Cardiovascular Complications of Obstructive Sleep Apnea in the Intensive Care Unit and Beyond
Abstract
:1. Introduction
2. Atrial Fibrillation
3. Non-AF Arrhythmias
4. Heart Failure
5. Acute Coronary Syndrome
6. Stroke
7. Pulmonary Hypertension
8. Thrombo-Embolic Disease
9. Screening and Treating OSA
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaneko, Y.; Floras, J.S.; Usui, K.; Plante, J.; Tkacova, R.; Kubo, T.; Ando, S.; Bradley, T.D. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N. Engl. J. Med. 2003, 348, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, R.S.; Bradley, T.D. Sleep apnea and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2001, 164, 2147–2165, quiz 554–545. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.D.; Brooks, D.; Kozar, L.F.; Render-Teixeira, C.L.; Horner, R.L.; Douglas Bradley, T.; Phillipson, E.A. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am. J. Respir. Crit. Care Med. 1999, 160, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Ramar, K.; Olson, E.J. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 2011, 86, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.M.; Ten Have, T.; Rein, J.; Vela-Bueno, A.; Kales, A. Prevalence of sleep-disordered breathing in women: Effects of gender. Am. J. Respir. Crit. Care Med. 2001, 163, 608–613. [Google Scholar] [CrossRef]
- Bolona, E.; Hahn, P.Y.; Afessa, B. Intensive care unit and hospital mortality in patients with obstructive sleep apnea. J. Crit. Care 2015, 30, 178–180. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef]
- Lin, C.M.; Davidson, T.M.; Ancoli-Israel, S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med. Rev. 2008, 12, 481–496. [Google Scholar] [CrossRef] [Green Version]
- Bertuzzi, F.; Santagostini, A.; Pollis, M.; Meola, F.; Segù, M. The Interaction of Craniofacial Morphology and Body Mass Index in Obstructive Sleep Apnea. Dent. J. 2022, 10, 136. [Google Scholar] [CrossRef]
- Roca, G.Q.; Redline, S.; Claggett, B.; Bello, N.; Ballantyne, C.M.; Solomon, S.D.; Shah, A.M. Sex-Specific Association of Sleep Apnea Severity with Subclinical Myocardial Injury, Ventricular Hypertrophy, and Heart Failure Risk in a Community-Dwelling Cohort: The Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation 2015, 132, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, S.; Galerneau, L.M.; Ruckly, S.; Seiller, A.; Terzi, N.; Schwebel, C.; Dupuis, C.; Tamisier, R.; Mourvillier, B.; Pepin, J.L.; et al. Impact of obstructive sleep apnea on the obesity paradox in critically ill patients. J. Crit. Care 2020, 56, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.C.; Anderson, W.M.; Chesson, A.L., Jr.; Nagel, C.L. Sleep-related breathing disorders in patients who are critically ill. J. Cardiovasc. Nurs. 2002, 17, 42–55. [Google Scholar] [CrossRef] [PubMed]
