Abstract
Background and objectives: Improving early diagnosis and advances in colorectal cancer (CRC) treatment leads to longer survival of these patients. The purpose of this study was to identify the main surgical factors affecting long-term Quality of life (QoL) among colorectal cancer patients after surgery. Materials and Methods: QoL was prospectively evaluated in patients undergoing elective colorectal cancer resection operations in three CRC surgery centers of Lithuania using EORTC generic (QLQC-30) and disease-specific (QLQ-CR29) questionnaires at the time of preoperative admission and 1, 24, and 72 months after surgery. QoL was evaluated among different patient groups, diagnostic and treatment modalities, disease, and postoperative complications. Non-parametric tests and multivariate logistic regression models were used for statistical analysis. Results: Eighty-eight consecutive CRC patients from three institutions were included in the study over a three-month inclusion period, 42 (47.73%) women and 46 (52.27%) men, mean age 64.2 ± 11.5 years. Most tumors were localized in the sigmoid colon and rectum. The largest number of patients had stage III cancer. Twenty-nine patients died—a 6-year survival rate was 67%. 50 of 59 live patients (84.8%) responded to the questionnaire 6 years after their operation. Evaluating changes in quality of life 72 months after surgery with assessments before surgery, both questionnaire responses revealed good long-term CRC surgical treatment results: improved general and functional scale estimates and decreased symptom scale ratings. The multivariate analysis found that age, stoma formation, and rectal cancer were independent risk factors for having worse QoL six years after surgical intervention. Conclusions: Six years after surgery, QoL returns to preoperative levels. Age, stoma formation, adjuvant treatment, and rectal cancer reduce long-term QoL.
1. Introduction
Colorectal cancer (CRC) is one of the most burdensome cancers in the Western world due to its high incidence, significant mortality, and increasing survivorship [1,2,3]. About 1.4 million people are diagnosed with new cases of colorectal cancer worldwide every year. Colon cancer is more common in developed countries and is associated with lifestyle [4]. Improving early diagnosis and advances in treatment leads to longer survival of these patients [5,6,7]. The emergence of new treatment options for CRC, such as laparoscopic and robotic surgery, transanal techniques, and neoadjuvant and total neoadjuvant chemo- and radiotherapy, result in better outcomes, as well as increasing the quality of life of the patients. Quality of life (QoL) is a multidimensional, dynamic, subjective, and patient-centered construct comprising physical, functional, emotional, and social or family well-being [8]. Not only does it provide patient-centered outcomes of cancer treatment, but it is also related to overall survival [9,10,11] and a good indicator of treatment quality [12,13]. In addition, QoL measurements have become particularly important in assessing the outcome of long-term treatment in chronic diseases or where improvement is only short-term and temporary, and where disease progression is unstoppable and only palliative treatment is possible. Health-related quality of life (HRQoL) research is of great significance and importance, as it helps to evaluate the effectiveness of treatment methods, health improvement, and disease prevention programs, and is useful in monitoring the state of public health and developing public health policy.
Our study aims to assess the long-term results of the quality of life after surgical treatment of CRC and to determine the factors associated with decreased quality of life in the long-term postoperative period.
2. Patients and Methods
A prospective snapshot cohort study was performed. Lithuanian bioethics committee approval (no. L-13-03/1) was obtained. The study included 88 patients operated on with curative intent for CRC in three major cancer centers of Lithuania: Vilnius University Hospital Santaros Clinics, Lithuanian University of Health Sciences Kaunas Clinics Hospital, and the National Cancer Institute. The patients were included in the study for three months, from September to December 2012. All patients older than 18 years admitted for elective curative surgery for colorectal cancer, with the diagnosis confirmed endoscopically and histologically, were included. Patients who underwent emergency surgery were excluded. Informed consent was obtained, and baseline demographic information was collected preoperatively using patient interviews. Clinical and operative details, American Society of Anesthesiologists (ASA) grade, preoperative radiological evaluation, neoadjuvant treatment, operation type (right, left, or rectal procedure), presence of a stoma (or not), final pathological diagnosis, and postoperative complications were also recorded and reported earlier [14].
Patients’ quality of life was assessed before surgery, and at 1, 24, and 72 months after the surgery. Validated Lithuanian translations of the EORTC QLQ-C30 (version 3.0) and QLQ-CR29 questionnaires were used in the current study. The collected data were analyzed according to EORTC scoring guidelines in the same way as we reported previously [14]. The higher estimates in assessing the overall state of health and functional scales indicated better results. Higher estimates indicated more pronounced symptoms and worse postoperative outcomes. The factors that resulted in a statistically significantly worse quality of life outcome in the long-term period were identified.
