Relative Leukocyte Telomere Length and Telomerase Complex Regulatory Markers Association with Leber’s Hereditary Optic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
- Under the age of 18;
- Systemic diseases (e.g., malignant tumors, systemic connective tissue diseases, diabetes mellitus, chronic infections, or conditions following organ or tissue transplantation).
2.2. Leber’s Hereditary Optic Neuropathy (LHON) Diagnostics
2.3. DNA Extraction, RLTL Measurement, and Genotyping
2.4. Statistical Analysis
3. Results
3.1. Influence of TEP1 rs1760904, rs1713418, TERC rs12696304 on LHON Development
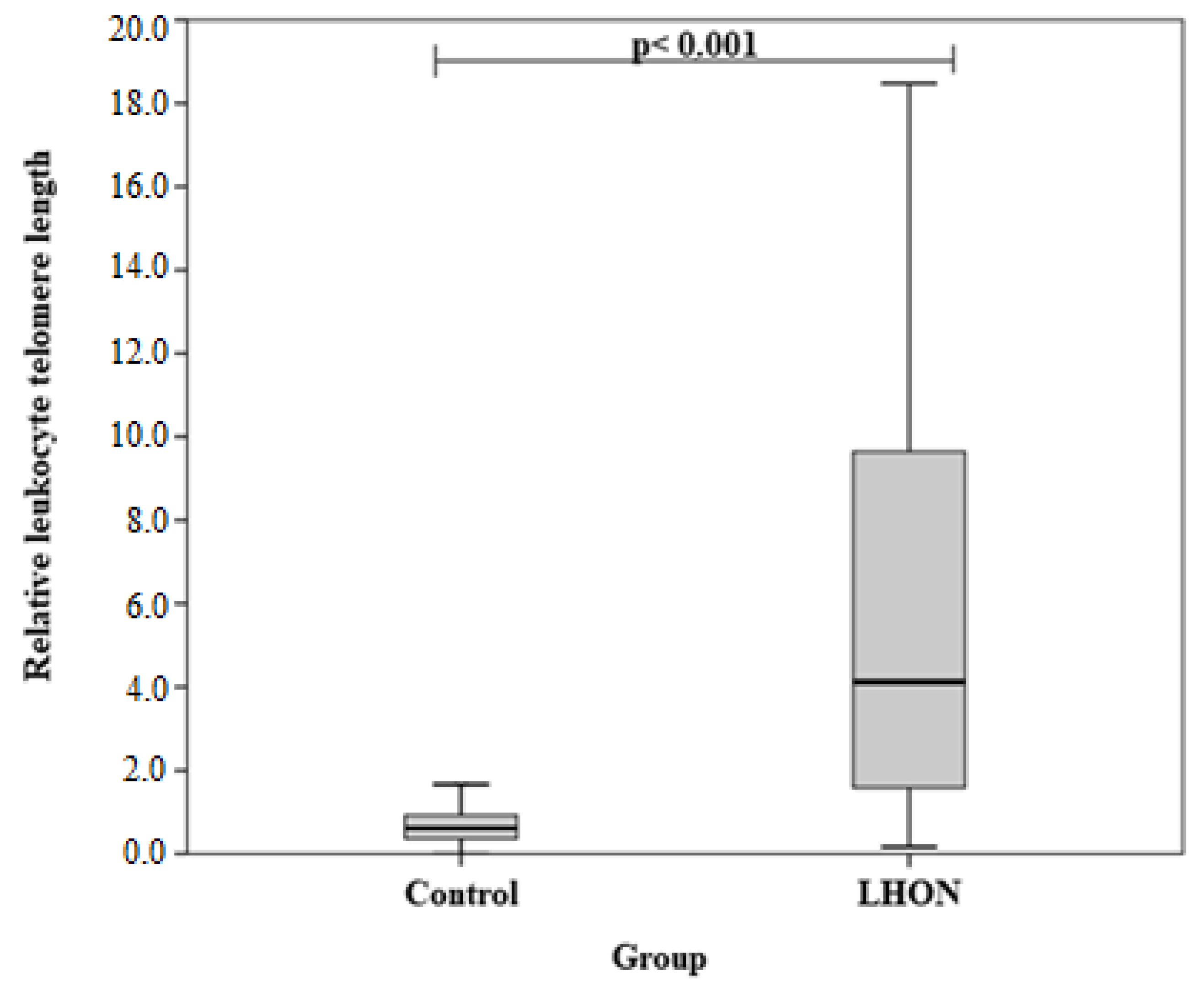
3.2. Differences in Relative Leukocyte Telomere Length (RLTL) between LHON and Healthy Subjects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J., II; Nikoskelainen, E.K. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Logan, I.; Yao, Y.-G. Leber Hereditary Optic Neuropathy: A Mitochondrial Disease Unique in Many Ways. Handb. Exp. Pharmacol. 2017, 240, 309–336. [Google Scholar] [PubMed]
- Mackey, D.A.; Oostra, R.-J.; Rosenberg, T.; Nikoskelainen, E.; Bronte-Stewart, J.; Poulton, J.; Harding, A.E.; Govan, G.; Bolhuis, P.A.; Norby, S. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am. J. Hum. Genet. 1996, 59, 481–485. [Google Scholar] [PubMed]
- Fraser, J.A.; Biousse, V.; Newman, N.J. The neuro-ophthalmology of mitochondrial disease. Surv. Ophthalmol. 2010, 55, 299–334. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Griffiths, P.G.; Chinnery, P.F. Mitochondrial optic neuropathies: Disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 2011, 30, 81–114. [Google Scholar] [CrossRef]
- Sadun, A.A.; La Morgia, C.; Carelli, V. Leber’s hereditary optic neuropathy. Curr. Treat. Options Neurol. 2011, 13, 109–117. [Google Scholar] [CrossRef]
- Nikoskelainen, E.K.; Huoponen, K.; Juvonen, V.; Lamminen, T.; Nummelin, K.; Savontaus, M.-L. Ophthalmologic Findings in Leber Hereditary Optic Neuropathy, with Special Reference to mtDNA Mutations. Ophthalmology 1996, 103, 504–514. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Griffiths, P.G.; Hudson, G.; Chinnery, P.F. Inherited mitochondrial optic neuropathies. J. Med. Genet. 2009, 46, 145–158. [Google Scholar] [CrossRef]
- Jancic, J.; Samardzic, J.; Stojanovic, S.; Stojanovic, A.; Milanovic, A.M.; Nikolić, B.; Ivancevic, N.; Kostic, V. Leber’s Hereditary Optic Neuropathy: Novel Views and Persisting Challenges. CNS Neurol. Disord. Drug Targets 2017, 16, 927–935. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Chinnery, P.F. Leber Hereditary Optic Neuropathy. GeneReviews 2000, 1, 1–20. [Google Scholar]