- BaHammam, A.; Syed, S.; Al-Mughairy, A. Sleep-related breathing disorders in obese patients presenting with acute respiratory failure. Respir. Med. 2005, 99, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Li, X.; Zhang, J.; Liang, Z. Association between Obstructive Sleep Apnea and Reduced Mortality in Critically Ill Patients: A Propensity Score-Based Analysis. Int. J. Gen. Med. 2021, 14, 4723–4729. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Kanji, S.; Williamson, D.R.; Yaghchi, B.M.; Albert, M.; McIntyre, L. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J. Crit. Care 2012, 27, e321–e328. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujii, T.; Uchino, S.; Takinami, M. Epidemiology, prevention, and treatment of new-onset atrial fibrillation in critically ill: A systematic review. J. Intensive Care 2015, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Traaen, G.M.; Øverland, B.; Aakerøy, L.; Hunt, T.E.; Bendz, C.; Sande, L.; Aakhus, S.; Zaré, H.; Steinshamn, S.; Anfinsen, O.G.; et al. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int J. Cardiol. Heart Vasc. 2020, 26, 100447. [Google Scholar] [CrossRef]
- May, A.M.; Van Wagoner, D.R.; Mehra, R. OSA and Cardiac Arrhythmogenesis: Mechanistic Insights. Chest 2017, 151, 225–241. [Google Scholar] [CrossRef]
- Klein Klouwenberg, P.M.; Frencken, J.F.; Kuipers, S.; Ong, D.S.; Peelen, L.M.; van Vught, L.A.; Schultz, M.J.; van der Poll, T.; Bonten, M.J.; Cremer, O.L. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am. J. Respir. Crit. Care Med. 2017, 195, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Lan, H.; Reynolds, D.J.; Gunderson, T.M.; Kashyap, R.; Gajic, O.; Caples, S.; Li, G.; Kashani, K.B. Association between Obstructive Sleep Apnea and Acute Kidney Injury in Critically Ill Patients: A Propensity-Matched Study. Nephron 2017, 135, 137–146. [Google Scholar] [CrossRef]
- Shukla, A.; Aizer, A.; Holmes, D.; Fowler, S.; Park, D.S.; Bernstein, S.; Bernstein, N.; Chinitz, L. Effect of Obstructive Sleep Apnea Treatment on Atrial Fibrillation Recurrence: A Meta-Analysis. JACC Clin. Electrophysiol. 2015, 1, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Caples, S.M.; Mansukhani, M.P.; Friedman, P.A.; Somers, V.K. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: A randomized controlled trial. Int. J. Cardiol. 2019, 278, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Takahashi, M.; Yaegashi, H.; Eda, S.; Tsunemoto, H.; Kamikozawa, M.; Koyama, J.; Yamazaki, K.; Ikeda, U. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels 2010, 25, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Traaen, G.M.; Aakerøy, L.; Hunt, T.E.; Øverland, B.; Bendz, C.; Sande, L.; Aakhus, S.; Fagerland, M.W.; Steinshamn, S.; Anfinsen, O.G.; et al. Effect of Continuous Positive Airway Pressure on Arrhythmia in Atrial Fibrillation and Sleep Apnea: A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 573–582. [Google Scholar] [CrossRef]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [Green Version]
- Raghuram, A.; Clay, R.; Kumbam, A.; Tereshchenko, L.G.; Khan, A. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. J. Clin. Sleep Med. 2014, 10, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Fernández, A.; García-Río, F.; Racionero, M.A.; Pino, J.M.; Ortuño, F.; Martínez, I.; Villamor, J. Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanisms. Chest 2005, 127, 15–22. [Google Scholar] [CrossRef]
