, B.A.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.P.; et al. Anti-inflammatory drugs and uterine cervical cancer cells: Antineoplastic effect of meclofenamic acid. Oncol. Lett. 2015, 10, 2574–2578. [Google Scholar] [CrossRef] [Green Version]
- Caglar, S.; Altay, A.; Kuzucu, M.; Caglar, B. In Vitro Anticancer Activity of Novel Co(II) and Ni(II) Complexes of Non-steroidal Anti-inflammatory Drug Niflumic Acid Against Human Breast Adenocarcinoma MCF-7 Cells. Cell Biophys. 2021, 79, 729–746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, M.; Ye, C.; Xu, Q.; Wang, L. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m6A abrogation in RNA. J. Biol. Chem. 2019, 294, 16908–16917. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Y.; He, Z.; Chen, X.; Wu, X.; Li, X.; Bai, X.; Liu, W.; Li, B.; Wang, S.; et al. Meclofenamic Acid Reduces Reactive Oxygen Species Accumulation and Apoptosis, Inhibits Excessive Autophagy, and Protects Hair Cell-Like HEI-OC1 Cells from Cisplatin-Induced Damage. Front. Cell. Neurosci. 2018, 12, 139. [Google Scholar] [CrossRef]
- Grande, F.; Giordano, F.; Occhiuzzi, M.; Rocca, C.; Ioele, G.; De Luca, M.; Ragno, G.; Panno, M.; Rizzuti, B.; Garofalo, A. Toward Multitasking Pharmacological COX-Targeting Agents: Non-Steroidal Anti-Inflammatory Prodrugs with Antiproliferative Effects. Molecules 2021, 26, 3940. [Google Scholar] [CrossRef]
- Arai, Y.; Tanaka, K.-I.; Ushijima, H.; Tomisato, W.; Tsutsumi, S.; Aburaya, M.; Hoshino, T.; Yokomizo, K.; Suzuki, K.; Katsu, T.; et al. Low Direct Cytotoxicity of Nabumetone on Gastric Mucosal Cells. Dig. Dis. Sci. 2005, 50, 1641–1646. [Google Scholar] [CrossRef]
- Frejborg, E.; Salo, T.; Salem, A. Role of Cyclooxygenase-2 in Head and Neck Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 9246. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wang, B.; Liang, N.; Zhou, Q.; Long, S. MicroRNA and cyclooxygenase-2 in breast cancer. Clin. Chim. Acta 2021, 522, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.S.; Chen, X.M.; Huang, Z.G.; Wang, Z.R.; Zhang, D.W.; Zhang, X. Rofecoxib augments anticancer effects by reversing intrinsic multidrug resistance gene expression in BGC-823 gastric cancer cells. J. Dig. Dis. 2010, 11, 34–42. [Google Scholar] [CrossRef]
- Yu, L.; Chen, M.; Li, Z.; Wen, J.; Fu, J.; Guo, D.; Jiang, Y.; Wu, S.; Cho, C.-H.; Liu, S. Celecoxib Antagonizes the Cytotoxicity of Cisplatin in Human Esophageal Squamous Cell Carcinoma Cells by Reducing Intracellular Cisplatin Accumulation. Mol. Pharmacol. 2010, 79, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.X.; Lee, K.Y.; Bertram, C.T.; Goldstein, J.L. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: Impact on NSAID and gastroprotective drug prescribing and utilization. Curr. Med Res. Opin. 2007, 23, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Brenner, G.B.; Makkos, A.; Nagy, C.T.; Onódi, Z.; Sayour, N.V.; Gergely, T.G.; Kiss, B.; Görbe, A.; Sághy, É.; Zádori, Z.S.; et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells 2020, 9, 551. [Google Scholar] [CrossRef] [Green Version]
