Vaping-Associated Lung Injury: A Review
Abstract
:1. Introduction
2. Materials and Methods
3. Background
4. Epidemiology of E-Cigarette Use
5. Mechanism of Injury with Vaping
6. Electronic Cigarette and Vaping-Associated Lung Injury: EVALI
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustamante, G.; Ma, B.; Yakovlev, G.; Yershova, K.; Le, C.; Jensen, J.; Hatsukami, D.K.; Stepanov, I. Presence of the Carcinogen N′-Nitrosonornicotine in Saliva of E-cigarette Users. Chem. Res. Toxicol. 2018, 31, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.M.; Wagener, T.L.; Peck, J.D.; Brame, L.S.; Thompson, D.M.; Stephens, L.D.; Campbell, J.E.; Beebe, L.A. Biomarkers of Exposure in ENDS Users, Smokers, and Dual Users of American Indian Descent. Tob. Regul. Sci. 2018, 4, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Luzardo, O.P.; González-Antuña, A.; Zumbado, M.; Rogozea, L.; Floroian, L.; Alexandrescu, D.; Moga, M.; Gaman, L.; Radoi, M.; et al. Body burden of toxic metals and rare earth elements in non-smokers, cigarette smokers and electronic cigarette users. Environ. Res. 2018, 166, 269–275. [Google Scholar] [CrossRef]
- Phung, B.; Lam, A. Pediatric Acute Respiratory Distress Syndrome and Hypersensitivity Pneumonitis Related to E-cigarette Vaping. J. Pediatr. Intensive Care 2020, 9, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome from E-Cigarette Use. Pediatrics 2018, 141, e20163927. [Google Scholar] [CrossRef]
- Wolf, M.; Richards, J. Acute Eosinophilic Pneumonia Due to Vaping-Associated Lung Injury. J. Crit. Care Med. 2020, 6, 259–262. [Google Scholar] [CrossRef]
- Thota, D.; Latham, E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J. Emerg. Med. 2014, 47, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.W.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Final Report. N. Engl. J. Med. 2020, 382, 903–916. [Google Scholar] [CrossRef]
- Abrams, D.B.; Glasser, A.M.; Pearson, J.L.; Villanti, A.C.; Collins, L.K.; Niaura, R.S. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu. Rev. Public Health 2018, 39, 193–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozier, J.; Chivers, E.K.; Chapman, D.G.; Larcombe, A.N.; Bastian, N.A.; Masso-Silva, J.A.; Byun, M.K.; McDonald, C.F.; Alexander, L.E.; Ween, M.P. The Evolving Landscape of e-Cigarettes: A Systematic Review of Recent Evidence. Chest 2020, 157, 1362–1390. [Google Scholar] [CrossRef] [PubMed]
- Blagev, D.P.; Harris, D.; Dunn, A.C.; Guidry, D.W.; Grissom, C.K.; Lanspa, M.J. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: A prospective observational cohort study. Lancet 2019, 394, 2073–2083. [Google Scholar] [CrossRef]
- Zou, R.H.; Tiberio, P.J.; Triantafyllou, G.A.; Lamberty, P.E.; Lynch, M.J.; Kreit, J.W.; McVerry, B.J.; Gladwin, M.T.; Morris, A.; Chiarchiaro, J.; et al. Clinical Characterization of E-Cigarette, or Vaping, Product Use-associated Lung Injury in 36 Patients in Pittsburgh, Pennsylvania. Am. J. Respir. Crit. Care Med. 2020, 201, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Sangani, R.; Rojas, E.; Forte, M.; Zulfikar, R.; Prince, N.; Tasoglou, A.; Goldsmith, T.; Casuccio, G.; Boyd, J.; Olfert, I.M.; et al. Electronic Cigarettes and Vaping-Associated Lung Injury (EVALI): A Rural Appalachian Experience. Hosp. Pract. 2021, 49, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; Navarette, K.A.; McGraw, M.D.; Croft, D.P. E-cigarette, or vaping, product use associated lung injury (EVALI): Case series and diagnostic approach. Lancet Respir. Med. 2019, 7, 1017–1026. [Google Scholar] [CrossRef]
- Doukas, S.G.; Kavali, L.; Menon, R.S.; Izotov, B.N.; Bukhari, A. E-cigarette or vaping induced lung injury: A case series and literature review. Toxicol. Rep. 2020, 7, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Kass, A.P.; Overbeek, D.L.; Chiel, L.E.; Boyer, E.W.; Casey, A.M.H. Case series: Adolescent victims of the vaping public health crisis with pulmonary complications. Pediatr. Pulmonol. 2020, 55, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Kaous, M.; Xian, J.; Rongo, D.; McDonald, M.; Ocasionez, D.; Mathew, R.; Estrada-Y-Martin, R.M.; Patel, B.; Cherian, S.V.; Jani, P.P. Clinical, radiology, pathologic patterns and outcomes of vaping related pulmonary injury in a single institution: A case series. Respir. Med. 2020, 173, 106153. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Carl, J.C.; Rezaee, F. The importance of anti-vaping vigilance-EVALI in seven adolescent pediatric patients in Northeast Ohio. Pediatr. Pulmonol. 2020, 55, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Parlette, K.; Kuntz, H.M. E-cigarettes and Vaping, Product-use Associated Lung Injury: A Case Series of Adolescents. Clin. Pract. Cases Emerg. Med. 2021, 5, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary Lipid-Laden Macrophages and Vaping. N. Engl. J. Med. 2019, 381, 1488–1489. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Steindor, M.; Stehling, F.; Dohna-Schwake, C. EVALI (E-cigarette or vaping product use associated lung injury): First case report of an adolescent in Europe. Pediatr. Pulmonol. 2021, 56, 1274–1275. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; Koritala, T.; Gotur, R.; Malayala, S.V.; Jain, N.K. EVALI—E-Cigarette or Vaping Product Use-Associated Lung Injury: A Case Report. Cureus 2021, 13, e13541. [Google Scholar] [CrossRef] [PubMed]
- Ganne, N.; Palraj, R.; Husted, E.; Shah, I. E-cigarette or vaping product use-associated lung injury (EVALI) masquerading as COVID-19. BMJ Case Rep. 2021, 14, e243885. [Google Scholar] [CrossRef] [PubMed]
