Localization Patterns of RAB3C Are Associated with Murine and Human Sperm Formation
Abstract
:1. Introduction
1.1. Male Infertility and Spermatogenesis
1.2. Septins
1.3. Biological Roles of the RAB3 Family
2. Materials and Methods
2.1. Sequencing Alignment and Data Mining of RAB3 Expression in Human Testis
2.2. Human Sperm Collection
2.3. Immunofluorescence Assay
2.4. Collection of Mature Sperm and Isolation of Testicular Germ Cells
2.5. Statistical Analysis
3. Results
3.1. RAB3C Is the Major Expressed Gene within the RAB3 Family during Human Spermatogenesis
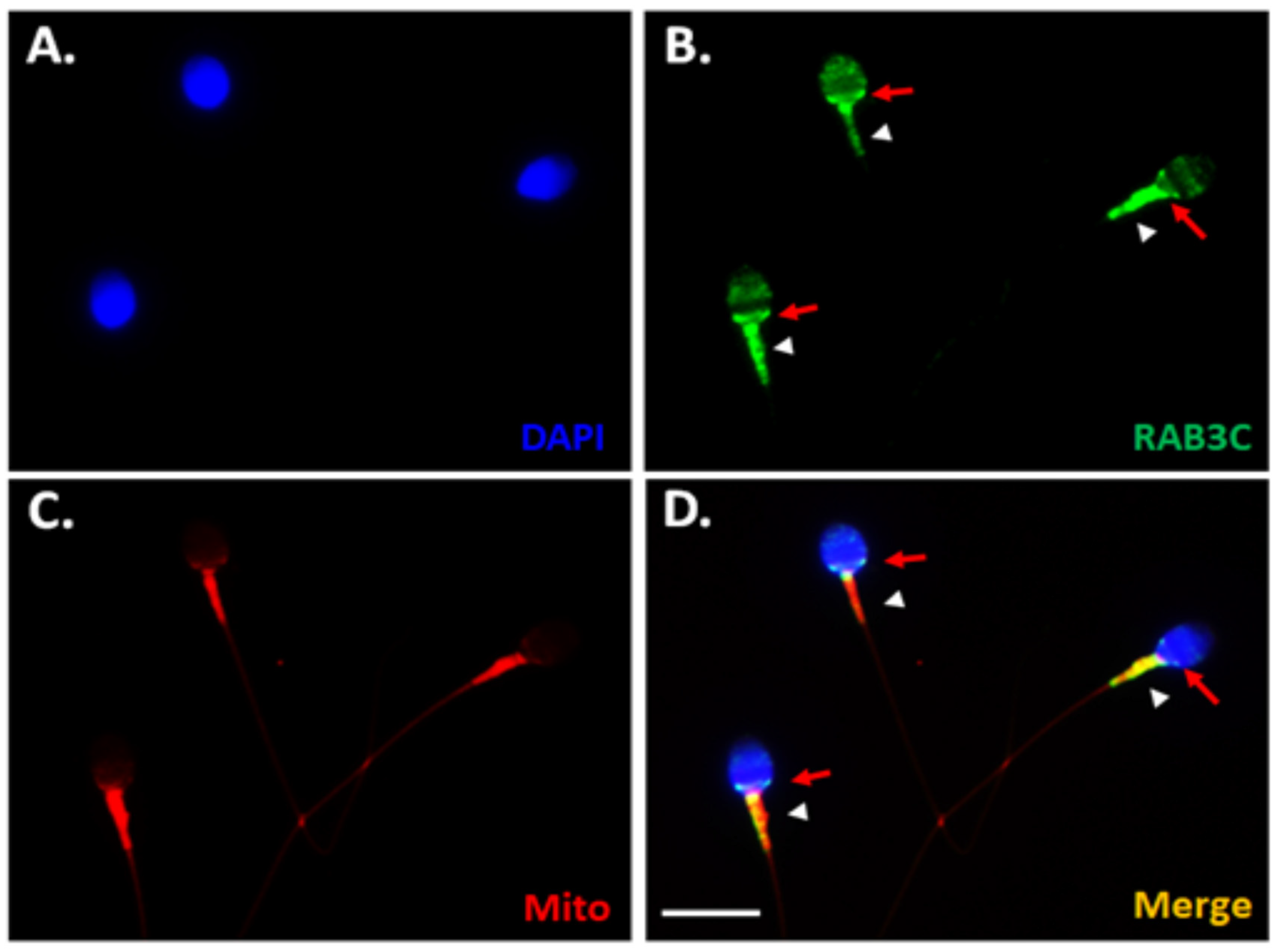
3.2. RAB3C Protein Expression in the Mature Human Sperm
3.3. Delocalized/Decreased RAB3C Signals in Sperm Harboring Mutated SEPT14
3.4. Dynamic Localization of RAB3C during Murine Spermiogenesis
4. Discussion
4.1. RAB3 Family Expression on Human Spermatogenesis
4.2. Functional Roles of the RAB3 Family in Male Germ Cells
4.3. Clinical Views of RAB3C Protein and SEPT14 Mutated Caused Teratozoospermia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Bieniek, J.M.; Lo, K.C. Recent advances in understanding & managing male infertility. F1000Research 2016, 5, 2756. [Google Scholar]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Lamb, D.J. Genetic dissection of mammalian fertility pathways. Nat. Med. 2002, 8, S40. [Google Scholar] [CrossRef]
- Gunes, S.; Esteves, S.C. Role of genetics and epigenetics in male infertility. Andrologia 2021, 53, e13586. [Google Scholar] [CrossRef]
- Kushnir, V.A.; Barad, D.H.; Albertini, D.F.; Darmon, S.K.; Gleicher, N. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod. Biol. Endocrinol. 2017, 15, 6. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.S.; Doody, K.J.; Schattman, G.L.; Adashi, E.Y. Public reporting of assisted reproductive technology outcomes: Past, present, and future. Am. J. Obstet. Gynecol. 2015, 212, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Wdowiak, A.; Bakalczuk, S.; Bakalczuk, G. The effect of sperm DNA fragmentation on the dynamics of the embryonic development in intracytoplasmatic sperm injection. Reprod. Biol. 2015, 15, 94–100. [Google Scholar] [CrossRef]
- Zini, A.; Boman, J.M.; Belzile, E.; Ciampi, A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum. Reprod. 2008, 23, 2663–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, G.D.; Neri, Q.V.; Cozzubbo, T.; Rosenwaks, Z. Perspectives on the assessment of human sperm chromatin integrity. Fertil. Steril. 2014, 102, 1508–1517. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005.e8. [Google Scholar] [CrossRef]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef]
