Botulinum Toxin Use for Modulating Neuroimmune Cutaneous Activity in Psoriasis
Abstract
:1. Introduction
2. Neuroimmune Pathways in the Pathogenesis of Psoriasis
3. Botulinum Toxin Biology
4. Preclinical and Clinical Research
4.1. Preclinical Research
4.2. Clinical Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taneda, K.; Tominaga, M.; Negi, O.; Tengara, S.; Kamo, A.; Ogawa, H.; Takamori, K. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br. J. Dermatol. 2011, 165, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kubanov, A.A.; Katunina, O.R.; Chikin, V.V. Expression of Neuropeptides, Neurotrophins, and Neurotransmitters in the Skin of Patients with Atopic Dermatitis and Psoriasis. Bull. Exp. Biol. Med. 2015, 159, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.; Kurian, J.; Warwick, D.J.; Friedmann, P.S. Unilateral remission of psoriasis following traumatic nerve palsy. Br. J. Dermatol. 2005, 152, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.P.; Farber, E.M. Are sensory nerves essential for the development of psoriatic lesions? J. Am. Acad. Dermatol. 1993, 28, 488–489. [Google Scholar] [CrossRef]
- Dewing, S.B. Remission of psoriasis associated with cutaneous nerve section. Arch. Dermatol. 1971, 104, 220–221. [Google Scholar] [CrossRef]
- Farber, E.M.; Lanigan, S.W.; Boer, J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int. J. Dermatol. 1990, 29, 418–420. [Google Scholar] [CrossRef]
- Qin, B.; Sun, C.; Chen, L.; Wang, S.; Yang, J.; Xie, Z.; Shen, Z. The nerve injuries attenuate the persistence of psoriatic lesions. J. Dermatol. Sci. 2021, 102, 85–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Jiang, B.; Yan, S.; Lu, J. A promising therapeutic target for psoriasis: Neuropeptides in human skin. Int. Immunopharmacol. 2020, 87, 106755. [Google Scholar] [CrossRef]
- Chen, S.Q.; Chen, X.Y.; Cui, Y.Z.; Yan, B.X.; Zhou, Y.; Wang, Z.Y.; Xu, F.; Huang, Y.Z.; Zheng, Y.X.; Man, X.Y. Cutaneous nerve fibers participate in the progression of psoriasis by linking epidermal keratinocytes and immunocytes. Cell Mol. Life Sci. 2022, 79, 267. [Google Scholar] [CrossRef]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. Botulin Toxin Use in Rosacea and Facial Flushing Treatment. Open Access Maced. J. Med. Sci. 2019, 7, 2985–2987. [Google Scholar] [CrossRef] [Green Version]
- Khattab, F.M. Evaluation of Botulinum Toxin A as an Optional Treatment for Atopic Dermatitis. J. Clin. Aesthet. Dermatol. 2020, 13, 32–35. [Google Scholar] [PubMed]
- Harries, M.J.; Wong, S.; Farrant, P. Frontal Fibrosing Alopecia and Increased Scalp Sweating: Is Neurogenic Inflammation the Common Link? Ski. Appendage Disord. 2016, 1, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, D.F.; Ramos, P.M.; Antelo, D.A.P.; Machado, C.J.; Barcaui, C.B. Is there a rationale for the use of botulinum toxin in the treatment of Androgenetic Alopecia? J. Cosmet. Dermatol. 2021, 20, 2093–2095. [Google Scholar] [CrossRef] [PubMed]