- Wekon-Kemeni, C.; Santhanam, P.; Halani, P.; Bradford, L.; Loughlin, C.E. A Gut Feeling: Abdominal Symptoms as an Initial Presentation of EVALI. Pediatrics 2021, 147, e20193834. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C.; Pedicelli, I.; Perna, F.; Di Spirito, V.; Fiorentino, G.; Procaccini, F.; Rea, G. E-cigarette, or vaping, product use Associated Lung Injury (EVALI): New scenarios for physicians and radiologists. Monaldi. Arch. Chest Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Colesar, M.T.; McCollum, D.J. E-Cigarette or Vaping Product Use-Associated Lung Injury (EVALI) in an Active Duty Service Member. Mil. Med. 2020, 186, e250–e253. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, O.; Sharma, K.; Fabre, A.; Murphy, D.J.; Keane, M.P.; McCarthy, C. Vaping-associated lung injury. Thorax 2020, 75, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Bozkanat, K.M.; Rao, D.R.; Lieu, T.J.; Rivera-Sanchez, Y.M. The perfect storm: A case of COVID-19 infection in an adolescent patient with EVALI. Respir. Med. Case Rep. 2020, 31, 101306. [Google Scholar] [CrossRef] [PubMed]
- Jankharia, B.; Rajan, S.; Angirish, B. Vaping associated lung injury (EVALI) as an organizing pneumonia pattern—A case report. Lung India 2020, 37, 533–535. [Google Scholar] [PubMed]
- Smith, E.; Cherian, R.; McGillen, B. A Case of E-cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) in a Previously Healthy Patient: Case Report and Literature Review. J. Gen. Intern. Med. 2020, 35, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Matta, P.; Hamati, J.N.; Unno, H.L.; Fox, M.D. E-cigarette or Vaping Product Use-Associated Lung Injury (EVALI) without Respiratory Symptoms. Pediatrics 2020, 145, e20193408. [Google Scholar] [CrossRef] [PubMed]
- Wellmann, K.F. Smoking and health. on the report of the advisory committee to the surgeon general of the public health service]. Dtsch. Med. Wochenschr. 1964, 89, 1085–1086. [Google Scholar] [PubMed]
- Thun, M.J.; Carter, B.D.; Feskanich, D.; Freedman, N.D.; Prentice, R.; Lopez, A.D.; Hartge, P.; Gapstur, S.M. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013, 368, 351–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, M.B.; Upson, D. Electronic cigarettes. Potential harms and benefits. Ann. Am Thorac. Soc. 2014, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.; Chapman, S. British American Tobacco on Facebook: Undermining Article 13 of the global World Health Organization Framework Convention on Tobacco Control. Tob. Control 2010, 19, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 1018–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Health at a Glance 2019: OECD Indicators; OECD Publishing: Paris, France, 2019; p. 243. [Google Scholar]
- Jha, P.; Ramasundarahettige, C.; Landsman, V.; Rostron, B.; Thun, M.; Anderson, R.N.; McAfee, T.; Peto, R. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 2013, 368, 341–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stead, L.F.; Perera, R.; Bullen, C.; Mant, D.; Hartmann-Boyce, J.; Cahill, K.; Lancaster, T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2012, 11, CD000146. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Glover, E.D.; Leischow, S.; Daughton, D.M.; Glover, P.N.; Muramoto, M.; Franzon, M.; Danielsson, T.; Landfeldt, B.; Westin, A. Efficacy of the nicotine inhaler in smoking reduction: A double-blind, randomized trial. Nicotine Tob. Res. 2006, 8, 555–564. [Google Scholar] [CrossRef]
- Bolliger, C.T.; Zellweger, J.P.; Danielsson, T.; van Biljon, X.; Robidou, A.; Westin, A.; Perruchoud, A.P.; Säwe, U. Smoking reduction with oral nicotine inhalers: Double blind, randomised clinical trial of efficacy and safety. BMJ 2000, 321, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, K.; Stevens, S.; Lancaster, T. Pharmacological treatments for smoking cessation. JAMA 2014, 311, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, N.A. Smoking cessation in patients with respiratory disease: Existing treatments and future directions. Lancet Respir. Med. 2013, 1, 241–250. [Google Scholar] [CrossRef]
- Oriakhi, M. Vaping: An Emerging Health Hazard. Cureus 2020, 12, e7421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnowski, T.R.; Odziomek, M. Particle Size Dynamics: Toward a Better Understanding of Electronic Cigarette Aerosol Interactions with the Respiratory System. Front. Physiol. 2018, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Salzman, G.A.; Alqawasma, M.; Asad, H. Vaping Associated Lung Injury (EVALI): An Explosive United States Epidemic. Mo. Med. 2019, 116, 492–496. [Google Scholar] [PubMed]
- Schier, J.G.; Meiman, J.G.; Layden, J.; Mikosz, C.A.; VanFrank, B.; King, B.A.; Salvatore, P.P.; Weissman, D.N.; Thomas, J.; Melstrom, P.C.; et al. Severe Pulmonary Disease Associated with Electronic-Cigarette-Product Use—Interim Guidance. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 787–790. [Google Scholar] [CrossRef]
- Lampos, S.; Kostenidou, E.; Farsalinos, K.; Zagoriti, Z.; Ntoukas, A.; Dalamarinis, K.; Savranakis, P.; Lagoumintzis, G. Real-Time Assessment of E-Cigarettes and Conventional Cigarettes Emissions: Aerosol Size Distributions, Mass and Number Concentrations. Toxics 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Balkissoon, R. Journal Club-Electronic Cigarettes and Vaping as a Harm Reduction Alternative: Really? Chronic. Obstr. Pulm. Dis. 2019, 6, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Kock, L.; Shahab, L.; West, R.; Brown, J. E-cigarette use in England 2014-17 as a function of socio-economic profile. Addiction 2019, 114, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Reid, J.L.; Rynard, V.L.; Fong, G.T.; Cummings, K.M.; McNeill, A.; Hitchman, S.; Thrasher, J.F.; Goniewicz, M.L.; Bansal-Travers, M.; et al. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: Repeat national cross sectional surveys. BMJ 2019, 365, l2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasza, K.A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Taylor, K.; Goniewicz, M.L.; Cummings, K.M.; Sharma, E.; Pearson, J.L.; Green, V.R.; et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N. Engl. J. Med. 2017, 376, 342–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann-Boyce, J.; McRobbie, H.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; Butler, A.R.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2020, 10, CD010216. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Habiballah, M.; Sayed, I.E. Efficacy of Electronic Cigarettes for Smoking Cessation: A Systematic Review and Meta-Analysis. Am. J. Health Promot. 2021, 35, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Phillips-Waller, A.; Przulj, D.; Pesola, F.; Myers Smith, K.; Bisal, N.; Li, J.; Parrott, S.; Sasieni, P.; Dawkins, L.; et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med. 2019, 380, 629–637. [Google Scholar] [CrossRef] [PubMed]