- Rossi, V.A.; Stradling, J.R.; Kohler, M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2013, 41, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.S.; Somers, V.K.; Knilans, T.K.; Ackerman, M.J.; Wang, Y.; Amin, R.S. Obstructive Sleep Apnea in Patients with Congenital Long QT Syndrome: Implications for Increased Risk of Sudden Cardiac Death. Sleep 2015, 38, 1113–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, H.; Brandenburg, U.; Conradt, R.; Köhler, U.; Peter, J.H.; Ploch, T.; von Wichert, P. Influence of nCPAP therapy on bradycardic arrhythmias in sleep apnea. Pneumologie 1993, 47 (Suppl. S4), 706–710. [Google Scholar]
- Ryan, C.M.; Usui, K.; Floras, J.S.; Bradley, T.D. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005, 60, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simantirakis, E.N.; Schiza, S.I.; Marketou, M.E.; Chrysostomakis, S.I.; Chlouverakis, G.I.; Klapsinos, N.C.; Siafakas, N.S.; Vardas, P.E. Severe bradyarrhythmias in patients with sleep apnoea: The effect of continuous positive airway pressure treatment: A long-term evaluation using an insertable loop recorder. Eur. Heart J. 2004, 25, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Sleep apnea and heart failure: Part, I.I. central sleep apnea. Circulation 2003, 107, 1822–1826. [Google Scholar] [CrossRef] [Green Version]
- Khattak, H.K.; Hayat, F.; Pamboukian, S.V.; Hahn, H.S.; Schwartz, B.P.; Stein, P.K. Obstructive Sleep Apnea in Heart Failure: Review of Prevalence, Treatment with Continuous Positive Airway Pressure, and Prognosis. Tex. Heart Inst. J. 2018, 45, 151–161. [Google Scholar] [CrossRef]
- Chiu, K.L.; Ryan, C.M.; Shiota, S.; Ruttanaumpawan, P.; Arzt, M.; Haight, J.S.; Chan, C.T.; Floras, J.S.; Bradley, T.D. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am. J. Respir. Crit. Care Med. 2006, 174, 1378–1383. [Google Scholar] [CrossRef]
- Chenuel, B.J.; Smith, C.A.; Skatrud, J.B.; Henderson, K.S.; Dempsey, J.A. Increased propensity for apnea in response to acute elevations in left atrial pressure during sleep in the dog. J. Appl. Physiol. 2006, 101, 76–83. [Google Scholar] [CrossRef]
- Chen, L.; Einbinder, E.; Zhang, Q.; Hasday, J.; Balke, C.W.; Scharf, S.M. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am. J. Respir. Crit. Care Med. 2005, 172, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Oldenburg, O.; Wellmann, B.; Buchholz, A.; Bitter, T.; Fox, H.; Thiem, U.; Horstkotte, D.; Wegscheider, K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur. Heart J. 2016, 37, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.E.; Belohlavek, M.; Romero-Corral, A.; Gami, A.S.; Gilman, G.; Svatikova, A.; Amin, R.S.; Lopez-Jimenez, F.; Khandheria, B.K.; Somers, V.K. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am. J. Cardiol. 2007, 99, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep J. Sleep Sleep Disord. Res. 2003, 26, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendjelid, K.; Schütz, N.; Suter, P.M.; Fournier, G.; Jacques, D.; Fareh, S.; Romand, J.A. Does continuous positive airway pressure by face mask improve patients with acute cardiogenic pulmonary edema due to left ventricular diastolic dysfunction? Chest 2005, 127, 1053–1058. [Google Scholar] [CrossRef]