- Cutrer, F.M.; Sandroni, P.; Wendelschafer-Crabb, G. Botulinum toxin treatment of cephalalgia alopecia increases substance P and calcitonin gene-related peptide-containing cutaneous nerves in scalp. Cephalalgia 2010, 30, 1000–1006. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hong, E.S.; Kim, H.S. Botulinum Toxin in the Field of Dermatology: Novel Indications. Toxins 2017, 9, 403. [Google Scholar] [CrossRef] [Green Version]
- Campanati, A.; Martina, E.; Giuliodori, K.; Consales, V.; Bobyr, I.; Offidani, A. Botulinum Toxin Off-Label Use in Dermatology: A Review. Ski. Appendage Disord. 2017, 3, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Martina, E.; Diotallevi, F.; Radi, G.; Campanati, A.; Offidani, A. Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review. Toxins 2021, 13, 120. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Brain, S.D. Neurovascular aspects of skin neurogenic inflammation. J. Investig. Dermatol. Symp. Proc. 2011, 15, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Siebenhaar, F.; Sharov, A.A.; Peters, E.M.; Sharova, T.Y.; Syska, W.; Mardaryev, A.N.; Freyschmidt-Paul, P.; Sundberg, J.P.; Maurer, M.; Botchkarev, V.A. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata). J. Investig. Dermatol. 2007, 127, 1489–1497. [Google Scholar] [CrossRef] [Green Version]
- Kothapalli, A.; Caccetta, T. Botulinum toxin type A for the first-line treatment of Hailey-Hailey disease. Australas. J. Dermatol. 2019, 60, 73–74. [Google Scholar] [CrossRef] [Green Version]
- Charlton, O.A.; Stewart, T.J.; Rosen, R.H. Treatment of Hailey-Hailey disease with botulinum toxin. Australas. J. Dermatol. 2018, 59, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Santiago-et-Sanchez-Mateos, J.L.; Bea, S.; Fernandez, M.; Perez, B.; Harto, A.; Jaen, P. Botulinum toxin type A for the preventive treatment of intertrigo in a patient with Darier’s disease and inguinal hyperhidrosis. Dermatol. Surg. 2008, 34, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Schultz, S.; Strouse, A.; Gater, D.R. Successful treatment of stage III hidradenitis suppurativa with botulinum toxin A. BMJ Case Rep. 2019, 12, e226064. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Martina, E.; Giuliodori, K.; Bobyr, I.; Consales, V.; Offidani, A. Two cases of Hidradenitis suppurativa and botulinum toxin type a therapy: A novel approach for a pathology that is still difficult to manage. Dermatol. Ther. 2019, 32, e12841. [Google Scholar] [CrossRef] [PubMed]
- Saraceno, R.; Kleyn, C.E.; Terenghi, G.; Griffiths, C.E. The role of neuropeptides in psoriasis. Br. J. Dermatol. 2006, 155, 876–882. [Google Scholar] [CrossRef]