- McMillen, R.; Klein, J.D.; Wilson, K.; Winickoff, J.P.; Tanski, S. E-Cigarette Use and Future Cigarette Initiation Among Never Smokers and Relapse Among Former Smokers in the PATH Study. Public Health Rep. 2019, 134, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Gomajee, R.; El-Khoury, F.; Goldberg, M.; Zins, M.; Lemogne, C.; Wiernik, E.; Lequy-Flahault, E.; Romanello, L.; Kousignian, I.; Melchior, M. Association Between Electronic Cigarette Use and Smoking Reduction in France. JAMA Intern. Med. 2019, 179, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Soneji, S.S.; Knutzen, K.E.; Villanti, A.C. Use of Flavored E-Cigarettes Among Adolescents, Young Adults, and Older Adults: Findings from the Population Assessment for Tobacco and Health Study. Public Health Rep. 2019, 134, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Notes from the field: Electronic cigarette use among middle and high school students—United States, 2011–2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 729–730. [Google Scholar]
- Villanti, A.C.; Johnson, A.L.; Ambrose, B.K.; Cummings, K.M.; Stanton, C.A.; Rose, S.W.; Feirman, S.P.; Tworek, C.; Glasser, A.M.; Pearson, J.L.; et al. Flavored Tobacco Product Use in Youth and Adults: Findings from the First Wave of the PATH Study (2013–2014). Am. J. Prev. Med. 2017, 53, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, B.K.; Day, H.R.; Rostron, B.; Conway, K.P.; Borek, N.; Hyland, A.; Villanti, A.C. Flavored Tobacco Product Use Among US Youth Aged 12–17 Years, 2013–2014. JAMA 2015, 314, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Soule, E.K.; Lopez, A.A.; Guy, M.C.; Cobb, C.O. Reasons for using flavored liquids among electronic cigarette users: A concept mapping study. Drug Alcohol Depend. 2016, 166, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenson, N.I.; Kirkpatrick, M.G.; Barrington-Trimis, J.L.; Pang, R.D.; McBeth, J.F.; Pentz, M.A.; Samet, J.M.; Leventhal, A.M. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug Alcohol Depend. 2016, 168, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion; Health OoSa. E-Cigarette Use Among Youth and Young Adults; A Report of the Surgeon General; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2016.
- Hsu, G.; Sun, J.Y.; Zhu, S.H. Evolution of Electronic Cigarette Brands From 2013–2014 to 2016–2017: Analysis of Brand Websites. J. Med. Internet Res. 2018, 20, e80. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.L.; Cummins, S.E.; Sun, J.Y.; Zhu, S.H. Long-term e-cigarette use and smoking cessation: A longitudinal study with US population. Tob. Control 2016, 25 (Suppl. 1), i90–i95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tackett, A.P.; Lechner, W.V.; Meier, E.; Grant, D.M.; Driskill, L.M.; Tahirkheli, N.N.; Wagener, T.L. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction 2015, 110, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Adkison, S.E.; O’Connor, R.J.; Bansal-Travers, M.; Hyland, A.; Borland, R.; Yong, H.H.; Cummings, K.M.; McNeill, A.; Thrasher, J.F.; Hammond, D.; et al. Electronic nicotine delivery systems: International tobacco control four-country survey. Am. J. Prev. Med. 2013, 44, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.; Reyes-Guzman, C.; Grana, R.; Choi, K.; Freedman, N.D. Demographic Characteristics, Cigarette Smoking, and e-Cigarette Use Among US Adults. JAMA Netw. Open 2020, 3, e2020694. [Google Scholar] [CrossRef]
- Pearson, J.L.; Richardson, A.; Niaura, R.S.; Vallone, D.M.; Abrams, D.B. E-Cigarette awareness, use, and harm perceptions in US adults. Am. J. Public Health 2012, 102, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.F.; Bullen, C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction 2011, 106, 2017–2028. [Google Scholar] [CrossRef] [Green Version]
- Dawkins, L.; Turner, J.; Roberts, A.; Soar, K. ‘Vaping’ profiles and preferences: An online survey of electronic cigarette users. Addiction 2013, 108, 1115–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goniewicz, M.L.; Lingas, E.O.; Hajek, P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: An internet survey. Drug Alcohol Rev. 2013, 32, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Glasser, A.M.; Abudayyeh, H.; Pearson, J.L.; Villanti, A.C. E-Cigarette Marketing and Communication: How E-Cigarette Companies Market E-Cigarettes and the Public Engages with E-cigarette Information. Nicotine Tob. Res. 2019, 21, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Counts, M.E.; Morton, M.J.; Laffoon, S.W.; Cox, R.H.; Lipowicz, P.J. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005, 41, 185–227. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jabłońska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehn, B.M. FDA: Electronic cigarettes may be risky. JAMA 2009, 302, 937. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems; Public Health Consequences of E-Cigarettes; Eaton, D.L.; Kwan, L.Y.; Stratton, K. (Eds.) Public Health Consequences of E-Cigarettes; National Academies Press: Washington, DC, USA, 2018.