- Tkacova, R.; Rankin, F.; Fitzgerald, F.S.; Floras, J.S.; Bradley, T.D. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation 1998, 98, 2269–2275. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, D.R.; Gollogly, N.C.; Kaye, D.M.; Richardson, M.; Bergin, P.; Naughton, M.T. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am. J. Respir. Crit. Care Med. 2004, 169, 361–366. [Google Scholar] [CrossRef]
- Gray, A.; Goodacre, S.; Newby, D.E.; Masson, M.; Sampson, F.; Nicholl, J. Noninvasive Ventilation in Acute Cardiogenic Pulmonary Edema. N. Engl. J. Med. 2008, 359, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.B.; Beanlands, R.S.; Yoshinaga, K.; Haddad, H.; Leech, J.; de Kemp, R.; Burwash, I.G. Acute and chronic effects of continuous positive airway pressure therapy on left ventricular systolic and diastolic function in patients with obstructive sleep apnea and congestive heart failure. Can. J. Cardiol. 2008, 24, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Cowie, M.R.; Woehrle, H.; Wegscheider, K.; Angermann, C.; d’Ortho, M.P.; Erdmann, E.; Levy, P.; Simonds, A.K.; Somers, V.K.; Zannad, F.; et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N. Engl. J. Med. 2015, 373, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Hla, K.M.; Young, T.; Hagen, E.W.; Stein, J.H.; Finn, L.A.; Nieto, F.J.; Peppard, P.E. Coronary heart disease incidence in sleep disordered breathing: The Wisconsin Sleep Cohort Study. Sleep 2015, 38, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Campos-Rodríguez, F.; Catalán-Serra, P.; Soler-Cataluña, J.J.; Almeida-Gonzalez, C.; De la Cruz Morón, I.; Durán-Cantolla, J.; Montserrat, J.M. Cardiovascular mortality in obstructive sleep apnea in the elderly: Role of long-term continuous positive airway pressure treatment: A prospective observational study. Am. J. Respir. Crit. Care Med. 2012, 186, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [PubMed]
- Shah, N.A.; Yaggi, H.K.; Concato, J.; Mohsenin, V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010, 14, 131–136. [Google Scholar] [CrossRef]
- Nakashima, H.; Katayama, T.; Takagi, C.; Amenomori, K.; Ishizaki, M.; Honda, Y.; Suzuki, S. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur. Heart J. 2006, 27, 2317–2322. [Google Scholar] [CrossRef] [Green Version]
- Yumino, D.; Tsurumi, Y.; Takagi, A.; Suzuki, K.; Kasanuki, H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am. J. Cardiol. 2007, 99, 26–30. [Google Scholar] [CrossRef]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive Sleep Apnea and Cardiovascular Events After Percutaneous Coronary Intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Khoo, S.M.; Chan, M.Y.; Wong, H.B.; Low, A.F.; Phua, Q.H.; Richards, A.M.; Tan, H.C.; Yeo, T.C. Severe obstructive sleep apnea and outcomes following myocardial infarction. J. Clin. Sleep Med. 2011, 7, 616–621. [Google Scholar] [CrossRef] [Green Version]
