Joint Dysfunctionality Alleviation along with Systemic Inflammation Reduction Following Arthrocen Treatment in Patients with Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
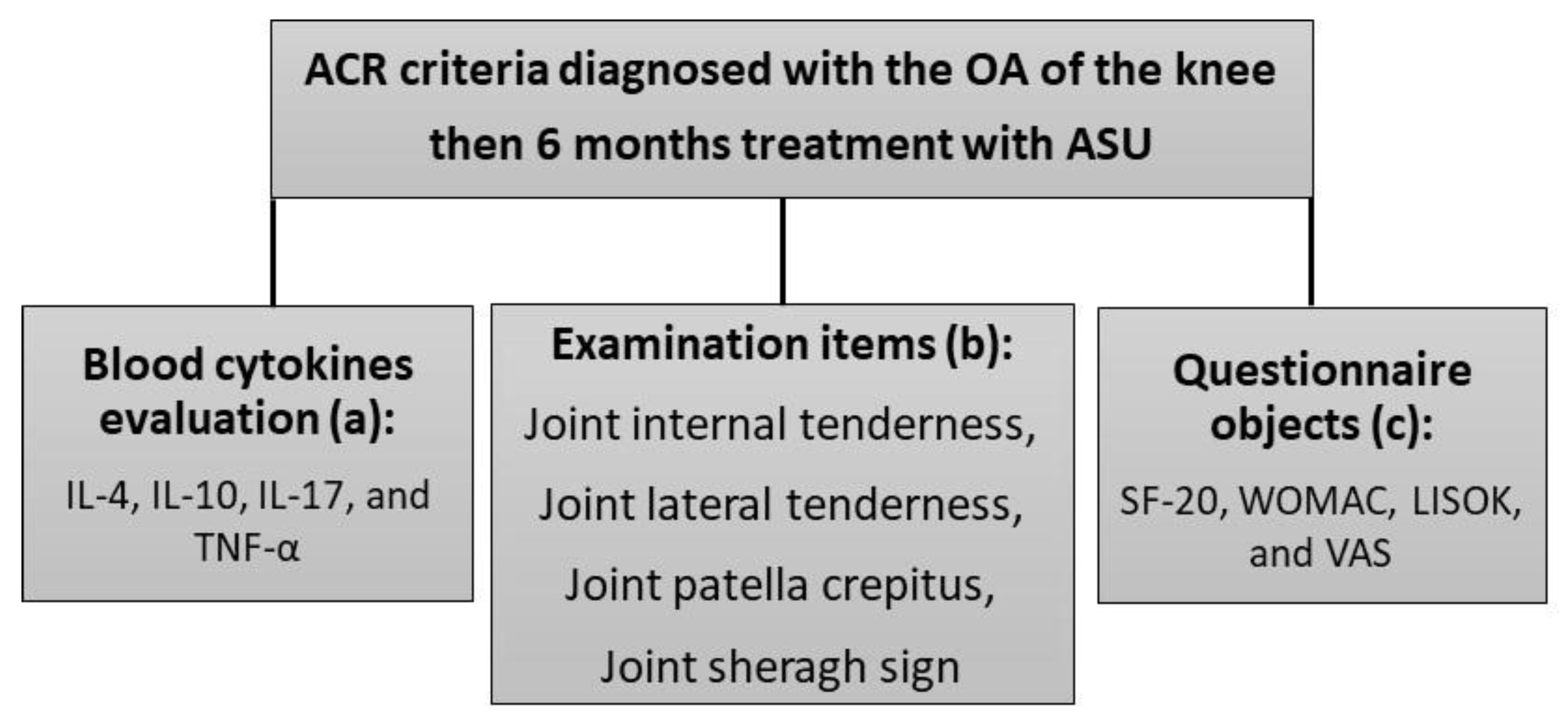
2.1. Study Design and Patients
2.2. Inflammatory Cytokines Evaluation
2.3. Clinical Assessments
3. Statistical Analysis
4. Results
4.1. Systemic Anti-Inflammatory Effect of ASU
4.2. OA Improvement in Patients Receiving Arthrocen
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Malemud, C.J. The biological basis of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 289. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Moskowitz, R.; Nuki, G.; Abramson, S.; Altman, R.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.; Croft, P.; Doherty, M. OARSI recommendations for the management of hip and knee osteoarthritis, part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthr. Cartil. 2007, 15, 981–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current treatment options for osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Latourte, A.; Frazier, A. Safety and efficacy of paracetamol and NSAIDs in osteoarthritis: Which drug to recommend? Expert Opin. Drug Saf. 2015, 14, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Losina, E.; Paltiel, A.D.; Weinstein, A.M.; Yelin, E.; Hunter, D.J.; Chen, S.P.; Klara, K.; Suter, L.G.; Solomon, D.H.; Burbine, S.A. Lifetime medical costs of knee osteoarthritis management in the United States: Impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015, 67, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Watt, F.E.; Gulati, M. New drug treatments for osteoarthritis: What is on the horizon? Eur. Med. J. Rheumatol. 2017, 2, 50. [Google Scholar]
- Trifunovic-König, M.; Klose, P.; Cramer, H.; Koch, A.K.; Dobos, G.; Langhorst, J. Phytotherapy for osteoarthritis: Evidence derived from two Cochrane reviews. Z. Fur Phytother. 2016, 37, 242–247. [Google Scholar]
- Goudarzi, R.; Reid, A.; McDougall, J.J. Evaluation of the novel avocado/soybean unsaponifiable Arthrocen to alter joint pain and inflammation in a rat model of osteoarthritis. PLoS ONE 2018, 13, e0191906. [Google Scholar] [CrossRef] [Green Version]
- Lippiello, L.; Nardo, J.V.; Harlan, R.; Chiou, T. Metabolic effects of avocado/soy unsaponifiables on articular chondrocytes. Evid.-Based Complement. Altern. Med. 2008, 5, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, M.; Sánchez-García, A.