- Atashbar, S.; Jamali, Z.; Khezri, S.; Salimi, A. Celecoxib decreases mitochondrial complex IV activity and induces oxidative stress in isolated rat heart mitochondria: An analysis for its cardiotoxic adverse effect. J. Biochem. Mol. Toxicol. 2021, 2021, e22934. [Google Scholar] [CrossRef]
- Wu, F.; Wang, W.; Duan, Y.; Guo, J.; Li, G.; Ma, T. Effect of Parecoxib Sodium on Myocardial Ischemia-Reperfusion Injury Rats. Med. Sci. Monit. 2020, 27, e928205-1. [Google Scholar] [CrossRef]
- Okamoto, K.; Saito, Y.; Narumi, K.; Furugen, A.; Iseki, K.; Kobayashi, M. Comparison of the nephroprotective effects of non-steroidal anti-inflammatory drugs on cisplatin-induced nephrotoxicity in vitro and in vivo. Eur. J. Pharmacol. 2020, 884, 173339. [Google Scholar] [CrossRef] [PubMed]
- El-Kader, M.A.; Taha, R.I. Comparative nephroprotective effects of curcumin and etoricoxib against cisplatin-induced acute kidney injury in rats. Acta Histochem. 2020, 122, 151534. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.W.; Pockley, A. Influence of opioids on immune function in patients with cancer pain: From bench to bedside. Br. J. Pharmacol. 2017, 175, 2726–2736. [Google Scholar] [CrossRef]
- Özgürbüz, U.; Gencür, S.; Kurt, F.Ö.; Özkalkanlı, M.; Vatansever, H.S. The effects of tramadol on cancer stem cells and metabolic changes in colon carcinoma cells lines. Gene 2019, 718, 144030. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Tong, X.; Wang, L.; Wang, Q.; Ye, H.; Liu, B.; Hong, X.; Tao, L.; Harris, A. Tramadol and Flurbiprofen Depress the Cytotoxicity of Cisplatin via Their Effects on Gap Junctions. Clin. Cancer Res. 2009, 15, 5803–5810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, D.P.; Walia, D.; Heller, N.M. Suppression of Human Natural Killer Cells by Different Classes of Opioids. Anesthesia Analg. 2019, 128, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, C.; Gao, W.; Liang, J.; Liu, C.; Yang, H.; Yan, Q.; Wen, Q. Fentanyl induces autophagy via activation of the ROS/MAPK pathway and reduces the sensitivity of cisplatin in lung cancer cells. Oncol. Rep. 2016, 36, 3363–3370. [Google Scholar] [CrossRef]
- El-Sheikh, A.A.K.; Khired, Z. Morphine Deteriorates Cisplatin-Induced Cardiotoxicity in Rats and Induces Dose-Dependent Cisplatin Chemoresistance in MCF-7 Human Breast Cancer Cells. Cardiovasc. Toxicol. 2021, 21, 553–562. [Google Scholar] [CrossRef]
- Michalska, M.; Schultze-Seemann, S.; Kuckuck, I.; Katzenwadel, A.; Wolf, P. Impact of Methadone on Cisplatin Treatment of Bladder Cancer Cells. Anticancer Res. 2018, 38, 1369–1375. [Google Scholar] [CrossRef]
- Landgraf, V.; Griessmann, M.; Roller, J.; Polednik, C.; Schmidt, M. DL-Methadone as an Enhancer of Chemotherapeutic Drugs in Head and Neck Cancer Cell Lines. Anticancer Res. 2019, 39, 3633–3639. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Garcez, F.; Leal, S.; Afonso, L.; Nascimento, A.; Moreira, R.; Pereira, F.; Queirós, O.; Carvalho, F.; et al. Repeated Administration of Clinically Relevant Doses of the Prescription Opioids Tramadol and Tapentadol Causes Lung, Cardiac, and Brain Toxicity in Wistar Rats. Pharmaceuticals 2021, 14, 97. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Garcez, F.; Leal, S.; Afonso, L.; Nascimento, A.; Moreira, R.; Queirós, O.; Carvalho, F.; Dinis-Oliveira, R. Repeated Administration of Clinical Doses of Tramadol and Tapentadol Causes Hepato- and Nephrotoxic Effects in Wistar Rats. Pharmaceuticals 2020, 13, 149. [Google Scholar] [CrossRef]