- Amalia, S.N.; Uchiyama, A.; Baral, H.; Inoue, Y.; Yamazaki, S.; Fujiwara, C.; Sekiguchi, A.; Yokoyama, Y.; Ogino, S.; Torii, R.; et al. Suppression of neuropeptide by botulinum toxin improves imiquimod-induced psoriasis-like dermatitis via the regulation of neuroimmune system. J. Dermatol. Sci. 2021, 101, 58–68. [Google Scholar] [CrossRef]
- Smolyannikova, V.A.; Kubanova, A.A.; Karamova, A.E.; Nefedova, M.A.; Chikin, V.V. Role of the skin expression of neuropeptides, neurotrophins and their receptors in the pathogenesis of dermatoses. Arkhiv Patol. 2015, 77, 33–39. [Google Scholar] [CrossRef]
- Guo, R.; Li, F.F.; Chen, M.L.; Ya, M.Z.; He, H.L.; Li, D. The role of CGRP and CALCA T-692C single-nucleotide polymorphism in psoriasis vulgaris. Die Pharm. 2015, 70, 88–93. [Google Scholar]
- Zhang, X.; He, Y. The Role of Nociceptive Neurons in the Pathogenesis of Psoriasis. Front. Immunol. 2020, 11, 1984. [Google Scholar] [CrossRef]
- Barros, P.O.; Ferreira, T.B.; Vieira, M.M.; Almeida, C.R.; Araujo-Lima, C.F.; Silva-Filho, R.G.; Hygino, J.; Andrade, R.M.; Andrade, A.F.; Bento, C.A. Substance P enhances Th17 phenotype in individuals with generalized anxiety disorder: An event resistant to glucocorticoid inhibition. J. Clin. Immunol. 2011, 31, 51–59. [Google Scholar] [CrossRef]
- Bera, M.M.; Lu, B.; Martin, T.R.; Cui, S.; Rhein, L.M.; Gerard, C.; Gerard, N.P. Th17 cytokines are critical for respiratory syncytial virus-associated airway hyperreponsiveness through regulation by complement C3a and tachykinins. J. Immunol. 2011, 187, 4245–4255. [Google Scholar] [CrossRef]
- Cunin, P.; Caillon, A.; Corvaisier, M.; Garo, E.; Scotet, M.; Blanchard, S.; Delneste, Y.; Jeannin, P. The tachykinins substance P and hemokinin-1 favor the generation of human memory Th17 cells by inducing IL-1beta, IL-23, and TNF-like 1A expression by monocytes. J. Immunol. 2011, 186, 4175–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, A.E.; Sumpter, T.L.; Rojas-Canales, D.M.; Bandyopadhyay, M.; Chen, Z.; Tkacheva, O.; Shufesky, W.J.; Wallace, C.T.; Watkins, S.C.; Berger, A.; et al. Neurokinin-1 Receptor Signaling Is Required for Efficient Ca(2+) Flux in T-Cell-Receptor-Activated T Cells. Cell Rep. 2020, 30, 3448–3465. [Google Scholar] [CrossRef] [Green Version]
- Mikami, N.; Watanabe, K.; Hashimoto, N.; Miyagi, Y.; Sueda, K.; Fukada, S.; Yamamoto, H.; Tsujikawa, K. Calcitonin gene-related peptide enhances experimental autoimmune encephalomyelitis by promoting Th17-cell functions. Int. Immunol. 2012, 24, 681–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Stohl, L.L.; Xu, L.; Zhou, X.K.; Manni, M.; Wagner, J.A.; Granstein, R.D. Calcitonin Gene-Related Peptide-Exposed Endothelial Cells Bias Antigen Presentation to CD4+ T Cells toward a Th17 Response. J. Immunol. 2016, 196, 2181–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schantz, E.J.; Johnson, E.A. Botulinum toxin: The story of its development for the treatment of human disease. Perspect. Biol. Med. 1997, 40, 317–327. [Google Scholar] [CrossRef]
