Predictive Factors in the Appearance and Evolution of Squamous Cell Carcinomas of the Oral Cavity
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Statistical Analysis
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/1-Lip-oral-cavity-fact-sheet.pdf (accessed on 4 March 2022).
- Hashibe, M. Risk factors for cancer of the mouth: Tobacco, betel quid, and alcohol. In Textbook of Oral Cancer; Warnakulasuriya, S., Greenspan, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 3, pp. 23–30. [Google Scholar]
- Diz, P.; Meleti, M.; Diniz-Freitas, M.; Vescovi, P.; Warnakulasuriya, S.; Johnson, N.W.; Kerr, A.R. Oral and pharyngeal cancer in Europe. Transl. Res. Oral Oncol. 2017, 2, 2057178. [Google Scholar] [CrossRef] [Green Version]
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H.; Subramanian, S.; Johnson, N. Oral Cancer: Prevention, Early Detection, and Treatment. In Cancer: Disease Control Priorities, 3rd ed.; Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- Lubin, J.H.; Muscat, J.; Gaudet, M.M.; Olshan, A.F.; Curado, M.P.; Maso, L.D.; Wünsch-Filho, V.; Sturgis, E.M.; Szeszenia-Dabrowska, N.; Castellsagué, X.; et al. An examination of male and female odds ratios by BMI, cigarette smoking, and alcohol consumption for cancers of the oral cavity, pharynx, and larynx in pooled data from 15 case–control studies. Cancer Causes Control 2011, 22, 1217–1231. [Google Scholar] [CrossRef] [PubMed]
- Eurohealth. Available online: https://www.eurohealthproject.com (accessed on 14 February 2022).
- Dumitrescu, A.L.; Ibric, S.; Ibric-Cioranu, V. Assessing Oral Cancer Knowledge in Romanian Undergraduate Dental Students. J. Cancer Educ. 2014, 29, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Nazar, H.; Shyama, M.; Ariga, J.; El-Salhy, M.; Soparkar, P.; Alsumait, A. Oral Cancer Knowledge, Attitudes and Practices among Primary Oral Health Care Dentists in Kuwait. Asian Pac. J. Cancer Prev. 2019, 20, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Lingen, M.W.; Kalmar, J.R.; Karrison, T.; Speight, P.M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008, 44, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Van Der Waal, I. Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e33–e37. [Google Scholar] [CrossRef]
- Cesar Rivera. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Rutkowska, M.; Hnitecka, S.; Nahajowski, M.; Dominiak, M.; Gerber, H. Oral cancer: The first symptoms and reasons for delaying correct diagnosis and appropriate treatment. Adv. Clin. Exp. Med. 2020, 29, 735–743. [Google Scholar] [CrossRef]
- Schwab, M. TNM Classification. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Worldlifeexpectancy. 2018. Available online: https://www.worldlifeexpectancy.com/ro/romania-oral-cancer (accessed on 6 April 2022).
- Healthdata. Available online: http://www.healthdata.org/gbd/gbd-2017-resources (accessed on 14 February 2022).
- Ritchie, H.; Roser, M. Our World in Data. 2019. Available online: https://ourworldindata.org/smoking (accessed on 14 February 2022).
- Dasanayake, A.P.; Silverman, A.J.; Warnakulasuriya, S. Maté drinking and oral and oro-pharyngeal cancer: A systematic review and meta-analysis. Oral Oncol. 2010, 46, 82–86. [Google Scholar] [CrossRef]
- World Dental Federation. Available online: https://www.fdiworlddental.org/news/20181011/lancet-report-ties-alcohol-use-to-oral-cancer-even-moderate-consumption-is-not-without (accessed on 11 October 2018).