- Kinoshita, M.; Noda, M. Roles of septins in the mammalian cytokinesis machinery. Cell Struct. Funct. 2001, 26, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, M.; Field, C.M.; Coughlin, M.L.; Straight, A.F.; Mitchison, T.J. Self- and actin-templated assembly of Mammalian septins. Dev. Cell 2002, 3, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Bowen, J.R.; Knox, T.K.; Zhou, K.; Pendziwiat, M.; Kuhlenbäumer, G.; Sindelar, C.V.; Spiliotis, E.T. Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J. Cell Biol. 2013, 203, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Dolat, L.; Angelis, D.; Forgacs, E.; Spiliotis, E.T.; Galkin, V.E. Septin 9 Exhibits Polymorphic Binding to F-Actin and Inhibits Myosin and Cofilin Activity. J. Mol. Biol. 2015, 427, 3273–3284. [Google Scholar] [CrossRef] [Green Version]
- Hall, P.A.; Jung, K.; Hillan, K.J.; Russell, S.E. Expression profiling the human septin gene family. J. Pathol. 2005, 206, 269–278. [Google Scholar] [CrossRef]
- Peterson, E.A.; Petty, E.M. Conquering the complex world of human septins: Implications for health and disease. Clin. Genet. 2010, 77, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Kuo, Y.C.; Chiang, H.S.; Kuo, P.L. The role of the septin family in spermiogenesis. Spermatogenesis 2011, 1, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Ihara, M.; Kinoshita, A.; Yamada, S.; Tanaka, H.; Tanigaki, A.; Kitano, A.; Goto, M.; Okubo, K.; Nishiyama, H.; Ogawa, O.; et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell 2005, 8, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Kissel, H.; Georgescu, M.-M.; Larisch, S.; Manova, K.; Hunnicutt, G.R.; Steller, H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev. Cell 2005, 8, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lhuillier, P.; Rode, B.; Escalier, D.; Lorès, P.; Dirami, T.; Bienvenu, T.; Gacon, G.; Dulioust, E.; Touré, A. Absence of annulus in human asthenozoospermia: Case report. Hum. Reprod. 2009, 24, 1296–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugino, Y.; Ichioka, K.; Soda, T.; Ihara, M.; Kinoshita, M.; Ogawa, O.; Nishiyama, H. Septins as diagnostic markers for a subset of human asthenozoospermia. J. Urol. 2008, 180, 2706–2709. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, Y.M.; Wang, Y.Y.; Yu, I.S.; Lin, Y.W.; Wang, Y.H.; Wu, C.M.; Pan, H.A.; Chao, S.C.; Yen, P.H.; et al. The expression level of septin12 is critical for spermiogenesis. Am. J. Pathol. 2009, 174, 1857–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-H.; Wang, Y.-Y.; Chen, H.-I.; Kuo, Y.-C.; Chiou, Y.-W.; Lin, H.-H.; Wu, C.-M.; Hsu, C.-C.; Chiang, H.-S.; Kuo, P.-L. SEPTIN12 Genetic Variants Confer Susceptibility to Teratozoospermia. PLoS ONE 2012, 7, e34011. [Google Scholar]