- Cepeda-Valery, B.; Acharjee, S.; Romero-Corral, A.; Pressman, G.S.; Gami, A.S. Obstructive sleep apnea and acute coronary syndromes: Etiology, risk, and management. Curr. Cardiol. Rep. 2014, 16, 535. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, M.; Sánchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Thunström, E.; Glantz, H.; Eulenburg, C. Effect of Obstructive Sleep Apnea and CPAP Treatment on Cardiovascular Outcomes in Acute Coronary Syndrome in the RICCADSA Trial. J. Clin. Med. 2020, 9, 4051. [Google Scholar] [CrossRef]
- Quan, W.; Zheng, D.; Douglas McEvoy, R.; Barbe, F.; Chen, R.; Liu, Z.; Loffler, K.; Lorenzi-Filho, G.; Luo, Y.; Mukherjee, S.; et al. High Risk Characteristics for Recurrent Cardiovascular Events among Patients with Obstructive Sleep Apnoea in the SAVE Study. EClinicalMedicine 2018, 2–3, 59–65. [Google Scholar] [CrossRef]
- Lisan, Q.; Van Sloten, T.; Marques Vidal, P.; Haba Rubio, J.; Heinzer, R.; Empana, J.P. Association of Positive Airway Pressure Prescription with Mortality in Patients with Obesity and Severe Obstructive Sleep Apnea: The Sleep Heart Health Study. JAMA Otolaryngol. Head Neck. Surg. 2019, 145, 509–515. [Google Scholar] [CrossRef]
- Pack, A.I.; Magalang, U.J.; Singh, B.; Kuna, S.T.; Keenan, B.T.; Maislin, G. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: Understanding and overcoming bias. Sleep 2021, 44, zsaa229. [Google Scholar] [CrossRef]
- Yasir, M.; Pervaiz, A.; Sankari, A. Cardiovascular Outcomes in Sleep-Disordered Breathing: Are We Under-estimating? Front. Neurol. 2022, 13, 801167. [Google Scholar] [CrossRef]
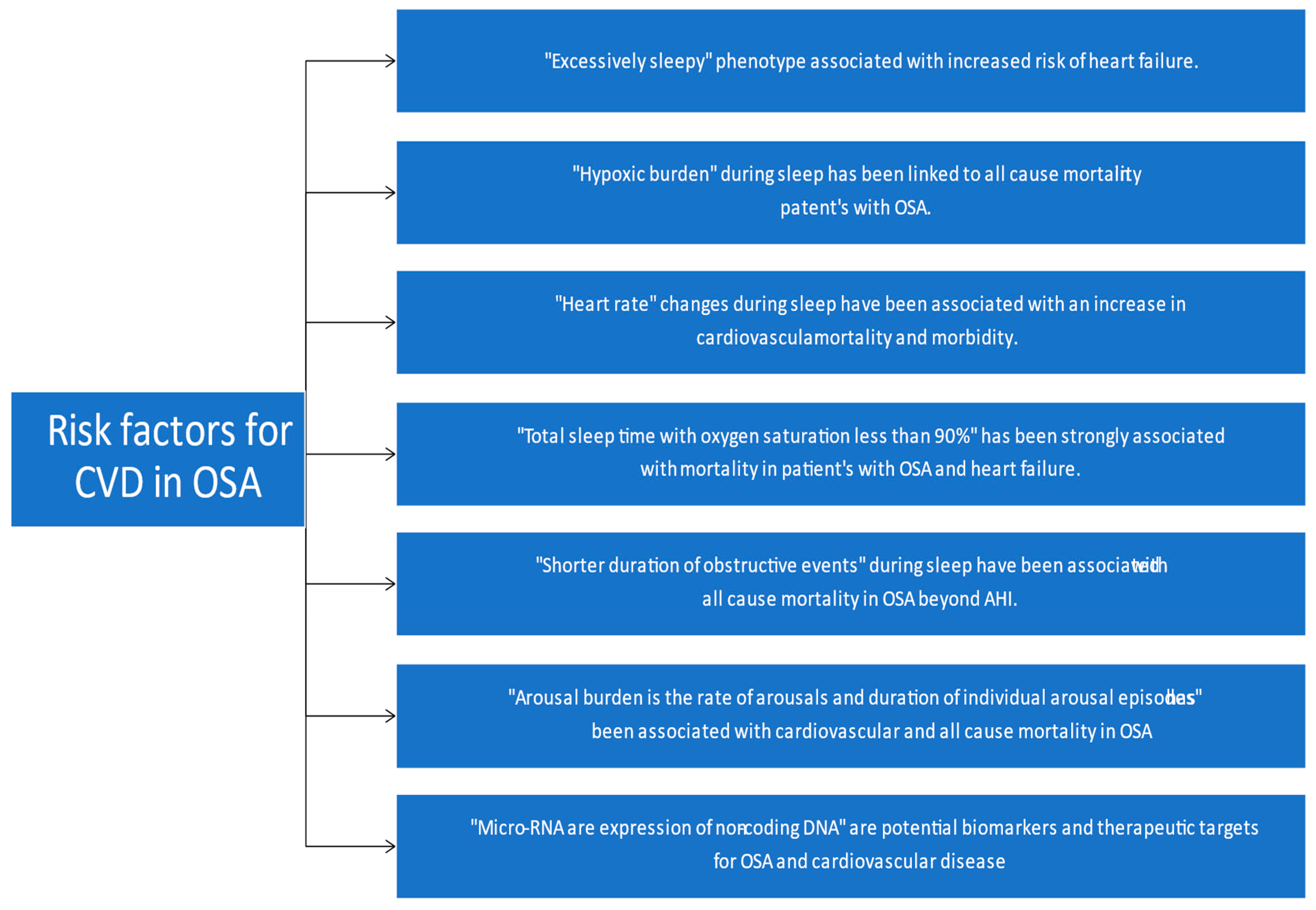
- Mazzotti, D.R.; Keenan, B.T.; Lim, D.C.; Gottlieb, D.J.; Kim, J.; Pack, A.I. Symptom Subtypes of Obstructive Sleep Apnea Predict Incidence of Cardiovascular Outcomes. Am. J. Respir. Crit. Care Med. 2019, 200, 493–506. [Google Scholar] [CrossRef]
- Corrigendum to: The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1157. [CrossRef]
- Sankari, A.; Ravelo, L.A.; Maresh, S.; Aljundi, N.; Alsabri, B.; Fawaz, S.; Hamdon, M.; Al-Kubaisi, G.; Hagen, E.; Badr, M.S.; et al. Longitudinal effect of nocturnal R-R intervals changes on cardiovascular outcome in a community-based cohort. BMJ Open. 2019, 9, e030559. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.P.; Emch, J.T.; Rueschman, M.; Sands, S.A.; Shea, S.A.; Wellman, A.; Redline, S. Apnea-Hypopnea Event Duration Predicts Mortality in Men and Women in the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2019, 199, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Shahrbabaki, S.S.; Linz, D.; Hartmann, S.; Redline, S.; Baumert, M. Sleep arousal burden is associated with long-term all-cause and cardiovascular mortality in 8001 community-dwelling older men and women. Eur. Heart J. 2021, 42, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Martos, F.; Benítez, I.; Ortega, F.; Zapater, A.; Giron, C.; Pinilla, L.; Pascual, L.; Cortijo, A.; Dalmases, M.; Fernandez-Real, J.M.; et al. Circulating microRNA profile as a potential biomarker for obstructive sleep apnea diagnosis. Sci. Rep. 2019, 9, 13456. [Google Scholar] [CrossRef] [Green Version]