- Poynton, S.; Sutton, J.; Goodall, S.; Margham, J.; Forster, M.; Scott, K.; Liu, C.; McAdam, K.; Murphy, J.; Proctor, C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): Product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017, 106 Pt A, 522–532. [Google Scholar] [CrossRef]
- Breheny, D.; Adamson, J.; Azzopardi, D.; Baxter, A.; Bishop, E.; Carr, T.; Crooks, I.; Hewitt, K.; Jaunky, T.; Larard, S.; et al. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 2): In vitro biological assessment and comparison with different tobacco-heating products. Food Chem. Toxicol. 2017, 106 Pt A, 533–546. [Google Scholar] [CrossRef]
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.Y.; Grudzinska, F.S.; Dosanjh, D.; Parekh, D.; Foronjy, R.; et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018, 73, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Behar, R.Z.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 2018, 8, 8288. [Google Scholar] [CrossRef] [Green Version]
- Hua, M.; Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci. Rep. 2019, 9, 2782. [Google Scholar] [CrossRef] [Green Version]
- Higham, A.; Bostock, D.; Booth, G.; Dungwa, J.V.; Singh, D. The effect of electronic cigarette and tobacco smoke exposure on COPD bronchial epithelial cell inflammatory responses. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 989–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasanthi Bathrinarayanan, P.; Brown, J.E.P.; Marshall, L.J.; Leslie, L.J. An investigation into E-cigarette cytotoxicity in-vitro using a novel 3D differentiated co-culture model of human airways. Toxicol. In Vitro 2018, 52, 255–264. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Sisler, J.D.; Shaffer, J.; Leonard, S.S.; Morris, A.M.; Qian, Y.; Bello, D.; Demokritou, P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 2018, 344, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Manyanga, J.; Brame, L.; McGuire, D.; Sadhasivam, B.; Floyd, E.; Rubenstein, D.A.; Ramachandran, I.; Wagener, T.; Queimado, L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS ONE. 2017, 12, e0177780. [Google Scholar] [CrossRef] [Green Version]
- Shaito, A.; Saliba, J.; Husari, A.; El-Harakeh, M.; Chhouri, H.; Hashem, Y.; Shihadeh, A.; El-Sabban, M. Electronic Cigarette Smoke Impairs Normal Mesenchymal Stem Cell Differentiation. Sci. Rep. 2017, 7, 14281. [Google Scholar] [CrossRef] [PubMed]
- Anthérieu, S.; Garat, A.; Beauval, N.; Soyez, M.; Allorge, D.; Garçon, G.; Lo-Guidice, J.-M. Comparison of cellular and transcriptomic effects between electronic cigarette vapor and cigarette smoke in human bronchial epithelial cells. Toxicol. In Vitro 2017, 45 Pt 3, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Majeste, A.; Hanus, J.; Wang, S. E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicol. Sci. 2016, 154, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016, 138, 386–396. [Google Scholar]
- Dicpinigaitis, P.V. Effect of tobacco and electronic cigarette use on cough reflex sensitivity. Pulm. Pharmacol. Ther. 2017, 47, 45–48. [Google Scholar] [CrossRef]
- Polosa, R.; Morjaria, J.B.; Prosperini, U.; Russo, C.; Pennisi, A.; Puleo, R.; Caruso, M.; Caponnetto, P. Health effects in COPD smokers who switch to electronic cigarettes: A retrospective-prospective 3-year follow-up. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2533–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polosa, R.; Morjaria, J.B.; Caponnetto, P.; Prosperini, U.; Russo, C.; Pennisi, A.; Bruno, C.M. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir. Res. 2016, 17, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maouche, K.; Medjber, K.; Zahm, J.M.; Delavoie, F.; Terryn, C.; Coraux, C.; Pons, S.; Cloëz-Tayarani, I.; Maskos, U.; Birembaut, P.; et al. Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 4099–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soule, E.K.; Maloney, S.F.; Spindle, T.R.; Rudy, A.K.; Hiler, M.M.; Cobb, C.O. Electronic cigarette use and indoor air quality in a natural setting. Tob. Control 2017, 26, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016, 94, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, L.; Suri, R.; Dearing, E.; Mudway, I.; Dove, R.E.; Neill, D.R.; Zyl-Smit, R.V.; Kadioglu, A.; Grigg, J. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018, 51, 1701592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cravo, A.S.; Bush, J.; Sharma, G.; Savioz, R.; Martin, C.; Craige, S.; Walele, T. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. 1), S1–S14. [Google Scholar] [CrossRef] [Green Version]