Statistical analysis was performed using SPSS® software version 23 (SPSS, Chicago, IL, USA). Non-parametric statistical tests and multivariate logistic regression models were used. Overall survival (OS) was calculated as the difference between the date of operation and the date of death (from any cause) or 72 months after the operation. Survival curves were estimated using the Kaplan–Meier estimator. Survival curves were compared with the log-rank test.
3. Results
Eighty-eight patients were included in the study. The demographic and clinical data are shown in Table 1.

Table 1.
Demographic and clinical data.
All patients were treated with surgery—58 (65.9%) patients with open-surgery, 26 (29.6%) with laparoscopic surgery, and 4 (4.5%) with transanal endoscopic microsurgery. There were no conversions in the laparoscopic group. A stoma was formed in 31 patients, with 19 (21.6%) preventive ileostomies and 12 (13.6%) end colostomies. Adjuvant chemotherapy was commenced 1-month post-operatively for 23 (26.1%) patients and radiation therapy for 5 (5.7%); in the third month, chemotherapy was used in 27 (30.68%) patients and radiotherapy in 2 (2.27%) patients.
Regarding survival, 29 patients died 72 months after surgery (33.0%), with a 6-year survival rate of 67% that was equal between men and women (p = 0.448) (Figure 1 and Figure 2).
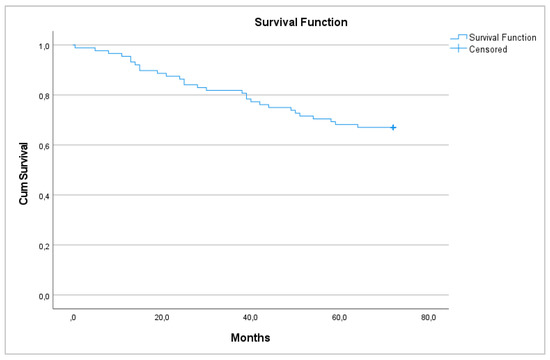
Figure 1.
6-year overall survival.
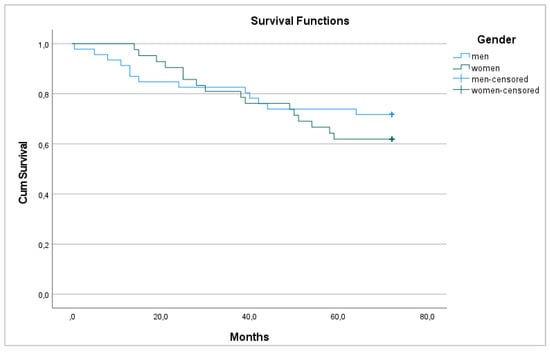
Figure 2.
6-year overall survival between men and women.
Most patients had stage III cancer—37 (42%). Patients with stage IV cancer did not survive after 6 years, although all patients with a tumor in situ survived (p < 0.001) (Figure 3).
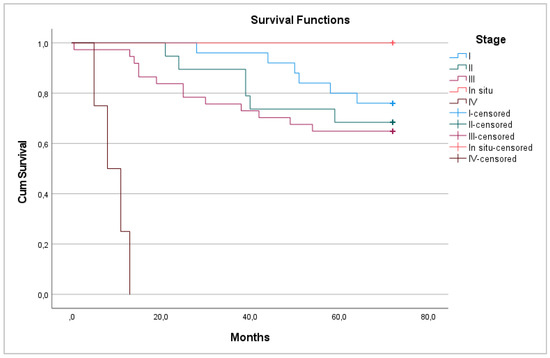
Figure 3.
6-year overall survival between pathological stages.
Fifty of the remaining 59 living patients (84.8%) responded to the questionnaire 72 months after surgery.
Evaluating changes in QoL 72 months after surgery with assessments before surgery both QLQ—C30 and QLQ—CR29 questionnaire responses revealed good long-term CRC surgical treatment results, showing improved general (60; 69.5; 65.33; p = 0.06) and functional (70.3; 79.8; 85.3; p = 0.041/72.9; 78.93; p = 0.049) scale estimates and decreased symptom scale ratings (24.3; 19; 17; p = 0.034)/22; 12.7; p = 0.025) (Figure 4 and Figure 5).