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Osuna-Garate, J.; Peña-Martínez, V.M.; Simental-Mendía, L.E. Efficacy and safety of avocado-soybean unsaponifiables for the treatment of hip and knee osteoarthritis: A systematic review and meta-analysis of randomized placebo-controlled trials. Int. J. Rheum. Dis. 2019, 22, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cudejko, T.; Van der Esch, M.; Van der Leeden, M.; Holla, J.; Roorda, L.D.; Lems, W.; Dekker, J. Proprioception mediates the association between systemic inflammation and muscle weakness in patients with knee osteoarthritis: Results from the Amsterdam Osteoarthritis cohort. J. Rehabil. Med. 2018, 50, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, T.; Brenner, H.; Koenig, W.; Günther, K. Severity and extent of osteoarthritis and low grade systemic inflammation as assessed by high sensitivity C reactive protein. Ann. Rheum. Dis. 2004, 63, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, B.A.; Bhatti, S.; Goudarzi, R.; Emami, S. Management of osteoarthritis with avocado/soybean unsaponifiables. Cartilage 2015, 6, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, R.; Nasab, M.E.; Saffari, P.M.; Zamanian, G.; Park, C.D.; Partoazar, A. Evaluation of ROCEN on Burn Wound Healing and Thermal Pain: Transforming Growth Factor-β1 Activation. Int. J. Low. Extrem. Wounds 2020, 20, 337–346. [Google Scholar] [CrossRef]
- Goudarzi, R.; Amini, S.; Dehpour, A.R.; Partoazar, A. Estimation of Anti-inflammatory and Analgesic Effects of Topical NANOCEN (Nanoliposomal Arthrocen) on Mice. AAPS PharmSciTech 2019, 20, 233. [Google Scholar] [CrossRef]
- Takahashi, A.; de Andres, M.; Hashimoto, K.; Itoi, E.; Oreffo, R. Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1946–1954. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, R.; Partoazar, A.; Mumtaz, F.; Yousefi-Manesh, H.; Abdollahi, A.; Dehpour, A.; Rashidian, A. Arthrocen, an avocado-soy unsaponifiable agent, improves acetic acid-induced colitis in rat by inhibition of NF-kB signaling pathway. J. Food Biochem. 2020, 44, e13244. [Google Scholar] [CrossRef]
- Mabey, T.; Honsawek, S. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Inoue, K.; Ushiyama, T.; Fukuda, S. Assessment of the American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Ryumachi. [Rheumatism] 1998, 38, 2–5. [Google Scholar] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Carver, D.J.; Chapman, C.A.; Thomas, V.S.; Stadnyk, K.J.; Rockwood, K. Validity and reliability of the Medical Outcomes Study Short Form-20 questionnaire as a measure of quality of life in elderly people living at home. Age Ageing 1999, 28, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Lequesne, M.; Mery, C.; Samson, M.; Gerard, P. Indexes of severity for osteoarthritis of the hip and knee: Validation–value in comparison with other assessment tests. Scand. J. Rheumatol. 1987, 16, 85–89. [Google Scholar] [CrossRef]