- D’Ruiz, C.D.; O’Connell, G.; Graff, D.W.; Yan, X.S. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regul. Toxicol. Pharmacol. 2017, 87, 36–53. [Google Scholar] [CrossRef]
- Ferrari, M.; Zanasi, A.; Nardi, E.; Morselli Labate, A.M.; Ceriana, P.; Balestrino, A.; Pisani, L.; Corcione, N.; Nava, S. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulm. Med. 2015, 15, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varughese, S.; Teschke, K.; Brauer, M.; Chow, Y.; van Netten, C.; Kennedy, S.M. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am. J. Ind. Med. 2005, 47, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, G.; Norbäck, D.; Lindgren, T. Experimental exposure to propylene glycol mist in aviation emergency training: Acute ocular and respiratory effects. Occup. Environ. Med. 2001, 58, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnasamy, V.P.; Hallowell, B.D.; Ko, J.Y.; Board, A.; Hartnett, K.P.; Salvatore, P.P.; Danielson, M.; Kite-Powell, A.; Twentyman, E.; Kim, L.; et al. Update: Characteristics of a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury—United States, August 2019–January 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basset-Léobon, C.; Lacoste-Collin, L.; Aziza, J.; Bes, J.C.; Jozan, S.; Courtade-Saïdi, M. Cut-off values and significance of Oil Red O-positive cells in bronchoalveolar lavage fluid. Cytopathology 2010, 21, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lozier, M.J.; Wallace, B.; Anderson, K.; Ellington, S.; Jones, C.M.; Rose, D.; Baldwin, G.; King, B.A.; Briss, P.; Mikosz, C.A. Update: Demographic, Product, and Substance-Use Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injuries—United States, December 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, K.; Buch, M.; Ramos-Ramirez, M.; Sharma, M.; Patel, S.; Choudhury, S.; Anjum, H.; Khan, A.; Surani, S. A Case Series of Vaping-Induced Lung Injury in a Community Hospital Setting. Case Rep. Pulmonol. 2020, 2020, 9631916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L585–L595. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Wiens, T.; Peterson, J.; Saravia, S.; Lunda, M.; Hanson, K.; Wogen, M.; D’Heilly, P.; Margetta, J.; Bye, M.; et al. Characteristics of E-cigarette, or Vaping, Products Used by Patients with Associated Lung Injury and Products Seized by Law Enforcement—Minnesota, 2018 and 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Karwowski, M.P.; Morel-Espinosa, M.; Rees, J.; Sosnoff, C.; Cowan, E.; Gardner, M.; Wang, L.; Valentin-Blasini, L.; Silva, L.; et al. Evaluation of Bronchoalveolar Lavage Fluid from Patients in an Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury—10 States, August–October 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
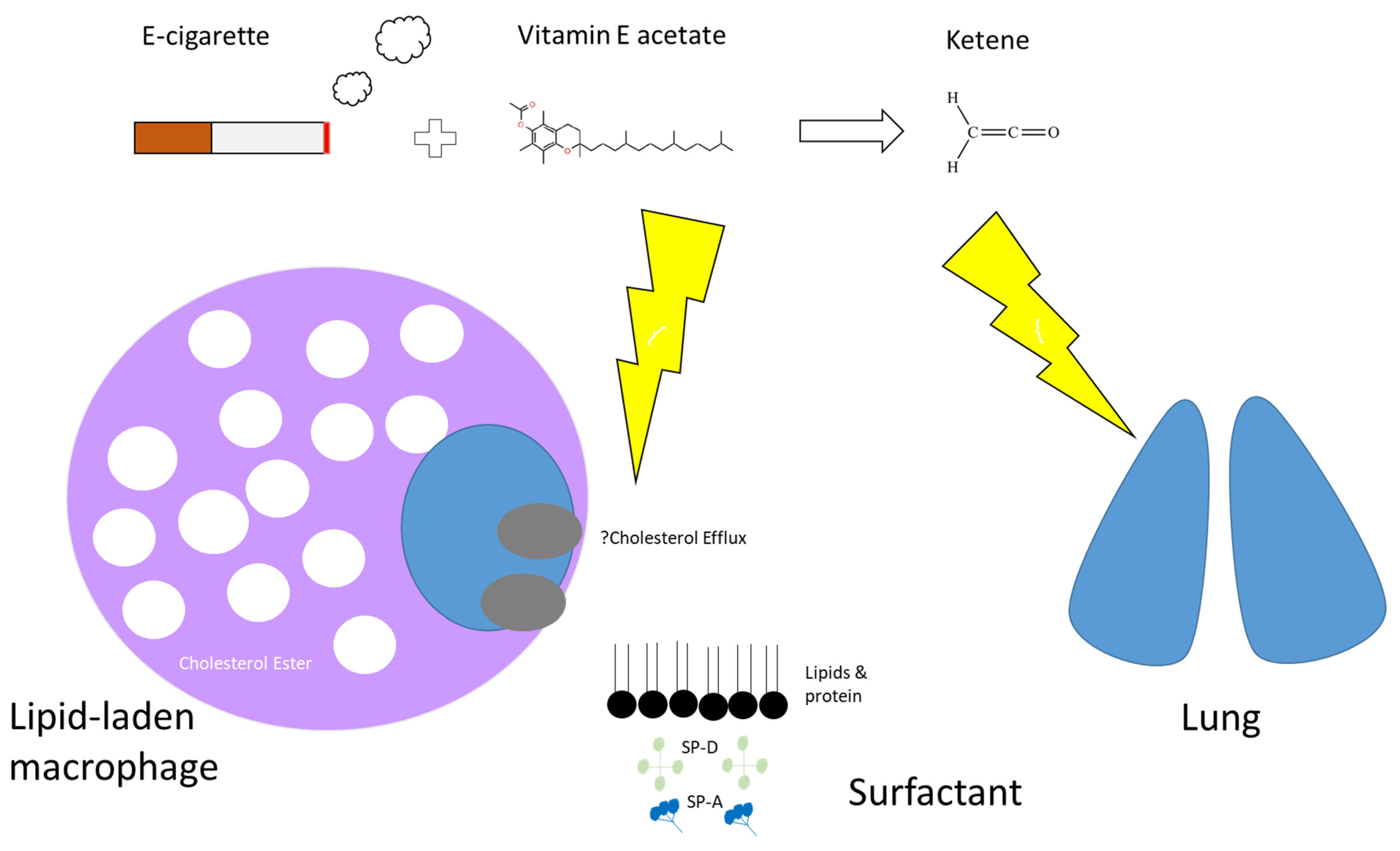
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Kalathil, S.G.; Bogner, P.N.; Blount, B.C.; Goniewicz, M.L.; Thanavala, Y.M. An Animal Model of Inhaled Vitamin E Acetate and EVALI-like Lung Injury. N. Engl. J. Med. 2020, 382, 1175–1177. [Google Scholar] [CrossRef]
- Attfield, K.R.; Chen, W.; Cummings, K.J.; Jacob, P.; O’Shea, D.F.; Wagner, J.; Wang, P.; Fowles, J. Potential of Ethenone (Ketene) to Contribute to Electronic Cigarette, or Vaping, Product Use-associated Lung Injury. Am. J. Respir. Crit. Care Med. 2020, 202, 1187–1189. [Google Scholar] [CrossRef]
- Lee, H. Vitamin E acetate as linactant in the pathophysiology of EVALI. Med. Hypotheses 2020, 144, 110182. [Google Scholar] [CrossRef]
- Wu, D.; O’Shea, D.F. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc. Natl. Acad. Sci. USA 2020, 117, 6349–6355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevention CfDCa. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Available online: https://www.cdc.gov (accessed on 25 February 2021).