- Tonska, K.; Kodron, A.; Bartnik, E. Genotype-phenotype correlationsin Leber hereditary optic neuropathy. Biochim. Biophys. Acta. Bioenerg. 2010, 1797, 1119–1123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heitz, F.D.; Erb, M.; Anklin, C.; Robay, D.; Pernet, V.; Gueven, N. Idebenone protects against retinal damage and loss of vision in a mouse mode of Leber’s hereditary optic neuropathy. PLoS ONE. 2012, 7, e45182. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; La Morgia, C.; Valentino, M.L.; Rizzo, G.; Carbonelli, M.; De Negri, A.M.; Sadun, F.; Carta, A.; Guerriero, S.; Simonelli, F.; et al. Idebenone treatment in Leber’s hereditary optic neuropathy. Brain. 2011, 134, e188. [Google Scholar] [CrossRef] [PubMed]
- Karanjia, R.; Chahal, J.; Ammar, M.; Sadun, A.A. Treatment of Leber’s hereditary optic neuropathy. Curr. Pharm. Des. 2017, 23, 624–628. [Google Scholar]
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Ludlow, A.T.; Ludlow, L.W.; Roth, S.M. Do Telomeres Adapt to Physiological Stress? Exploring the Effect of Exercise on Telomere Length and Telomere-Related Proteins. Biomed. Res. Int. 2013, 2013, 601368. [Google Scholar] [CrossRef] [PubMed]
- Vazirpanah, N.; Verhagen, F.H.; Rothova, A.; Missotten, T.O.A.R.; van Velthoven, M.; Den Hollander, A.I.; Hoyng, C.B.; Radstake, T.R.D.J.; Broen, J.C.A.; Kuiper, J.J.W. Kuiper, Aberrant leukocyte telomere length in Birdshot Uveitis. PLoS ONE 2017, 12, e0176175. [Google Scholar] [CrossRef]
- Giardini, M.A.; Segatto, M.; da Silva, M.S.; Nunes, V.S.; Cano, M.I. Telomere and telomerase biology. Prog. Mol. Biol. Transl. Sci. 2014, 125, 1–40. [Google Scholar]
- Terada, K.; Miyake, K.; Yamaguchi, H.; Miyake, N.; Yamanaka, K.; Kojima, S.; Ito, E.; Inokuchi, K.; Okada, T. TERT and TERC mutations detected in cryptic dyskeratosis congenita suppress telomerase activity. Int. J. Lab. Hematology. 2020, 42, 316–321. [Google Scholar] [CrossRef]
- Laudadio, I.; Orso, F.; Azzalin, G.; Calabrò, C.; Berardinelli, F.; Coluzzi, E.; Gioiosa, S.; Taverna, D.; Sgura, A.; Carissimi, C.; et al. AGO2 promotes telomerase activity and interaction between the telomerase components TERT and TERC. EMBO Rep. 2019, 20, e45969. [Google Scholar] [CrossRef]
- Cimino-Reale, G.; Gandellini, P.; Santambrogio, F.; Recagni, M.; Zaffaroni, N.; Folini, M. miR-380-5p-mediated repression of TEP1 and TSPYL5 interferes with telomerase activity and favours the emergence of an “ALT-like” phenotype in diffuse malignant peritoneal mesothelioma cells. J. Hematol. Oncol. 2017, 10, 140. [Google Scholar] [CrossRef]
- Kirkman, M.A.; Yu-Wai-Man, P.; Korsten, A.; Leonhardt, M.; Dimitriadis, K.; De Coo, I.F.; Klopstock, T.; Chinnery, P.F. Gene– environment interactions in Leber hereditary optic neuropathy. Brain 2009, 132, 2317–2326. [Google Scholar] [CrossRef]