- Seiler, A.; Camilo, M.; Korostovtseva, L.; Haynes, A.G.; Brill, A.K.; Horvath, T.; Egger, M.; Bassetti, C.L. Prevalence of sleep-disordered breathing after stroke and TIA: A meta-analysis. Neurology 2019, 92, e648–e654. [Google Scholar] [CrossRef]
- Brown, D.L.; Shafie-Khorassani, F.; Kim, S.; Chervin, R.D.; Case, E.; Morgenstern, L.B.; Yadollahi, A.; Tower, S.; Lisabeth, L.D. Sleep-Disordered Breathing Is Associated with Recurrent Ischemic Stroke. Stroke 2019, 50, 571–576. [Google Scholar] [CrossRef]
- Loke, Y.K.; Brown, J.W.; Kwok, C.S.; Niruban, A.; Myint, P.K. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef] [Green Version]
- Lyons, O.D.; Ryan, C.M. Sleep Apnea and Stroke. Can. J. Cardiol. 2015, 31, 918–927. [Google Scholar] [CrossRef]
- Brill, A.K.; Horvath, T.; Seiler, A.; Camilo, M.; Haynes, A.G.; Ott, S.R.; Egger, M.; Bassetti, C.L. CPAP as treatment of sleep apnea after stroke: A meta-analysis of randomized trials. Neurology 2018, 90, e1222–e1230. [Google Scholar] [CrossRef]
- Chaouat, A.; Weitzenblum, E.; Krieger, J.; Oswald, M.; Kessler, R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 1996, 109, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Shiomi, T.; Sasanabe, R.; Hasegawa, R.; Ootake, K.; Banno, K.; Wakayama, H.; Katada, M.; Kobayashi, T. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin. Neurosci. 2002, 56, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Jilwan, F.N.; Escourrou, P.; Garcia, G.; Jaïs, X.; Humbert, M.; Roisman, G. High Occurrence of Hypoxemic Sleep Respiratory Disorders in Precapillary Pulmonary Hypertension and Mechanisms. Chest 2013, 143, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kauppert, C.A.; Dvorak, I.; Kollert, F.; Heinemann, F.; Jörres, R.A.; Pfeifer, M.; Budweiser, S. Pulmonary hypertension in obesity-hypoventilation syndrome. Respir. Med. 2013, 107, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Kholdani, C.; Fares, W.H.; Mohsenin, V. Pulmonary hypertension in obstructive sleep apnea: Is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ. 2015, 5, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Mullin, C.J.; Ventetuolo, C.E. Critical Care Management of the Patient with Pulmonary Hypertension. Clin. Chest. Med. 2021, 42, 155–165. [Google Scholar] [CrossRef]
- Leuchte, H.H.; Baumgartner, R.A.; Nounou, M.E.; Vogeser, M.; Neurohr, C.; Trautnitz, M.; Behr, J. Brain natriuretic peptide is a prognostic parameter in chronic lung disease. Am. J. Respir. Crit. Care Med. 2006, 173, 744–750. [Google Scholar] [CrossRef]
- Minai, O.A.; Ricaurte, B.; Kaw, R.; Hammel, J.; Mansour, M.; McCarthy, K.; Golish, J.A.; Stoller, J.K. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am. J. Cardiol. 2009, 104, 1300–1306. [Google Scholar] [CrossRef]
- Nagaoka, M.; Goda, A.; Takeuchi, K.; Kikuchi, H.; Finger, M.; Inami, T.; Soejima, K.; Satoh, T. Nocturnal Hypoxemia, But Not Sleep Apnea, Is Associated with a Poor Prognosis in Patients with Pulmonary Arterial Hypertension. Circ. J. 2018, 82, 3076–3081. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Martínez, I.; Villamor, J. Pulmonary hypertension in obstructive sleep apnoea: Effects of continuous positive airway pressure: A randomized, controlled cross-over study. Eur. Heart J. 2006, 27, 1106–1113. [Google Scholar] [CrossRef] [Green Version]