- Ustrell-Borràs, M.; Traboulsi-Garet, B.; Escoda, C.G. Alcohol-based mouthwash as a risk factor of oral cancer: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e1–e12. [Google Scholar] [CrossRef]
- Amagasa, T.; Yamashiro, M.; Uzawa, N. Oral Premalignant Lesions: From a Clinical Perspective. Int. J. Clin. Oncol. 2011, 16, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Sabiha, B.; Jan, H.U.; Haider, S.A.; Khan, A.A.; Ali, S.S. Genetic etiology of oral cancer. Oral Oncol. 2017, 70, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- Pires, F.R.; Ramos, A.B.; De Oliveira, J.B.C.; Tavares, A.S.; Da Luz, P.S.R.; Dos Santos, T.C.R.B. Oral squamous cell carcinoma: Clinicopathological features from 346 cases from a single Oral Pathology service during an 8-year period. J. Appl. Oral Sci. 2013, 21, 460–467. [Google Scholar] [CrossRef]
- Globocan. Available online: https://www.uicc.org/new-global-cancer-data-globocan-2018 (accessed on 14 April 2022).
- Matsuo, K.; Gallus, S.; Negri, E.; Kawakita, D.; Oze, I.; Hosono, S.; Ito, H.; Hatooka, S.; Hasegawa, Y.; Shinoda, M.; et al. Time to first cigarette and upper aerodigestive tract cancer risk in Japan. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1986–1992. [Google Scholar] [CrossRef] [Green Version]
- Scully, C.; Porter, S. ABC of oral health. Oral cancer. BMJ 2000, 321, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.S.; Lo, H.-I.; Wong, T.-Y.; Huang, C.-C.; Lee, W.-T.; Tsai, S.-T.; Chen, K.-C.; Yen, C.-J.; Wu, Y.-H.; Hsueh, W.-T.; et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013, 49, 1010–1017. [Google Scholar] [CrossRef]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case–control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef]
- Mathur, R.; Singhavi, H.R.; Malik, A.; Nair, S.V.; Chaturvedi, P. Role of Poor Oral Hygiene in Causation of Oral Cancer—a Review of Literature. Indian J. Surg. Oncol. 2018, 10, 184–195. [Google Scholar] [CrossRef]
- Zeng, X.-T.; Deng, A.-P.; Li, C.; Xia, L.-Y.; Niu, Y.-M.; Leng, W.-D. Periodontal Disease and Risk of Head and Neck Cancer: A Meta-Analysis of Observational Studies. PLoS ONE 2013, 8, e79017. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sanchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Group, E.W. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Limesand, K.H.; Ann, D.K. State of Knowledge on Salivary Gland Cancers. Crit. Rev. Oncog. 2018, 23, 139–151. [Google Scholar] [CrossRef]