- Carelli, V.; Ross-Cisneros, F.N.; Sadun, A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004, 23, 53–89. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Carbonelli, M.; Barboni, P.; Sadun, A.A.; Carelli, V. Medical management of hereditary optic neuropathies. Front. Neurol. 2014, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Carbonelli, M.; de Coo, I.F.; Kawasaki, A.; Klopstock, T.; Lagreze, W.A.; La Morgia, C.; Newman, N.J.; Orssaud, C.; Pott, J.W.R.; et al. International consensus statement on the clinical and therapeutic management of leber hereditary optic neuropathy. J. Neuro-Ophthalmol. 2017, 37, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Armstrong, L.L.; Bradford, Y.; Carlson, C.S.; Crawford, D.C.; Crenshaw, A.T.; de Andrade, M.; Doheny, K.F.; Haines, J.L.; Hayes, G. Quality control procedures for genome-wide association studies. Curr. Protoc. Hum. Genet. 2011, 68, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Griffiths, P.G.; Brown, D.T.; Howell, N.; Turnbull, D.M.; Chinnery, P.F. The epidemiology of leber hereditary optic neuropathy in the north east of England. Am. J. Hum. Genet. 2003, 72, 333–339. [Google Scholar] [CrossRef]
- Rosenberg, T.; Nørby, S.; Schwartz, M.; Saillard, J.; Magalhães, P.J.; Leroy, D.; Kann, E.C.; Duno, M. Prevalence and genetics of Leber hereditary optic neuropathy in the Danish population. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1370–1375. [Google Scholar] [CrossRef]
- Barboni, P.; Savini, G.; Valentino, M.L.; Montagna, P.; Cortelli, P.; De Negri, A.M.; Sadun, F.; Bianchi, S.; Longanesi, L.; Zanini, M. Retinal nerve fiber layer evaluation by optical coherence tomography in leber’s hereditary optic neuropathy. Ophthalmology 2005, 112, 120–126. [Google Scholar] [CrossRef]
- Barboni, P.; Carbonelli, M.; Savini, G.; Ramos, C.D.V.; Carta, A.; Berezovsky, A.; Salomao, S.R.; Carelli, V.; Sadun, A.A. Natural history of leber’s hereditary optic neuropathy: Longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology 2010, 117, 623–627. [Google Scholar] [CrossRef]
- Balducci, N.; Savini, G.; Cascavilla, M.L.; La Morgia, C.; Triolo, G.; Giglio, R.; Carbonelli, M.; Parisi, V.; Sadun, A.A.; Bandello, F.; et al. Macular nerve fibre and ganglion cell layer changes in acute leber’s hereditary optic neuropathy. Br. J. Ophthalmol. 2016, 100, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Lott, M.T. Leber hereditary optic neuropathy: Exemplar of an mtDNA disease. Handb. Exp. Pharmacol. 2017, 240, 339–376. [Google Scholar] [PubMed]