- Aoki, K.R.; Guyer, B. Botulinum toxin type A and other botulinum toxin serotypes: A comparative review of biochemical and pharmacological actions. Eur. J. Neurol. 2001, 8, 21–29. [Google Scholar] [CrossRef]
- Chen, S. Clinical uses of botulinum neurotoxins: Current indications, limitations and future developments. Toxins 2012, 4, 913–939. [Google Scholar] [CrossRef] [Green Version]
- Ababneh, O.H.; Cetinkaya, A.; Kulwin, D.R. Long-term efficacy and safety of botulinum toxin A injections to treat blepharospasm and hemifacial spasm. Clin. Exp. Ophthalmol. 2014, 42, 254–261. [Google Scholar] [CrossRef]
- Rowe, F.J.; Noonan, C.P. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst. Rev. 2017, 3, CD006499. [Google Scholar] [CrossRef]
- Popescu, M.N.; Petca, R.C.; Beiu, C.; Dumitrascu, M.C.; Petca, A.; Mehedintu, C.; Farcasanu, P.D.; Sandru, F. Efficiency of Different Preparations of Botulinum Toxin Type A, Xeomin and Dysport, in the Management of Spastic Upper Limb After Stroke. Rev. Chim. 2019, 70, 3490–3494. [Google Scholar] [CrossRef]
- Barbu, M.G.; Thompson, D.C.; Popescu, M.N.; Beiu, C.; Mihai, M.M.; Enachescu, C.I. A Romanian survey on the impact of SARS-CoV-2 pandemic on dystonia patients. Rom. J. Leg. Med. 2020, 28, 208–211. [Google Scholar]
- Kattimani, V.; Tiwari, R.V.C.; Gufran, K.; Wasan, B.; Shilpa, P.H.; Khader, A.A. Botulinum Toxin Application in Facial Esthetics and Recent Treatment Indications (2013–2018). J. Int. Soc. Prev. Community Dent. 2019, 9, 99–105. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.R.; Montagner, S. Botulinum toxin for axillary hyperhidrosis. Dermatol. Clin. 2014, 32, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Giuliodori, K.; Martina, E.; Giuliano, A.; Ganzetti, G.; Offidani, A. Onabotulinumtoxin type A (Botox®) versus Incobotulinumtoxin type A (Xeomin®) in the treatment of focal idiopathic palmar hyperhidrosis: Results of a comparative double-blind clinical trial. J. Neural Transm. 2014, 121, 21–26. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Lawrence, G.; Dolly, J.O. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J. Cell Sci. 2007, 120, 2864–2874. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, N.M.E.; Dostrovsky, J.O.; Charlton, M.P. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain 2010, 149, 316–324. [Google Scholar] [CrossRef]