- da Silva Lins, L.S.; Bezerra, N.V.F.; Freire, A.R.; de Almeida, L.D.F.D.; de Lucena, E.H.G.; Cavalcanti, Y.W. Socio-demographic characteristics are related to the advanced clinical stage of oral cancer. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e759–e763. [Google Scholar] [CrossRef]
- Schwab, M. Broder Histological Classification. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
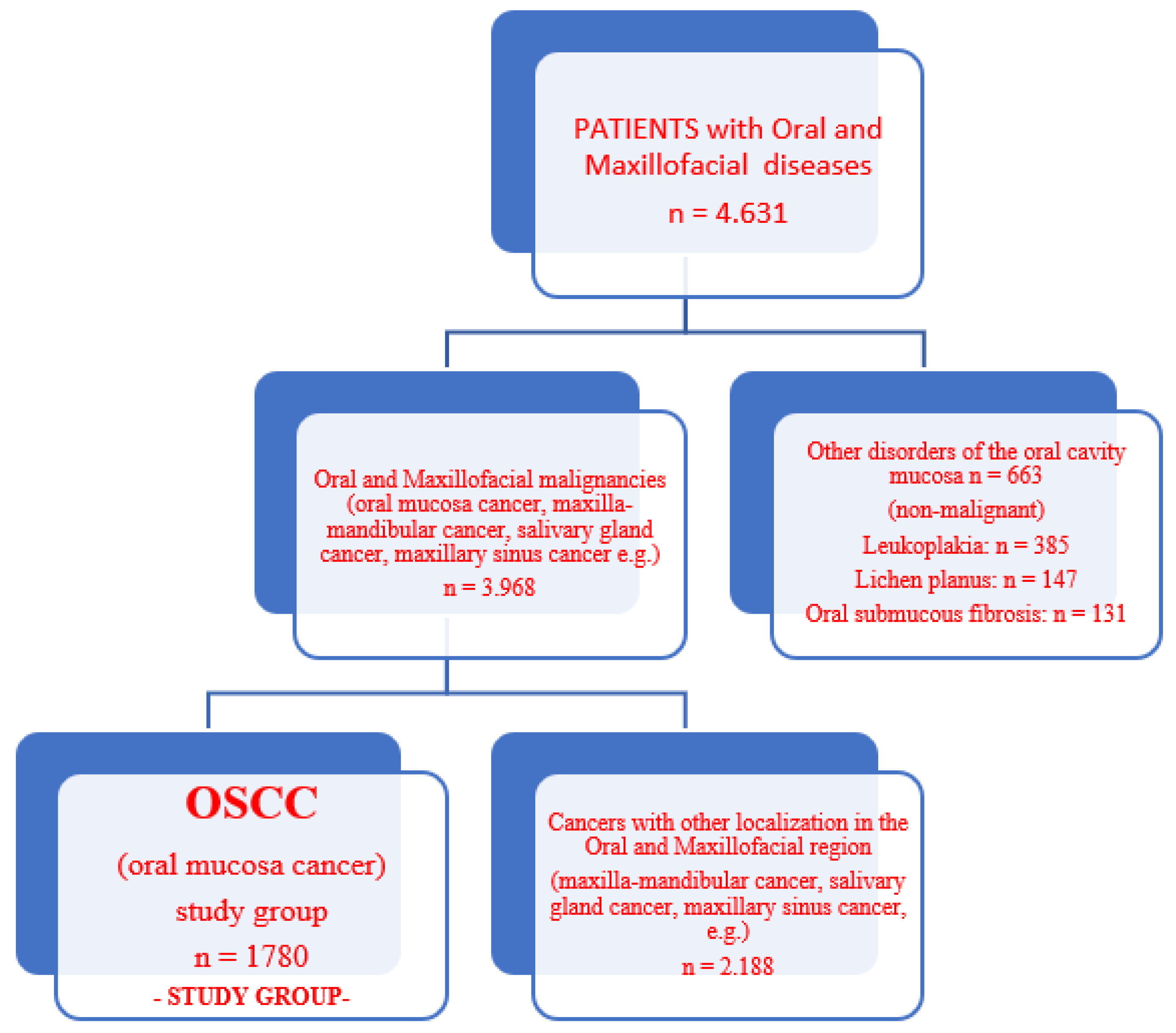
| Baseline Characteristics | All Groups (n = 2443) | p-Value | |
|---|---|---|---|
| Study Group (n = 1780) | Control Group (n = 663) | ||
| Demographic characteristics | |||
| Age in years, mean ± SD | 61.3 ± 11.3 | 60.5 ± 11.9 | 0.004 |
| <60 years, n (%) ≥60 years, n (%) | 762 (42.8) 1018 (57.2) | 339 (51.1) 324 (48.9) | 0.014 |
| Gender, male/female, n (%) | 1492/288 (83.8/16.2) | 399/264 (60.2/39.8) | <0.001 |
| Tobacco use (at presentation) (Yes/No), n (%) | 1473/307 (82.7/17.3) | 412/251 (62.1/37.9) | 0.002 |
| Alcohol abuse or dependence (Yes/No), n (%) | 1285/495 (72.2/27.8) | 104/559 (15.7/84.3) | <0.001 |
| Poor oral hygiene (Yes/No), n (%) | 564/1216 (31.7/68.3) | 121(18.3/81.7) | 0.004 |
| Comorbidities (Yes/No), n (%) | 761/1019 (42.8/57.2) | 268/395 (40.4/59.6) | 0.082 |
| Anatomical-clinical and histopathological characteristics | |||
| ANATOMIC SITE | |||
| Lip | 362 (20.3) | - | |
| Pelvilingual | 374 (21) | - | |
| Tongue | 412 (23.2) | - | |
| Floor of the mouth | 221 (12.4) | - | |
| Gingival-alveolar | 187 (10.5) | - | |
| Gingival-alveolar extended in Floor of the mouth and vice versa | 91 (5.1) | - | |
| Soft palate | 79 (4.4) | - | |
| Intermaxillary commissure with a jugal extension/toward the sidewall of the pharynx | 54 (3) | - | |
| Clinical anatomical types—advanced stages | |||
| Ulcero-vegetant | 420 (23.6) | - | |
| Ulcero-destructive | 396 (22.3) | - | |
| Infiltrative-diffuse | 256 (14.4) | - | |
| Sclerosal form | 127 (7.1) | - | |
| Lymphoganglionary Dissemination | |||
| Metastatic lymphadenopathy | 1321 (74.2) | - | |
| No dissemination | 459 (25.8) | - | |
| Distribution of patients by clinical stage | |||
| Stage I | 221 (12.4) | ||
| Stage II | 651 (36.6) | ||
| Stage III | 487 (27.4) | ||