- Dimitriadis, K.; Leonhardt, M.; Yu-Wai-Man, P.; Kirkman, M.A.; Korsten, A.; De Coo, I.F.; Chinnery, P.F.; Klopstock, T. Leber’s hereditary optic neuropathy with late disease onset: Clinical and molecular characteristics of 20 patients. Orphanet J. Rare Dis. 2014, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Mashima, Y.; Yamada, K.; Wakakura, M.; Kigasawa, K.; Kudoh, J.; Shimizu, N.; Oguchi, Y. Spectrum of pathogenic mitochondrial DNA mutations and clinical features in Japanese families with Leber’s hereditary optic neuropathy. Curr Eye Res. 1998, 17, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L. DNAJC30 biallelic mutations extend mitochondrial complex I-deficient phenotypes to include recessive Leber’s hereditary optic neuropathy. J. Clin. Investig. 2021, 131, e147734. [Google Scholar] [CrossRef]
- Gonzales-Ebsen, A.C.; Gregersen, N.; Olsen, R.K. Linking telomere loss and mitochondrial dysfunction in chronic disease. Front. Biosci. 2017, 22, 117–127. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Saretzki, G.; Docke, W.; Lotze, C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp. Cell Res. 1995, 220, 186–193. [Google Scholar] [CrossRef]
- Oikawa, S.; Kawanishi, S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999, 453, 365–368. [Google Scholar] [CrossRef]
- Rovcanin, B.; Jancic, J.; Pajic, J.; Rovcanin, M.; Samardzic, J.; Djuric, V.; Nikolic, B.; Ivancevic, N.; Novakovic, I.; Kostic, V. Oxidative Stress Profile in Genetically Confirmed Cases of Leber’s Hereditary Optic Neuropathy. J. Mol. Neurosci. 2021, 71, 1070–1081. [Google Scholar] [CrossRef]
- Bahr, T.; Welburn, K.; Donnelly, J.; Bai, Y. Emerging model systems and treatment approaches for Leber’s hereditary optic neuropathy: Challenges and opportunities. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165743. [Google Scholar] [CrossRef]
- Telomeres Mendelian Randomization Collaboration; Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh-Far, R.; Lin, J.; Epel, E.S.; Harris, W.S.; Blackburn, E.H.; Whooley, M.A. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA 2010, 303, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef]
- Desgarnier, M.C.D.; Zinflou, C.; Mallet, J.D.; Gendron, S.P.; Méthot, S.J.; Rochette, P.J. Telomere Length Measurement in Different Ocular Structures: A Potential Implication in Corneal Endothelium Pathogenesis. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta- analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Polat, F.; Yilmaz, M.; Budak Diler, S. The Association of MYNN and TERC Gene Polymorphisms and Bladder Cancer in a Turkish Population. Urol. J. 2019, 16, 50–55. [Google Scholar]
- Jones, A.M.; Beggs, A.D.; Carvajal-Carmona, L.; Farrington, S.; Tenesa, A.; Walker, M.; Howarth, K.; Ballereau, S.; Hodgson, S.V.; Zauber, A.; et al. TERC polymorphisms are associated both with susceptibility to colorectal cancer and with longer telomeres. Gut 2012, 61, 248–254. [Google Scholar] [CrossRef]