- Sajkov, D.; Wang, T.; Saunders, N.A.; Bune, A.J.; McEvoy, R.D. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2002, 165, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Franchini, M. Sleep apnea and venous thromboembolism. A systematic review. Thromb. Haemost. 2015, 114, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, D.; Campobasso, A.; Suriano, C.; Lo Muzio, E.; Guida, L.; Salcuni, F.; Laurenziello, M.; Illuzzi, G.; Tepedino, M. A new design of mandibular advancement device (IMYS) in the treatment of obstructive sleep apnea. CRANIO® 2022, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Selection bias |
|
| Adherence to PAP therapy was sub optimal. | Patients recruited had established cardiovascular disease and did not present with OSA initially. Hence, adherence to PAP therapy was sub-optimal and invariably less than 4 h/night and hence may have impacted the results of the clinical trials. |
| Lack of adequate numbers and hence void of statistical power to show difference in outcome | The clinical trials were small in number and lacked statistical power. All the studies had this limitation. |
| “Composite end point” as outcome was a limitation | The studies chose “Composite end point” as the outcome which consisted of various competing events such as stroke, myocardial infarction, heart failure, angina, etc., which were not equal in terms of weightage. Small sample size, inadequate power and smaller number of individual events altogether impacted the final outcome. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahab, A.; Chowdhury, A.; Jain, N.K.; Surani, S.; Mushtaq, H.; Khedr, A.; Mir, M.; Jama, A.B.; Rauf, I.; Jain, S.; et al. Cardiovascular Complications of Obstructive Sleep Apnea in the Intensive Care Unit and Beyond. Medicina 2022, 58, 1390. https://doi.org/10.3390/medicina58101390
Wahab A, Chowdhury A, Jain NK, Surani S, Mushtaq H, Khedr A, Mir M, Jama AB, Rauf I, Jain S, et al. Cardiovascular Complications of Obstructive Sleep Apnea in the Intensive Care Unit and Beyond. Medicina. 2022; 58(10):1390. https://doi.org/10.3390/medicina58101390
Chicago/Turabian StyleWahab, Abdul, Arnab Chowdhury, Nitesh Kumar Jain, Salim Surani, Hisham Mushtaq, Anwar Khedr, Mikael Mir, Abbas Bashir Jama, Ibtisam Rauf, Shikha Jain, and et al. 2022. "Cardiovascular Complications of Obstructive Sleep Apnea in the Intensive Care Unit and Beyond" Medicina 58, no. 10: 1390. https://doi.org/10.3390/medicina58101390
APA StyleWahab, A., Chowdhury, A., Jain, N. K., Surani, S., Mushtaq, H., Khedr, A., Mir, M., Jama, A. B., Rauf, I., Jain, S., Korsapati, A. R., Chandramouli, M. S., Boike, S., Attallah, N., Hassan, E., Chand, M., Bawaadam, H. S., & Khan, S. A. (2022). Cardiovascular Complications of Obstructive Sleep Apnea in the Intensive Care Unit and Beyond. Medicina, 58(10), 1390. https://doi.org/10.3390/medicina58101390