- Ward, N.L.; Kavlick, K.D.; Diaconu, D.; Dawes, S.M.; Michaels, K.A.; Gilbert, E. Botulinum neurotoxin A decreases infiltrating cutaneous lymphocytes and improves acanthosis in the KC-Tie2 mouse model. J. Investig. Dermatol. 2012, 132, 1927–1930. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, S.M.; Belkadi, A.; Loyd, C.M.; Diaconu, D.; Ward, N.L. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J. Investig. Dermatol. 2011, 131, 1530–1538. [Google Scholar] [CrossRef] [Green Version]
- Zanchi, M.; Favot, F.; Bizzarini, M.; Piai, M.; Donini, M.; Sedona, P. Botulinum toxin type-A for the treatment of inverse psoriasis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 431–436. [Google Scholar] [CrossRef]
- Saber, M.; Brassard, D.; Benohanian, A. Inverse psoriasis and hyperhidrosis of the axillae responding to botulinum toxin type A. Arch. Dermatol. 2011, 147, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.; Ward, N.L. Efficacy of botulinum neurotoxin type A for treating recalcitrant plaque psoriasis. J. Drugs Dermatol. 2014, 13, 1407–1408. [Google Scholar] [PubMed]
- Aschenbeck, K.A.; Hordinsky, M.K.; Kennedy, W.R.; Wendelschafer-Crabb, G.; Ericson, M.E.; Kavand, S.; Bertin, A.; Dykstra, D.D.; Panoutsopoulou, I.G. Neuromodulatory treatment of recalcitrant plaque psoriasis with onabotulinumtoxinA. J. Am. Acad. Dermatol. 2018, 79, 1156–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.; Franco, M.; Londono, A.; Valenzuela, F. Breaking paradigms in the treatment of psoriasis: Use of botulinum toxin for the treatment of plaque psoriasis. Dermatol. Ther. 2020, 33, e14319. [Google Scholar] [CrossRef] [PubMed]
- Todberg, T.; Zachariae, C.; Bregnhoj, A.; Hedelund, L.; Bonefeld, K.K.; Nielsen, K.; Iversen, L.; Skov, L. The effect of botulinum neurotoxin A in patients with plaque psoriasis—An exploratory trial. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e81–e82. [Google Scholar] [CrossRef]
- Botsali, A.; Erbil, H. Management of nail psoriasis with a single injection of abobotulinum toxin. J. Cosmet. Dermatol. 2021, 20, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
| First Author [Ref.], Year | Study Design | Number of Patients Included | Affected Sites | Type and Doses of BoNT-A injected | Follow-Up | Results |
|---|---|---|---|---|---|---|
| Zanchi [50], 2008 | Single-arm (BoNT-A) pilot study | 15 | Inverse psoriasis: axillae (7), inframammary folds (6), intergluteal sulcus (7), groin skin folds (5), and umbilicus (1) | OnabotulinumtoxinA: 50–100 U per patient | 12 weeks | VAS score for itching and pain improved in all patients, and 87% of patients showed reduction in skin erythema and infiltration |
| Saber [51], 2011 | Case report | 1 | Inverse psoriasis: axillary regions | OnabotulinumtoxinA: 100 U per axilla | 1 month | From large brightly erythematous well-demarcated axillary plaques to only minimal residual erythema |