- Evans, M.E.; Twentyman, E.; Click, E.S.; Goodman, A.B.; Weissman, D.N.; Kiernan, E.; Adkins Hocevar, S.; Mikosz, C.A.; Danielson, M.; Anderson, K.N.; et al. Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use-Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge—United States, December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 68, 1189–1194. [Google Scholar] [PubMed]
- Pacula, R.L. The need to more effectively regulate END markets: A primary public health lesson of the U.S. vaping associated lung injury outbreak. Addiction 2021, 116, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Palamar, J.J. Increases in Frequent Vaping of Cannabis among High School Seniors in the United States, 2018–2019. J. Adolesc. Health 2021, 69, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.A. Regulation of E-Cigarettes in the United States and Its Role in a Youth Epidemic. Children 2019, 6, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghinai, I.; Pray, I.W.; Navon, L.; O’Laughlin, K.; Saathoff-Huber, L.; Hoots, B.; Kimball, A.; Tenforde, M.W.; Chevinsky, J.R.; Layer, M.; et al. E-cigarette Product Use, or Vaping, Among Persons with Associated Lung Injury—Illinois and Wisconsin, April-September 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 865–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navon, L.; Jones, C.M.; Ghinai, I.; King, B.A.; Briss, P.A.; Hacker, K.A.; Layden, J.E. Risk Factors for E-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) among Adults Who Use E-Cigarette, or Vaping, Products—Illinois, July–October 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1034–1039. [Google Scholar] [CrossRef] [PubMed]

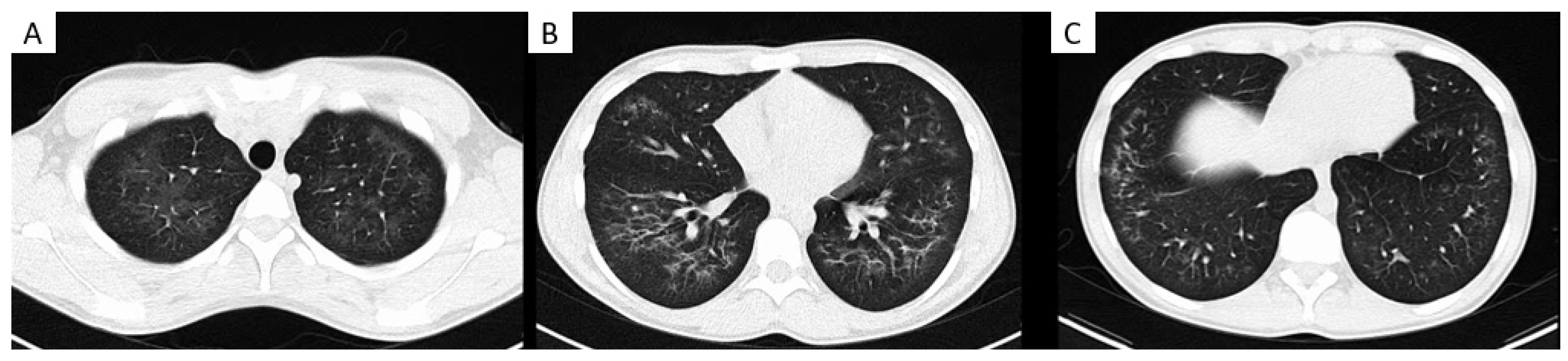
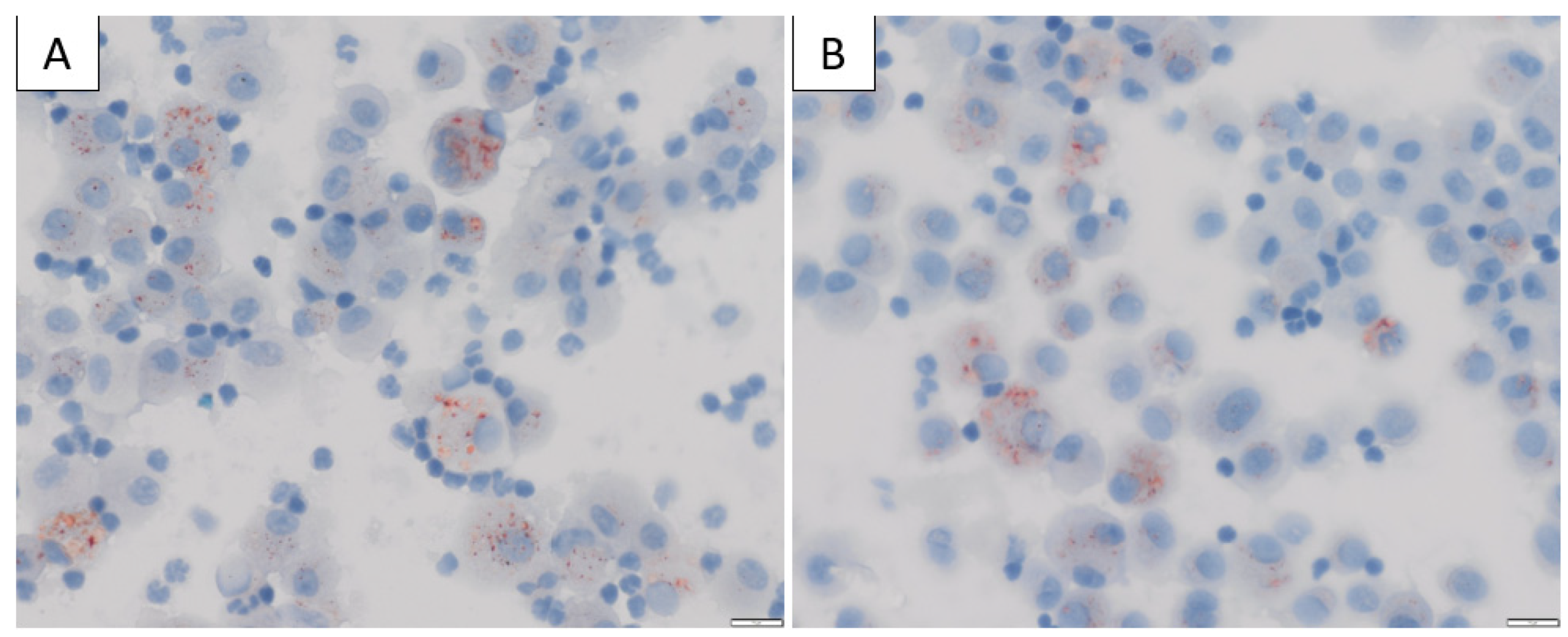
| Study | # | Symptoms | Vital Signs | Radiology (CT Chest Findings) | Laboratory Findings | BAL Findings | Clinical Course and Outcomes |
|---|---|---|---|---|---|---|---|
| Layden et al., 2020; USA [9] | 98 | Respiratory SOB 83/98 (85%) Chest pain 51/98 (52%) Cough 83/98 (85%) Hemoptysis 8/98 (8%) GI Nausea 65/98 (66%) Vomiting 60/98 (61%) Diarrhoea 43/98 (44%) Abdominal pain 33/98 (34%) Constitutional Fever 82/98 (84%) Weight loss 25/98 (26%) Fatigue 46/98 (34%) | Pyrexia 33% Hypoxia (SpO2 < 95%) 58% | Bilateral infiltrates 100% | ESR > 30 mm/h in 90% | 2–68% macrophages 56% of those reported presence of LLMs | Admitted 93/98 Intubation 25/98 Antibiotics 86/93 Steroids 78/93 Died 2/98 |
| Blagev et al., 2019; USA [12] | 60 | Respiratory SOB 51/60 (85%) Chest pain 26/60 (43%) Cough 47/60 (78%) Hemoptysis 7/60 (12%) GI Nausea 45/60 (75%) Vomiting 43/60 (72%) Abdominal pain 28/60 (47%) Constitutional Fever 46/60 (78%) Weight loss 7/60 (12%) Fatigue 29/60 (48%) | Pyrexia 57% Hypoxia (SpO2 < 95%) 87% | Abnormal Chest CT 100% | Mean CRP 31 mg/L ESR > 3.0 mm/h in 92% | 63% (12/19) neutrophil predominant BAL21% (4/19) macrophage predominant BAL89% (8/9) reported presence of LLMs | Admitted 54/60 Intubation 10/60 Antibiotics 54/60 Steroids 57/60 Died 2/60 |
| Zou et al., 2020; USA [13] | 36 | - | Mean fever 38.1 Mean SpO2 94% | Abnormal 97% | - | - | Admitted 36/36 Intubation 7/36 Antibiotics 28/36 Steroids 26/36 |