- Li, Y.; Cheang, I.; Zhang, Z.; Yao, W.; Zhou, Y.; Zhang, H.; Liu, Y.; Zuo, X.; Li, X.; Cao, Q. Prognostic Association of TERC, TERT Gene Polymorphism, and Leukocyte Telomere Length in Acute Heart Failure: A Prospective Study. Front Endocrinol. 2021, 12, 650922. [Google Scholar] [CrossRef]
- Gu, C.; Li, Q.; Zhu, Y.; Qu, Y.; Zhang, G.; Wang, M.; Yang, Y.; Wang, J.; Jin, L.; Wei, Q.; et al. Genetic variants in the TEP1 gene are associated with prostate cancer risk and recurrence. Prostate Cancer Prostatic Dis. 2015, 18, 310–316. [Google Scholar] [CrossRef][Green Version]
- Yan, L.; Wu, S.; Zhang, S.; Ji, G.; Gu, A. Genetic variants in telomerase reverse transcriptase (TERT) and telomerase-associated protein 1 (TEP1) and the risk of male infertility. Gene 2014, 534, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, H.; Yang, Y.; Wang, P.; Zhang, H.; Liu, B.; Wei, W.; Yao, W.; Zhou, X.; Zhao, J.; et al. Genetic variants in telomerase-associated protein 1 are associated with telomere damage in PAH- exposed workers. Ecotoxicol. Environ. Saf. 2021, 223, 112558. [Google Scholar] [CrossRef] [PubMed]
- Codd, V.; Mangino, M.; van der Harst, P.; Braund, P.S.; Kaiser, M.; Beveridge, A.J.; Rafelt, S.; Moore, J.; Nelson, C.; Soranzo, N.; et al. Common variants near TERC are associated with mean telomere length. Nat. Genet. 2010, 42, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Müezzinler, U.; Mons, A.K.; Dieffenbach, K.; Butterbach, K.-U.; Saum, M.; Schick, H.; Stammer, P.; Boukamp, B.; Holleczek, C.; Stegmaier, H.; et al. Smoking habits and leukocyte telomere length dynamics among older adults: Results from the ESTHER cohort. Exp. Gerontol. 2015, 70, 18–25. [Google Scholar] [CrossRef]
| Characteristics | Group | p-Value * | ||
|---|---|---|---|---|
| LHON n (%) | Control n (%) | |||
| Gender | Males | 10 (71.4) | 61 (46.9) | 0.081 |
| Females | 4 (28.6) | 69 (53.1) | ||
| Age, mean (IQR) | 40.6 (13.1) | 39.9 (17.9) | 0.843 | |
| RLTL, mean (SD) | 7.31 (7.28) | 0.77 (0.75) | <0.001 | |
| Gene, SNP | Allele Frequencies | Genotype Distribution | p-Value * | |
|---|---|---|---|---|
| TEP1 rs1760904 | 0.49 (A) | 0.51 (G) | 30/69/31 | 0.482 |
| TEP1 rs1713418 | 0.43 (G) | 0.57 (A) | 21/71/38 | 0.204 |
| TERC rs12696304 | 0.27 (G) | 0.73 (C) | 8/54/68 | 0.526 |
| TERC rs35073794 | 0.19 (A) | 0.81 (G) | 0/49/81 | <0.05 |
| Model | Genotype/Allele | OR (95% CI) | p-Value | AIC |
|---|---|---|---|---|
| TEP1 rs1760904 | ||||
| Codominant | AG vs. GG AA vs. GG | 1.647 (0.429–6.323) 0.000 (0.000–) | 0.467 0.998 | 88.358 |
| Dominant | AG+AA vs. GG | 1.148 (0.301–4.380) | 0.840 | 93.812 |
| Recessive | AA vs. GG+AG | 0.000 (0.000–) | 0.998 | 86.926 |
| Overdominant | AG vs. AA+GG | 3.242 (0.864–12.162) | 0.081 | 90.283 |