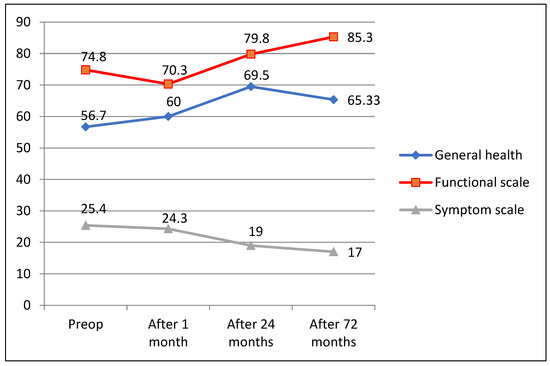
Figure 4.
QLQ-C30 questionnaire outcomes 72 months after surgery.
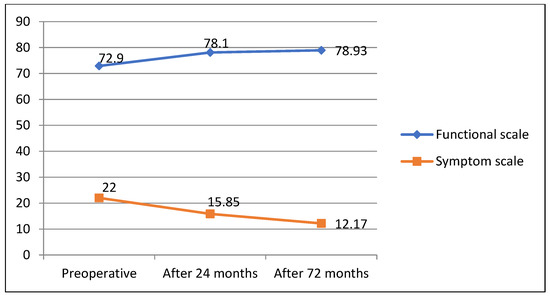
Figure 5.
QLQ—CR29 questionnaire outcomes 72 months after surgery.
Evaluating the QoL 72 months after surgery between stages of both overall health status and overall QoL scores, we did not find any significant differences (p = 0.687; p = 0.457) (Figure 6 and Figure 7).
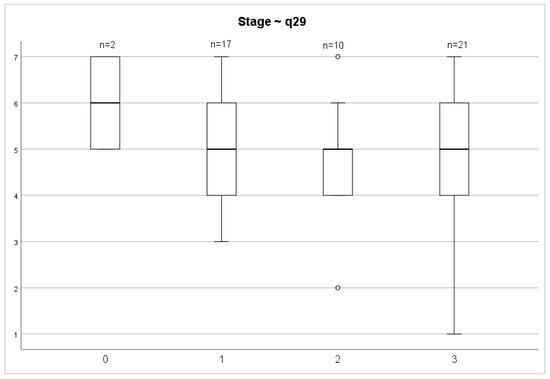
Figure 6.
Stage and general health status.
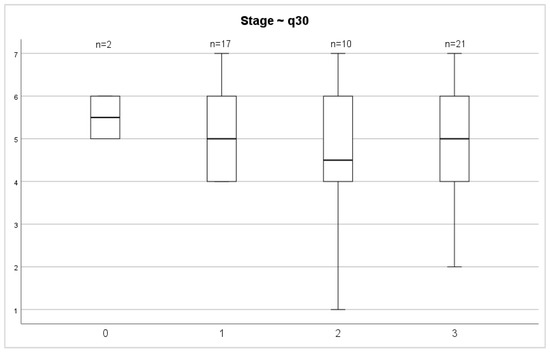
Figure 7.
Stage and overall quality of life.
Forty-six (52.3%) patients had rectal cancer. This localization of tumors is associated with worse overall health status and overall QoL scores (Figure 8 and Figure 9). However, no significant differences between these groups were found (p = 0.2035; p = 0.1002).
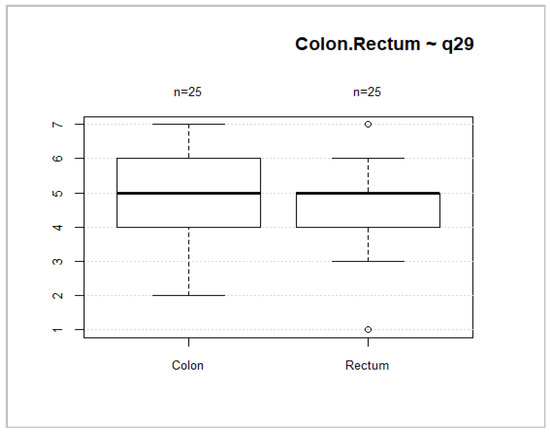
Figure 8.
Tumor location and general health status.