- Lang, M.; Bischl, B.; Surmann, D. Batchtools: Tools for R to work on batch systems. J. Open Source Softw. 2017, 2, 135. [Google Scholar] [CrossRef]
- Mosteller, F.; Tukey, J.W. Data Analysis and Regression: A Second Course in Statistics; Pearson: London, UK, 1977. [Google Scholar]
- Daghestani, H.N.; Kraus, V.B. Inflammatory biomarkers in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Guo, T.-M.; Zhu, C. Effects of nonsteroidal anti-inflammatory drugs on serum proinflammatory cytokines in the treatment of ankylosing spondylitis. Biochem. Cell Biol. 2018, 96, 450–456. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef] [Green Version]
- Van Roon, J.; Van Roy, J.; Duits, A.; Lafeber, F.; Bijlsma, J. Proinflammatory cytokine production and cartilage damage due to rheumatoid synovial T helper-1 activation is inhibited by interleukin-4. Ann. Rheum. Dis. 1995, 54, 836–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Roon, J.A.; van Roy, J.L.; Gmelig-Meyling, F.H.; Lafeber, F.P.; Bijlsma, J.W. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996, 39, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Jansen, N.W.; Roosendaal, G.; Hooiveld, M.J.; Bijlsma, J.W.; Van Roon, J.A.; Theobald, M.; Lafeber, F.P. Interleukin-10 protects against blood-induced joint damage. Br. J. Haematol. 2008, 142, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Shingu, M.; Miyauchi, S.; Nagai, Y.; Yasutake, C.; Horie, K. The role of IL-4 and IL-6 in IL-1-dependent cartilage matrix degradation. Rheumatology 1995, 34, 101–106. [Google Scholar] [CrossRef]
- Lacraz, S.; Nicod, L.; Chicheportiche, R.; Welgus, H.; Dayer, J. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J. Clin. Investig. 1995, 96, 2304–2310. [Google Scholar] [CrossRef] [Green Version]
- Pustjens, M.; Mastbergen, S.; Steen-Louws, C.; van Roon, J.; Hack, E.; Lafeber, F. IL4-10 synerkine induces direct and indirect structural cartilage repair in osteoarthritis. Osteoarthr. Cartil. 2016, 24, S532. [Google Scholar] [CrossRef]
- Iannone, F.; De Bari, C.; Dell Accio, F.; Covelli, M.; Cantatore, F.P.; Patella, V.; Bianco, G.L.; Lapadula, G. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin. Exp. Rheumatol. 2001, 19, 139–146. [Google Scholar]
- Au, R.; Al-Talib, T.; Au, A.; Phan, P.; Frondoza, C. Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartil. 2007, 15, 1249–1255. [Google Scholar] [CrossRef] [Green Version]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [Green Version]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Miller, F.G.; Colloca, L.; Kaptchuk, T.J. The placebo effect: Illness and interpersonal healing. Perspect. Biol. Med. 2009, 52, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Item | Group † | Before Treatment (±S.E.M) | After Treatment (±S.E.M) | p-Value ‡ |