| Name of NSAID | Organ/Tissue | Effect | Type of Experiment | Ref. |
|---|---|---|---|---|
| Acetaminophen | Kidney | Nephrotoxicity | Animal study (rat) | [23] |
| Liver | Hepatotoxicity | |||
| NSAIDs 1 | Kidney | Nephro-protective | Animal study (rat) | [48] |
| 1. Salicylate | Auditory system | Protect against ototoxicity | Human study | [49] |
| Neurons | Neuro-protective | In vitro | [50] | |
| 2. Propionic acid-derived NSAIDs | ||||
| Fluoro-loxoprofen | Stomach | Gastroprotective | Animal study (rats) | [34] |
| Ibuprofen | Kidney | Nephrotoxicity | Animal study (rat) | [57] |
| Liver cells | Hepatotoxicity | In vitro | [58] | |
| Ketoprofen | Kidney | Nephro-protective | Animal studies (rat and pig) | [60,61] |
| 3. Acetic acid-derived NSAIDS | ||||
| Indomethacin | Stomach cells | Gastric ulceration | In vitro | [35] |
| Diclofenac | Kidney | Nephrotoxicity | Human (review) | [31] |
| Liver | Hepatotoxicity | Animal study (rat) | [76] | |
| 4. Enolic acid-derived NSAIDs | ||||
| Meloxicam | Kidney | Nephroprotective | Animal study (mouse) | [78] |
| Piroxicam | Stomach | Gastric ulceration | Human (review) | [30] |
| Kidney | Nephrotoxicity | Animal study (rat) | [81] | |
| Tenoxicam | Liver | Hepatotoxicity | Animal study (rat) | [83] |
| 5. Anthranilic acid-derived NSAIDs | ||||
| Meclofenamic acid | Kidney | Nephrotoxicity | Animal study (mouse) and in vitro | [88] |
| Cochlear hair cell | Protect against ototoxicity | In vitro | [89] | |
| 6. COX-II 2 selective NSAIDS | ||||
| Valdecoxib | Heart | Cardiotoxicity | Human (review) | [96] |
| Rofecoxib | Heart | Cardiotoxicity | Animal study (rat) | [97] |
| Celecoxib | Cardiomyocytes | Cardiotoxicity | In vitro | [98] |
| Kidney | Nephroprotective | Animal study (rat) and in vitro | [100] | |
| Parecoxib | Heart | Cardio-protective | Animal study (rat) | [99] |
| Narcotic analgesics | ||||
| Morphine | Heart | Cardiotoxicity | Animal study (rat) | [13] |
| Liver | Hepatotoxicity | Animal study (rat) | [107] | |
| Tapentadol | Lung, heart, and neurons | Lung, heart, and neuronal toxicities | Animal study (rat) | [110] |
| Liver, Kidney | Hepato- and nephrotoxicity | Animal study (rat) | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheikh, A.; Khired, Z. Interactions of Analgesics with Cisplatin: Modulation of Anticancer Efficacy and Potential Organ Toxicity. Medicina 2022, 58, 46. https://doi.org/10.3390/medicina58010046
El-Sheikh A, Khired Z. Interactions of Analgesics with Cisplatin: Modulation of Anticancer Efficacy and Potential Organ Toxicity. Medicina. 2022; 58(1):46. https://doi.org/10.3390/medicina58010046
Chicago/Turabian StyleEl-Sheikh, Azza, and Zenat Khired. 2022. "Interactions of Analgesics with Cisplatin: Modulation of Anticancer Efficacy and Potential Organ Toxicity" Medicina 58, no. 1: 46. https://doi.org/10.3390/medicina58010046
APA StyleEl-Sheikh, A., & Khired, Z. (2022). Interactions of Analgesics with Cisplatin: Modulation of Anticancer Efficacy and Potential Organ Toxicity. Medicina, 58(1), 46. https://doi.org/10.3390/medicina58010046