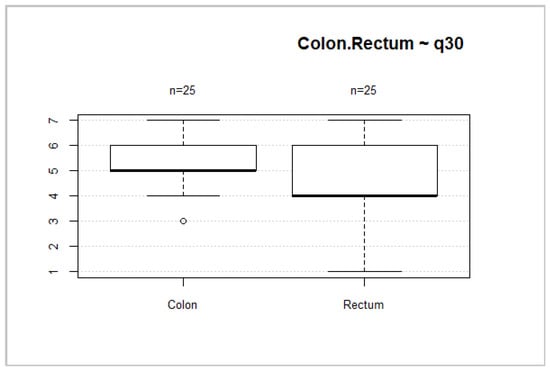
Figure 9.
Tumor localization and overall QoL.
We performed multivariate logistic regression models, which revealed that age (≥ 65 years), stoma formation, and rectal cancer are significant predictors of worse QoL (Table 2).

Table 2.
Multivariate analysis of factors predicting poorer quality of life.
4. Discussion
We found that the QoL of patients increased two years after surgery and was maintained for up to six years. In a German study, QoL was assessed one and three years after diagnosis. Most patients with CRC reported high overall QoL and only small deficits in physical functioning, but deficits in emotional and social functioning persisted over years in patients with CRC. Improvements in QoL from the first to the third year after diagnosis in patients who remained free of disease were very modest and limited to fewer financial difficulties, a better future perspective, and fewer stoma-related problems [15].
We found that decreased long-term QoL was associated with age, stoma formation, and the use of radiotherapy. The study that investigated HRQoL in terms of symptoms and functional outcomes in disease-free survivors of rectal cancer showed that age, female sex, stoma, late complications predicted worse physical functioning; stoma and chemoradiotherapy—worse body image and age, female sex, and late major complications worse sexual functioning [16]. Another study revealed that patients with ostomies who had any late complications had lower overall HRQoL (OR 1.5; 95% CI 0.9–2.6). This was not the case for patients with anastomoses (OR 0.9; 95% CI 0.5–1.5) [17].
Comparing laparoscopic vs. open surgery 18 months after surgery, any differences in QoL between patients randomized to laparoscopic-assisted colectomy (LAC) or open colectomy favored LAC. However, the magnitude of the benefits was small; only age and activity were predictive of poor QoL [18]. We also didn’t find any influence of the type of surgery on QoL. The COLORII trial revealed that HRQoL after rectal cancer surgery was not affected by the surgical approach [19]. Other studies have shown the opposite. Elderly patients undergoing laparoscopic colectomy for cancer experience fewer postoperative local complications than elderly patients undergoing an open colectomy [20]. Nevertheless, in the first postoperative month, these patients experienced worse global QoL than younger patients undergoing the same operation with impairment of all functions and the presence of fatigue, sleep disturbances, appetite loss, and dyspnea [21]. HRQoL generally improved over the first year after laparoscopic colectomy, reaching even better levels than before surgery. There was an early postoperative improvement in the patients’ emotional status [22].
A prospective survey of a population-based sample of 763 colorectal cancer patients assessed sociodemographic variables, health behaviors, optimism, threat appraisal, and perceived social support at 5 months post-diagnosis as predictors of QoL and psychological distress 5 years post-diagnosis. Risk factors for worse QoL and/or greater psychological distress included later-stage disease, having a permanent stoma, rectal cancer, fatigue, smoking, being single, low social support, low optimism, and a more negative cancer threat appraisal [23].
The association between a post-diagnosis lifestyle score and HRQoL in the long-term CRC survivals indicated that lifestyle behaviors, such as a body mass index (BMI) < 30 kg/m2, dietary intake, physical activity, and smoking status, were associated with HRQol among CRC long-term survivors in a cross-sectional study [24].
The six-year OS of patients in our cohort (67%) was similar to those found in hospital-based studies in Brazil (63.5%) [25], Italy (66.45%) [26], and Taiwan (68.7%) [27]. We did not identify differences based on gender or tumor location, but found differences between clinical stages in OS. Similar findings were obtained in a Brazilian study [25].
The main disadvantage of this study is its small cohort. We tried to capture a snapshot of short-term surgical practice in the country, providing data on the long-term survival and QoL of CRC patients. We also included only patients undergoing elective surgery with curative intent. Emergency surgery for colorectal cancer does not influence overall and disease-free survival [28]; the data on the QoL of this small sub-group of patients should be studied further.