|---|---|---|---|---|
| Joint internal tenderness | Placebo | 0.45 ± 0.07 | 0.24 ± 0.05 | p < 0.01 |
| Arthrocen | 0.38 ± 0.07 | 0.08 ± 0.04 | p < 0.001 | |
| p-value | p = 0.6 | p = 0.0139 | - | |
| Joint lateral tenderness | Placebo | 0.34 ± 0.06 | 0.14 ± 0.04 | p < 0.05 |
| Arthrocen | 0.29 ± 0.06 | 0.03 ± 0.02 | p < 0.001 | |
| p-value | p = 0.64 | p = 0.04 | - | |
| Joint patella crepitus | Placebo | 1.29 ± 0.08 | 1 ± 0.05 | p < 0.001 |
| Arthrocen | 1.15 ± 0.06 | 0.9 ± 0.05 | p < 0.001 | |
| p-value | p = 0.16 | p = 0.12 | - | |
| Joint shrug sign | Placebo | 0.48 ± 0.09 | 0.16 ± 0.05 | p < 0.001 |
| Arthrocen | 0.38 ± 0.07 | 0.12 ± 0.04 | p < 0.001 | |
| p-value | p = 0.46 | p = 0.59 | - |
| Item | Group † | Before Treatment (±S.E.M) | After Treatment (±S.E.M) | p-Value ‡ |
|---|---|---|---|---|
| SF-20 | Placebo | 46.09 ± 15.8 | 48.69 ± 14.8 | p = 0.116 |
| Arthrocen | 45.41 ± 15.9 | 52.29 ± 14.9 | p < 0.001 | |
| p-value | p = 0.147 | p = 0.295 | - | |
| WOMAC | Placebo | 63.98 ± 16- 1 | 55.85 ± 18.9 | p < 0.79 |
| Arthrocen | 67.56 ± 15.3 | 56.59 ± 17.8 | p < 0.001 | |
| p-value | p = 0.312 | p = 0.83 | - | |
| LISOK | Placebo | 21.06 ± 0.6 | 20.4 ± 0.6 | p < 0.29 |
| Arthrocen | 21.1 ± 0.6 | 20.9 ± 0.6 | p = 0.69 | |
| p-value | p = 0.9 | p = 0.5 | - | |
| VAS (pain) | Placebo | 4.6 ± 0.3 | 3.76 ± 0.3 | p < 0.01 |
| Arthrocen | 4.4 ± 0.3 | 3.56 ± 0.2 | p < 0.001 | |
| p-value | p = 0.67 | p = 0.66 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goudarzi, R.; Thomas, P.; Ryan, S. Joint Dysfunctionality Alleviation along with Systemic Inflammation Reduction Following Arthrocen Treatment in Patients with Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Medicina 2022, 58, 228. https://doi.org/10.3390/medicina58020228
Goudarzi R, Thomas P, Ryan S. Joint Dysfunctionality Alleviation along with Systemic Inflammation Reduction Following Arthrocen Treatment in Patients with Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Medicina. 2022; 58(2):228. https://doi.org/10.3390/medicina58020228
Chicago/Turabian StyleGoudarzi, Ramin, Peter Thomas, and Sandra Ryan. 2022. "Joint Dysfunctionality Alleviation along with Systemic Inflammation Reduction Following Arthrocen Treatment in Patients with Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial" Medicina 58, no. 2: 228. https://doi.org/10.3390/medicina58020228
APA StyleGoudarzi, R., Thomas, P., & Ryan, S. (2022). Joint Dysfunctionality Alleviation along with Systemic Inflammation Reduction Following Arthrocen Treatment in Patients with Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Medicina, 58(2), 228. https://doi.org/10.3390/medicina58020228