| Additive | A | 0.622 (0.266–1.454) | 0.274 | 92.626 |
| TEP1 rs1713418 | ||||
| Codominant | AG vs. AA GG vs. AA | 1.070 (0.303–3.786) 0.905 (0.153–5.360) | 0.916 0.912 | 95.809 |
| Dominant | AG+GG vs. AA | 1.033 (0.305–3.496) | 0.959 | 93.851 |
| Recessive | GG vs. AA+AG | 0.865 (0.180–4.150) | 0.856 | 93.820 |
| Overdominant | AG vs. AA+GG | 1.108 (0.364–3.373) | 0.857 | 93.821 |
| Additive | G | 0.972 (0.421–2.248) | 0.948 | 92.849 |
| TERC rs12696304 | ||||
| Codominant | CG vs. CC GG vs. CC | 0.720 (0.200–2.586) 3.643 (0.782–16.961) | 0.6140.100 | 92.529 |
| Dominant | CG+GG vs. CC | 1.097 (0.364–3.304) | 0.870 | 93.827 |
| Recessive | GG vs. CC+CG | 4.159 (0.963–17.969) | 0.056 | 90.789 |
| Overdominant | CG vs. CC+GG | 0.563 (0.168–1.890) | 0.352 | 92.935 |
| Additive | C | 1.513 (0.662–3.458) | 0.326 | 92.917 |
| Genotype | RLTL | p-Value * | |
|---|---|---|---|
| LHON Group Median (IQR) | Control Group Median (IQR) | ||
| TEP1 rs1760904 | |||
| GG | 0.725 (-) | 0.441 (0.511) | 0.738 |
| GA | 4.505 (15.405) | 0.696 (0.549) | <0.001 |
| AA | - | 0.512 (0.858) | NA |
| TEP1 rs1713418 | |||
| AA | 3.474 (16.635) | 0.506 (0.682) | 0.011 |
| AG | 7.940 (12.964) | 0.644 (0.565) | <0.001 |
| GG | 1.629 (-) | 0.695 (0.573) | 0.913 |
| TERC rs12696304 | |||
| CC | 3.097 (3.780) | 0.456 (0.652) | 0.005 |
| CG | 12.105 (16.187) | 0.694 (0.573) | 0.008 |
| GG | 9.764 (-) | 0.982 (1.529) | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liutkeviciene, R.; Mikalauskaite, R.; Gedvilaite, G.; Glebauskiene, B.; Kriauciuniene, L.; Žemaitienė, R. Relative Leukocyte Telomere Length and Telomerase Complex Regulatory Markers Association with Leber’s Hereditary Optic Neuropathy. Medicina 2022, 58, 1240. https://doi.org/10.3390/medicina58091240
Liutkeviciene R, Mikalauskaite R, Gedvilaite G, Glebauskiene B, Kriauciuniene L, Žemaitienė R. Relative Leukocyte Telomere Length and Telomerase Complex Regulatory Markers Association with Leber’s Hereditary Optic Neuropathy. Medicina. 2022; 58(9):1240. https://doi.org/10.3390/medicina58091240
Chicago/Turabian StyleLiutkeviciene, Rasa, Rasa Mikalauskaite, Greta Gedvilaite, Brigita Glebauskiene, Loresa Kriauciuniene, and Reda Žemaitienė. 2022. "Relative Leukocyte Telomere Length and Telomerase Complex Regulatory Markers Association with Leber’s Hereditary Optic Neuropathy" Medicina 58, no. 9: 1240. https://doi.org/10.3390/medicina58091240
APA StyleLiutkeviciene, R., Mikalauskaite, R., Gedvilaite, G., Glebauskiene, B., Kriauciuniene, L., & Žemaitienė, R. (2022). Relative Leukocyte Telomere Length and Telomerase Complex Regulatory Markers Association with Leber’s Hereditary Optic Neuropathy. Medicina, 58(9), 1240. https://doi.org/10.3390/medicina58091240