5. Conclusions
In conclusion, most patients return to a stable QoL within 24 months after the operation, and it remains stable by 72 months. Older age, stoma formation, adjuvant treatment, and rectal cancer influence long-term quality of life. Lifestyle adjustments and social support are important in improving quality of life and should be further investigated.
Author Contributions
Study conception design: T.P., K.S., Z.S., A.T. and N.E.S. Data acquisition: G.V.-T. and A.K. Data analysis and interpretation: G.V.-T., A.K. and T.P. Drafting the article: G.V.-T. and T.P. Critical revision for intellectual content: T.P., E.P., A.D., K.S., V.J., A.T., Z.S. and N.E.S. Final approval of the manuscript: G.V.-T., A.K., E.P., V.J., Z.S., A.T., P.L., A.D., N.E.S., K.S. and T.P. Agree to be accountable for all aspects of work to ensure that questions regarding accuracy & integrity investigated and resolved: G.V.-T., A.K., E.P., V.J., Z.S., A.T., P.L., A.D., N.E.S., K.S. and T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This quality of life study was funded by a grant (no. LIG-01/2011) from the Research Council of Lithuania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Lithuanian bioethics committee (approval no. L-13-03/1, 26 April 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to institutional restrictions.
Acknowledgments
The study was sponsored by the Research Council of Lithuania, grant no LIG01/2011.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lunevicius, R.; Poskus, T.; Samalavicius, N.E. National burden of colorectal cancer in Lithuania and country’s ranking across 45 European nations. Oncol. Lett. 2015, 10, 433–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase. No 9; International Agency for Research on Cancer: Lyon, France, 2021; Available online: http://ci5.iarc.fr (accessed on 12 April 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Poskus, T.; Strupas, K.; Mikalauskas, S.; Bitinaitė, D.; Kavaliauskas, A.; Samalavicius, N.E.; Saladzinskas, Z. Initial results of the National Colorectal Cancer Screening Program in Lithuania. Eur. J. Cancer Prev. 2015, 24, 76–80. [Google Scholar] [CrossRef]
- Poskus, E.; Kryzauskas, M.; Poškus, T.; Mikalauskas, S.; Samalavičius, N.E.; Aliosin, O.; Dailidėnas, S.; Tamelis, A.; Saladžinskas, Z.; Lizdenis, P.; et al. Improved perioperative care is associated with improved long-term survival in colorectal cancer. Int. J. Color. Dis. 2018, 33, 779–785. [Google Scholar] [CrossRef]
- Dulskas, A.; Gaizauskas, V.; Kildusiene, I.; Samalavicius, N.E.; Smailyte, G. Improvement of Survival over Time for Colorectal Cancer Patients: A Population-Based Study. J. Clin. Med. 2020, 9, 4038. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S. Quality of Life in Cancer: Definition, Purpose, and Method of Measurement. Cancer Investig. 1993, 11, 327–336. [Google Scholar] [CrossRef]
- Gotay, C.C.; Kawamoto, C.T.; Bottomley, A.; Efficace, F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J. Clin. Oncol. 2008, 26, 1355–1363. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Reyes, M.; Ye, Y.; Zhou, Y.; Liang, A.; Kopetz, S.; Rodriquez, M.A.; Wu, X.; Hildebrandt, M.A.T. Predictors of health-related quality of life and association with survival may identify colorectal cancer patients at high risk of poor prognosis. Qual. Life Res. 2016, 26, 319–330. [Google Scholar] [CrossRef]