| Sangani et al., 2020; USA [14] | 17 | Respiratory SOB 17/17 (100%) Chest pain 6/17 (35%) Cough 12/17 (71%) GI symptoms 9/17 (53%) Fever 12/60 (71%) Constitutional symptoms 12/17 (71%) | - | Bilateral GGO 82% Consolidation 41% | - | 24% (4/15) Neutrophil predominant BAL 87% (13/15) had ORO staining on BAL sample | Admitted 17/17 Intubation 7/17 |
| Kalininskiy et al., 2019; USA [15] | 12 | Respiratory SOB 10/11 (91%) Cough 9/11 (82%) Pleuritic pain 6/11 (55%) Sputum 4/11 (36%) Haemoptysis 1/11 (9%) GI Vomiting 10/11 (91%) Nausea 7/11 (64%) Abdominal pain 3/11 (27%) Diarrhoea 3/11 (27%) Constitutional Fever 10/12 (83%) Malaise 9/12 (75%) Sweats 5/12 (42%) Myalgia 2/12 (17%) | Pyrexia 75% Hypoxia (SpO2 < 95%) 75% | Bilateral GGO 100% Subpleural sparing 64% Fibrotic features 18% | Median Eos 0.03 × 109 Median CRP 232 mg/L Median ESR 80.5 mm/h | - | Admitted 12/12 Intubation 1/12 Antibiotics 11/12 Steroids 8/12 |
| Doukas et al., 2020; USA [16] | 10 | Respiratory symptoms 80% (8/10) GI symptoms 60% (6/10) Constitutional symptoms 60% (6/10) | Pyrexia (40%) Hypoxia (SpO2 < 95%) 60% | Bilateral GGO 100% | - | - | Admitted 9/10 Antibiotics 10/10 IV steroids 4/10 |
| Kass et al., 2020; USA [17] | 10 | Respiratory SOB 5/10 (50%) Cough 6/10 (60%) Pleuritic pain 3/10 (30%) Haemoptysis 3/10 (30%) GI Vomiting 5/10 (50%) Nausea 4/10 (40%) Diarrhoea 3/10 (30%) Weight loss 2/10 (20%) Constitutional Fever 3/10 (30%) Fatigue 1/10 (10%) Sweats 2/10 (20%) | Hypoxia (SpO2 < 95%) 60% | Bilateral GGO 50% Consolidation 10% Cavitation 10% Bronchiectasis 30% | Mean CRP (9/10) 8.93 mg/dL Mean ESR (4/10) 11.25 mm/h | 6/10 patients 66% macrophage predominant BAL All with LLMs 16% neutrophil and eosinophilic predominant BAL | Admitted 10/10 Intubated 1/10 Antibiotics 10/10 Steroids 6/10 |
| Kaous et al., 2020; USA [18] | 8 | Respiratory SOB 8/8 (100%) Chest pain 3/8 (37.5%) GI Nausea 4/8 (50%) Vomiting 1/8 (12.5%) Constitutional Fever 6/8 (75%) Myalgia 3/8 (37.5%) | Hypoxia (not defined) 87.5% | Bilateral opacities 100% | - | 50% (3/6) Macrophage predominance on BAL 100% (6/6) LLMs on BAL | Admitted 8/8 Steroids 6/8 |
| Corcoran et al., 2020; USA [19] | 7 | Respiratory SOB 5/7 (71%) Cough 6/7 (85%) Chest pain 3/7 (42%) Haemoptysis 1/7 (14%) GI Vomiting 5/7 (71%) Constitutional Fevers 4/7 (57%) | Pyrexia 42% Hypoxia (SpO2 < 95%) 57% | Bilateral GGO 85% Consolidation 42% Nodules 14% | Median CRP 34.9 mg/dL | - | Admitted 7/7 Antibiotics 6/7 Steroids 3/7 |
| Khan et al., 2021; USA [20] | 7 | Respiratory SOB 5/7 (71%) Cough 6/7 (85%) Chest pain 2/7 (28%) GI Abdo. Pain 1/7 (14%) Nausea 4/7 (57%) Vomiting 3/7 (42%) Diarrhoea 1/7 (14%) Constitutional Fevers 4/7 (57%) | Pyrexia 42% Hypoxia (SpO2 < 95%) 85% | Bilateral GGO 71% Consolidation 42% Subpleural sparing 14% | - | - | Admitted 7/7 Intubated 1/7 Steroids 6/7 |
| Maddock et al., 2019; USA [21] | 6 | Respiratory SOB 2/6 (33%) Cough 4/6 (66%) Wheeze 1/6 (16%) GI Abdo. Pain 3/6 (50%) Nausea 3/6 (50%) Vomiting 2/6 (33%) Weight loss 1/6 (16%) Constitutional Fevers 4/6 (66%) Sweats 3/6 (50%) Myalgia 4/6 (66%) Weakness 3/6 (50%) | Pyrexia (83%) | Bilateral infiltrates 100% | Eos 0.0–2.9 × 109 CRP 20.4–30.7 ESR 60–128 mm/h | 32–79% macrophages on BAL 25–75% LLMs | Admitted 6/6 Intubation 1/6 Antibiotics 5/6 Steroids 4/6 |