| Gilbert [52], 2014 | Case report | 1 | Left buttock | AbobotulinumtoxinA: 30 U | 7 months | Total remission of the injected plaque was achieved, and the effect was maintained for 7 months post-treatment; local relapse was noticed at 8 months post-injection |
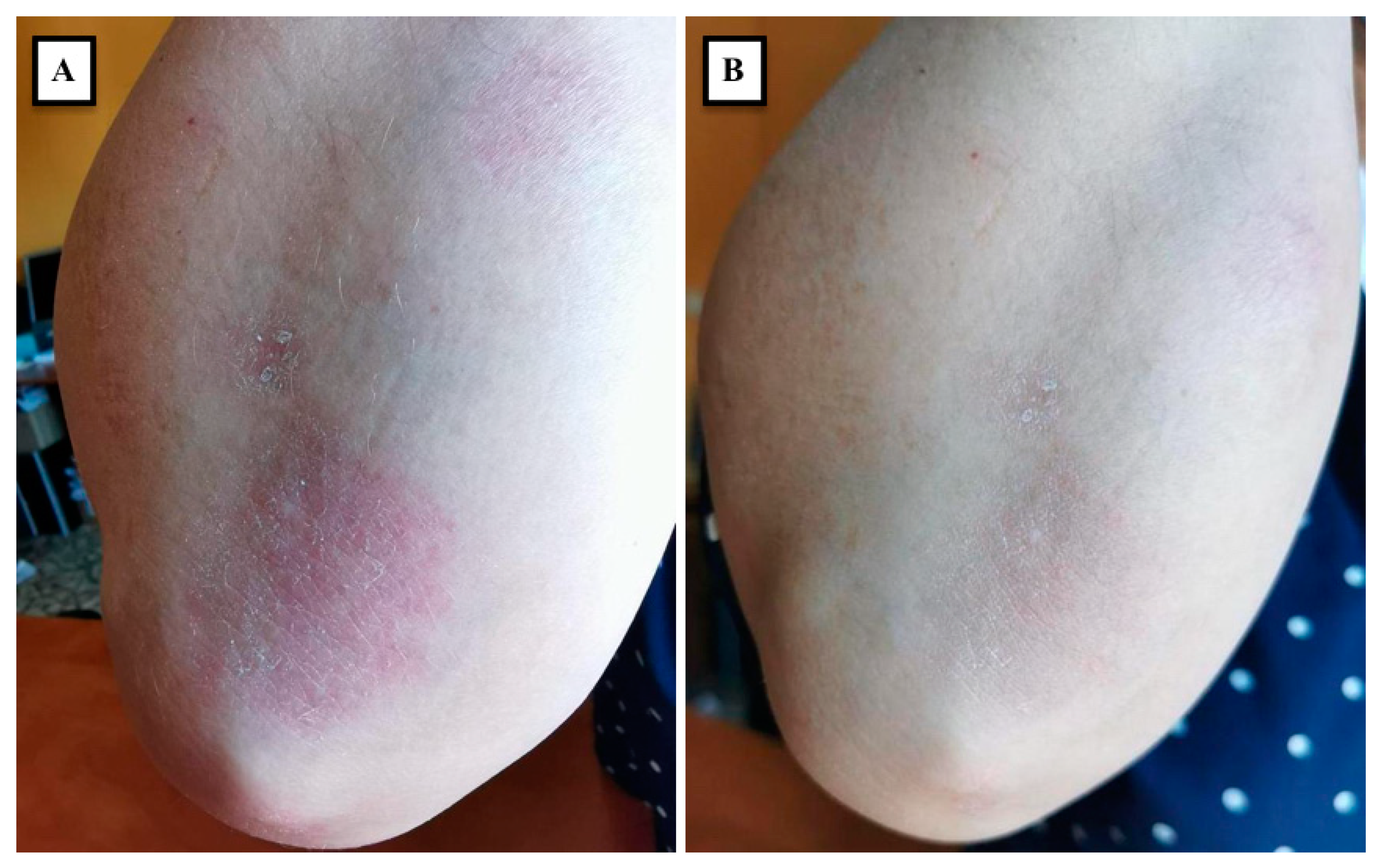
| Aschenbeck [53], 2018 | Single-center pilot study | 8 | Elbow (4), back (1), knee (1), leg (1), foot (1) | OnabotulinumtoxinA: 53 U in average (range, 25–98 U) | 10 weeks | Clinically, PASI and PGA score were significantly decreased; Immunohistochemically, increased ENF density and decreased expression of the neuropeptides SP and CGRP were detected 8 weeks after the injection |
| Gonzalez [54], 2020 | Descriptive cross-sectional study | 8 | Specific sites were not reported (12 plaques included in total) | AbobotulinumtoxinA: 5 U per cm2 of lesional skin (a maximum of 50 U) | 4 weeks | Average TCS showed statistically significant clinical improvement; two patients reported a significant reduction in pruritus |
| Todberg [55], 2018 | Randomized double-blinded trial | 10 | Specific sites were not reported (up to two target lesions were selected per patient) | AbobotulinumtoxinA: 36 U for a psoriatic plaque | 8 Weeks | No clinical or histopathological statistically significant differences between BoNT-A and placebo groups |
| Botsali [56], 2020 | Case report | 2 | Nail psoriasis | AbobotulinumtoxinA: Patient 1: 30 U per affected nail; Patient 2: 15 U per affected nail | Patient 1: 8 months; Patient 2: 6 months | Patient 1: VAS score decreased from 9 to 3 Patient 2: VAS score decreased from 6 to 2 on the 4th month, and was consistent with 3 on month 6 post-injection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.N.; Beiu, C.; Iliescu, M.G.; Mihai, M.M.; Popa, L.G.; Stănescu, A.M.A.; Berteanu, M. Botulinum Toxin Use for Modulating Neuroimmune Cutaneous Activity in Psoriasis. Medicina 2022, 58, 813. https://doi.org/10.3390/medicina58060813
Popescu MN, Beiu C, Iliescu MG, Mihai MM, Popa LG, Stănescu AMA, Berteanu M. Botulinum Toxin Use for Modulating Neuroimmune Cutaneous Activity in Psoriasis. Medicina. 2022; 58(6):813. https://doi.org/10.3390/medicina58060813
Chicago/Turabian StylePopescu, Marius Nicolae, Cristina Beiu, Mădălina Gabriela Iliescu, Mara Mădălina Mihai, Liliana Gabriela Popa, Ana Maria Alexandra Stănescu, and Mihai Berteanu. 2022. "Botulinum Toxin Use for Modulating Neuroimmune Cutaneous Activity in Psoriasis" Medicina 58, no. 6: 813. https://doi.org/10.3390/medicina58060813
APA StylePopescu, M. N., Beiu, C., Iliescu, M. G., Mihai, M. M., Popa, L. G., Stănescu, A. M. A., & Berteanu, M. (2022). Botulinum Toxin Use for Modulating Neuroimmune Cutaneous Activity in Psoriasis. Medicina, 58(6), 813. https://doi.org/10.3390/medicina58060813