| Stage IV | 421 (23.7) | ||
| Degree of differentiation | |||
| well-differentiated | 985 (55.3) | - | |
| moderately differentiated | 598 (33.6) | - | |
| poorly differentiated | 176 (9.9) | - | |
| Undifferentiated | 21 (1.2) | - | |
| Segment | Age (Years) | APC | APC | Test Statistic (t) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Lower Endpoint | Upper Endpoint | Lower CI | Upper CI | ||||
| Slope 1 | 16 | 46 | −2.8 | −24.4 | −7.1 | −3.42 | 0.0012 |
| Slope 2 | 46 | 49 | 9.6 | 6.7 | 10.4 | 9.38 | 0.0081 |
| Slope 3 | 49 | 64 | −2.4 | −5.3 | −1.6 | −1.65 | 0.1239 |
| Slope 4 | 64 | 74 | 4.6 | 1.4 | 6.9 | 11.59 | 0.0108 |
| Slope 5 | 74 | 80 | −8.8 | −9.1 | −5.4 | −7.36 | 0.00251 |
| Slope 6 | 80 | 91 | 8.1 | 6.4 | 14.2 | 12.91 | 0.0024 |
| Logistic Regression | Odds Ratio (95%CI) | SE | p-Value |
|---|---|---|---|
| Univariate analysis | |||
| Age ≥ 60 years (reference: age < 60) | 1.95 (1.38–2.59) | 0.011 | 0.003 |
| Gender, male (reference: female) | 4.43 (3.84–5.80) | 0.152 | <0.001 |
| Tobacco use (at presentation) (Yes) | 2.46 (1.15–3.63) | 0.024 | 0.001 |
| Alcohol abuse or dependence (Yes) | 2.79 (1.27–4.05) | 0.211 | 0.002 |
| Poor oral hygiene (Yes) | 1.19 (0.87–2.99) | 0.096 | 0.083 |
| Comorbidities (Yes) | 1.24 (0.97–6.14) | 0.084 | 0.076 |
| Multivariate analysis, Method: Enter | |||
| Age ≥ 60 years (reference: age < 60) | 1.48 (1.22–3.19) | 0.054 | 0.009 |
| Gender, male (reference: female) | 3.61 (2.06–5.81) | 0.043 | 0.036 |
| Tobacco use (at presentation) (Yes) | 2.97 (1.42–3.94) | 0.021 | 0.001 |
| Alcohol abuse or dependence (Yes) | 1.85 (1.11–3.46) | 0.045 | 0.015 |
| Poor oral hygiene (Yes) | 1.04 (0.45–5.13) | 0.106 | 0.058 |
| Comorbidities (Yes) | 1.32 (0.47–2.54) | 0.084 | 0.096 |
| Time Limit (Moment of Evaluation) (Months) | % Cumulative Surviving RT + CT | % Cumulative Surviving S + RT + CT | % Cumulative Surviving S + RT | % Cumulative Surviving RT |
|---|---|---|---|---|
| 5 | 100 | 100. | 100.0000 | 100.0000 |
| 7 | 100. | 100 | 83.3333 | 96.4286 |
| 9 | 100 | 100 | 83.3333 | 89.2857 |
| 11 | 100 | 66.7 | 83.3333 | 78.3528 |
| 13 | 100 | 66.6667 | 83.3333 | 65.9813 |
| 15 | 85.7 | 66.6667 | 83.3333 | 56.5554 |
| 17 | 85.7 | 66.6667 | 83.3333 | 51.8424 |
| 19 | 54.5 | 66.6667 | 83.3333 | 51.8424 |
| 21 | 54.5 | 66.6667 | 83.3333 | 51.8424 |