| Schäfer et al., 2021; Germany [22] | 1 | SOB, dry cough, weight loss, fatigue | Hypoxia (SpO2 < 95%) | Bilateral GGO Interlobular septal thickening | Eos 0.1 × 109 CRP 11.9 mg/dl | 88% neutrophils on BAL | Admitted IV steroids |
| Adhikari et al., 2021; USA [23] | 1 | SOB, tachypnoea, nausea, diarrhoea, fever | Pyrexia Hypoxia (SpO2 < 95%) | Bilateral infiltrates | CRP 35 mg/dL ESR 97 mm/h | - | Admitted Antibiotics Steroids |
| Ganne et al., 2021; USA [24] | 1 | SOB, cough, fevers, myalgia, fatigue | Hypoxia (SpO2 < 95%) | Bilateral infiltrates | CRP > 400 mg/L | - | Admitted Antibiotics Steroids |
| Wekon-Kemeni et al., 2021; USA [25] | 1 | Nausea, vomiting, abdominal pain, fevers, headaches | Pyrexia Hypoxia (SpO2 < 95%) | Multifocal GGO, crazy paving | CRP 303 mg/L ESR 86 mm/h | - | Admitted Antibiotics Steroids |
| Guarino et al., 2021; Italy [26] | 1 | SOB, cough | - | Focal GGO, Consolidation, nodular change | CRP 0.4 mg/dLESR 17 mm/h | 95% macrophages on BAL ORO stain positive | Admitted Antibiotics Steroids |
| Colesar et al., 2021; USA [27] | 1 | SOB, cough, chest pain, vomiting, fevers, headache | Pyrexia | Bilateral GGO | - | LLM identified on BAL cytology | Admitted Intubated Antibiotics IV steroids |
| O’Carroll et al., 2020; Ireland [28] | 1 | Cough, weight loss, sweats, fever | Pyrexia | Bilateral GGO Subpleural sparing | Eos 0.85 × 109 CRP normal ESR 100 mm/h | 66% macrophages on BAL 25% LLMs | Admitted |
| Wolf et al., 2020; USA [7] | 1 | SOB, sore throat, fevers | Hypoxia (SpO2 < 95%) | Bilateral nodular GGO Basal consolidation | Eos 5.8 × 109 | 36% eosinophils on BAL | Admitted Intubated IV steroids |
| Bozkanat et al., 2020; USA [29] | 1 | SOB, cough, abdominal pain, diarrhoea, weight loss | Pyrexia Hypoxia (SpO2 < 95%) | Bilateral GGO and scattered opacities | CRP 33 mg/dL | - | Admitted Steroids |
| Jankharia et al., 2020; India [30] | 1 | Cough | - | Bilateral GGO and opacities | - | - | Antibiotics |
| Smith et al., 2020; USA [31] | 1 | SOB, chest pain, nausea, vomiting, diarrhoea, fevers, headache | Pyrexia | Bilateral GGO Subpleural sparing | CRP 30.56 mg/dL ESR 124 mm/h | - | Admitted Antibiotics |
| Matta et al., 2020; USA [32] | 1 | Nausea, vomiting, weight loss, fever, headache | Pyrexia | Diffuse patchy GGO Interlobular septal thickening Subpleural sparing | CRP 22.0 mg/dL ESR 46 mm/h | - | Admitted IV steroids |
| Thota et al., 2014; USA [8] | 1 | SOB, cough, facial flushing | - | Bilateral GGO Upper and middle lobe predominance | Eos 2% | 74% eosinophils on BAL | Admitted Antibiotics Steroids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O'Callaghan, M.; Boyle, N.; Fabre, A.; Keane, M.P.; McCarthy, C. Vaping-Associated Lung Injury: A Review. Medicina 2022, 58, 412. https://doi.org/10.3390/medicina58030412
O'Callaghan M, Boyle N, Fabre A, Keane MP, McCarthy C. Vaping-Associated Lung Injury: A Review. Medicina. 2022; 58(3):412. https://doi.org/10.3390/medicina58030412
Chicago/Turabian StyleO'Callaghan, Marissa, Niamh Boyle, Aurelie Fabre, Michael P. Keane, and Cormac McCarthy. 2022. "Vaping-Associated Lung Injury: A Review" Medicina 58, no. 3: 412. https://doi.org/10.3390/medicina58030412
APA StyleO'Callaghan, M., Boyle, N., Fabre, A., Keane, M. P., & McCarthy, C. (2022). Vaping-Associated Lung Injury: A Review. Medicina, 58(3), 412. https://doi.org/10.3390/medicina58030412