- Tsunoda, A.; Nakao, K.; Hiratsuka, K.; Tsunoda, Y.; Kusano, M. Prospective analysis of quality of life in the first year after colo-rectal cancer surgery. Acta Oncol. 2007, 46, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Arndt, V.; Merx, H.; Stegmaier, C.; Ziegler, H.; Brenner, H. Quality of Life in Patients with Colorectal Cancer 1 Year After Diagnosis Compared with the General Population: A Population-Based Study. J. Clin. Oncol. 2004, 22, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Lizdenis, P.; Birutis, J.; Čelkienė, I.; Samalavičius, N.; Kuliavas, J.; Slunskis, V.; Poškus, T.; Jotautas, V.; Poškus, E.; Strupas, K.; et al. Short-term results of quality of life for curatively treated colorectal cancer patients in Lithuania. Medicina 2015, 51, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Arndt, V.; Merx, H.; Stegmaier, C.; Ziegler, H.; Brenner, H. Restrictions in quality of life in colorectal cancer patients over three years after diagnosis: A population based study. Eur. J. Cancer 2006, 42, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Pucciarelli, S.; Del Bianco, P.; Toppan, P.; Serpentini, S.; Efficace, F.; Pasetto, L.M.; Friso, M.L.; De Salvo, G.L.; Nitti, N. Health-Related Quality of Life Outcomes in Disease-Free Survivors of Mid-Low Rectal Cancer After Curative Surgery. Ann. Surg. Oncol. 2008, 15, 1846–1854. [Google Scholar] [CrossRef]
- Liu, L.; Herrinton, L.J.; Hornbrook, M.C.; Wendel, C.S.; Grant, M.; Krouse, R.S. Early and Late Complications Among Long-Term Colorectal Cancer Survivors with Ostomy or Anastomosis. Dis. Colon Rectum 2010, 53, 200–212. [Google Scholar] [CrossRef]
- Stucky, C.-C.H.; Pockaj, B.A.; Ms, P.J.N.; Sloan, J.A.; Sargent, D.; O’Connell, M.J.; Beart, R.W.; Skibber, J.M.; Nelson, H.; Weeks, J.C. Long-Term Follow-Up and Individual Item Analysis of Quality of Life Assessments Related to Laparoscopic-Assisted Colectomy in the COST Trial 93-46-53 (INT 0146). Ann. Surg. Oncol. 2011, 18, 2422–2431. [Google Scholar] [CrossRef]
- Andersson, J.; Angenete, E.; Gellerstedt, M.; Angerås, U.; Jess, P.; Rosenberg, J.; Fürst, A.; Bonjer, J.; Haglind, E. Health-related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br. J. Surg. 2013, 100, 941–949. [Google Scholar] [CrossRef]
- Kryzauskas, M.; Bausys, A.; Degutyte, A.E.; Abeciunas, V.; Poskus, E.; Bausys, R.; Dulskas, A.; Strupas, K.; Poskus, T. Risk factors for anastomotic leakage and its impact on long-term survival in left-sided colorectal cancer surgery. World J. Surg. Oncol. 2020, 18, 205. [Google Scholar] [CrossRef]
- Scarpa, M.; Di Cristofaro, L.; Cortinovis, M.; Pinto, E.; Massa, M.; Alfieri, R.; Cagol, M.; Saadeh, L.; Costa, A.; Castoro, C.; et al. Minimally invasive surgery for colorectal cancer: Quality of life and satisfaction with care in elderly patients. Surg. Endosc. 2013, 27, 2911–2920. [Google Scholar] [CrossRef]
- Theodoropoulos, G.E.; Karantanos, T.; Stamopoulos, P.; Zografos, G. Prospective evaluation of health-related quality of life after laparoscopic colectomy for cancer. Tech. Coloproctol. 2012, 17, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.K.; Meng, X.; Youl, P.; Aitken, J.; Dunn, J.; Baade, P. A five-year prospective study of quality of life after colorectal cancer. Qual. Life Res. 2011, 21, 1551–1564. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Walter, J.; Hampe, J.; von Schönfels, W.; Hinz, S.; Küchler, T.; Jacobs, G.; Schafmayer, C.; Nöthlings, U. Lifestyle factors and health-related quality of life in colorectal cancer survivors. Cancer Causes Control 2013, 25, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Aguiar Junior, S.; Oliveira, M.M.; Silva, D.R.M.E.; Mello, C.A.L.; Calsavara, V.F.; Curado, M.P. Survival of patients with colorectal can-cer in a cancer center. Arq. Gastroenterol. 2020, 57, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Micu, B.V.; Vesa, S.C.; Pop, T.R.; Micu, C.M. Evaluation of prognostic factors for 5 year-survival after surgery for colorectal cancer. Ann. Ital. Chir. 2020, 91, 41–48. [Google Scholar] [PubMed]
- Lee, C.-H.; Cheng, S.-C.; Tung, H.-Y.; Chang, S.-C.; Ching, C.-Y.; Wu, S.-F. The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran. J. Public Health 2018, 47, 519–530. [Google Scholar]
- Weixler, B.; Warschkow, R.; Ramser, M.; Droeser, R.; Von Holzen, U.; Oertli, D.; Kettelhack, C. Urgent surgery after emergency presentation for colorectal cancer has no impact on overall and disease-free survival: A propensity score analysis. BMC Cancer 2016, 16, 208. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).