| 24 | 54.5 | 66.6667 | 83.3333 | 51.8424 |
| Parameters | N | EFS n (%) | † EFS: Kaplan–Meier Method Cumulative Proportion Surviving: 24 Months | p-Value | |
|---|---|---|---|---|---|
| Estimate | Std. Error | ||||
| Gender, male | 56 | 8 (14.3%) | 83.2% | 0.057 | <0.001 |
| Age ≥ 60 years | 24 | 14 (58.3%) | 37.7% | 0.031 | 0.292 |
| Tobacco use | 119 | 28 (23.5%) | 71.1% | 0.075 | 0.113 |
| Anatomical-clinical forms | 0.109 | ||||
| Ulcero-vegetant | 34 | 13 (38.2%) | 54.7% | 0.042 | |
| Ulcero- destructive | 21 | 5 (23.8%) | 71.9% | 0.074 | |
| Infiltrative-diffuse | 14 | 5 (35.7%) | 46.4% | 0.051 | |
| Sclerotic shape | 6 | 3 (50%) | 50% | 0.104 | |
| Lymph node dissemination | |||||
| Metastatic lymphadenopathy | 33 | 10 (30.3%) | 69.1% | 0.040 | 0.327 |
| No metastatic lymphadenopathy | 31 | 10 (32.3% | 59.2% | 0.041 | |
| Clinical Stage | 0.159 | ||||
| Stage I | 81 | 17 (20.9%) | 75.6% | 0.029 | |
| Stage II | 44 | 14 (31.8%) | 57.2% | 0.036 | |
| Stage III | 61 | 11 (18.1%) | 72.6% | 0.037 | |
| Stage IV | 107 | 25 (23.4%) | 70.8% | 0.018 | |
| Degree of differentiation | 0.327 | ||||
| well-differentiated | 119 | 28 (23.5%) | 71.1% | 0.075 | |
| moderately differentiated | 6 (33.3%) | 64.9% | 0.046 | ||
| poorly differentiated | 24 (29.6%) | 62.7% | 0.022 | ||
| Undifferentiated | 7 (15.9%) | 81.6% | 0.049 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carp, A.; Nicolau, A.; Moscalu, M.; Popescu, E. Predictive Factors in the Appearance and Evolution of Squamous Cell Carcinomas of the Oral Cavity. Medicina 2022, 58, 570. https://doi.org/10.3390/medicina58050570
Carp A, Nicolau A, Moscalu M, Popescu E. Predictive Factors in the Appearance and Evolution of Squamous Cell Carcinomas of the Oral Cavity. Medicina. 2022; 58(5):570. https://doi.org/10.3390/medicina58050570
Chicago/Turabian StyleCarp, Alexandra, Andrei Nicolau, Mihaela Moscalu, and Eugenia Popescu. 2022. "Predictive Factors in the Appearance and Evolution of Squamous Cell Carcinomas of the Oral Cavity" Medicina 58, no. 5: 570. https://doi.org/10.3390/medicina58050570
APA StyleCarp, A., Nicolau, A., Moscalu, M., & Popescu, E. (2022). Predictive Factors in the Appearance and Evolution of Squamous Cell Carcinomas of the Oral Cavity. Medicina, 58(5), 570. https://doi.org/10.3390/medicina58050570