- Kuo, Y.-C.; Lin, Y.-H.; Chen, H.-I.; Wang, Y.-Y.; Chiou, Y.-W.; Lin, H.-H.; Pan, H.-A.; Wu, C.-M.; Su, S.-M.; Hsu, C.-C.; et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012, 33, 710–719. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chou, C.-K.; Hung, Y.-C.; Yu, I.-S.; Pan, H.-A.; Lin, S.-W.; Kuo, P.-L. SEPT12 deficiency causes sperm nucleus damage and developmental arrest of preimplantation embryos. Fertil. Steril. 2011, 95, 363–365. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lai, T.-H.; Chen, M.-F.; Lee, H.-L.; Kuo, P.-L.; Lin, Y.-H. SEPT14 Mutations and Teratozoospermia: Genetic Effects on Sperm Head Morphology and DNA Integrity. J. Clin. Med. 2019, 8, 1297. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Huang, C.-Y.; Ke, C.-C.; Wang, Y.-Y.; Lai, T.-H.; Liu, H.-C.; Ku, W.-C.; Chan, C.-C.; Lin, Y.-H. ACTN4 Mediates SEPT14 Mutation-Induced Sperm Head Defects. Biomedicines 2020, 8, 518. [Google Scholar] [CrossRef]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi-Komi, R.; Levay, M.; Ligeti, E. Small G proteins and their regulators in cellular signalling. Mol. Cell Endocrinol. 2012, 353, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, N.A.; Progida, C.; Rab, G.T. Pases: Switching to Human Diseases. Cells 2019, 8, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleuger, C.; Lehti, M.S.; Dunleavy, J.E.; Fietz, D.; O’Bryan, M.K. Haploid male germ cells-the Grand Central Station of protein transport. Hum. Reprod. Updat. 2020, 26, 474–500. [Google Scholar] [CrossRef]
- Fischer von Mollard, G.; Sudhof, T.C.; Jahn, R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature 1991, 349, 79–81. [Google Scholar] [CrossRef]
- Geppert, M.; Goda, Y.; Stevens, C.F.; Sudhof, T.C. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 1997, 387, 810–814. [Google Scholar] [CrossRef]
- Geppert, M.; Bolshakov, V.Y.; Siegelbaum, S.A.; Takei, K.; De Camilli, P.; Hammer, R.E.; Südhof, T.C. The role of Rab3A in neurotransmitter release. Nature 1994, 369, 493–497. [Google Scholar] [CrossRef]
- Wang, Y.; Okamoto, M.; Schmitz, F.; Hofmann, K.; Sudhof, T.C. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature 1997, 388, 593–598. [Google Scholar] [CrossRef]
- Schluter, O.M.; Khvotchev, M.; Jahn, R.; Sudhof, T.C. Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J. Biol. Chem. 2002, 277, 40919–40929. [Google Scholar] [CrossRef] [Green Version]
- Riedel, D.; Antonin, W.; Fernandez-Chacon, R.; de Toledo, G.A.; Naranjo, G.A.D.T.; Geppert, M.; Valentijn, J.A.; Valentijn, K.; Jamieson, J.D.; Südhof, T.C.; et al. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol. Cell Biol. 2002, 22, 6487–6497. [Google Scholar] [CrossRef] [Green Version]
- Yunes, R.; Michaut, M.; Tomes, C.; Mayorga, L.S. Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol. Reprod. 2000, 62, 1084–1089. [Google Scholar] [CrossRef]
- Iida, H.; Yoshinaga, Y.; Tanaka, S.; Toshimori, K.; Mori, T. Identification of Rab3A GTPase as an acrosome-associated small GTP-binding protein in rat sperm. Dev. Biol. 1999, 211, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Ke, C.-C.; Chen, Y.-L.; Lin, Y.-H.; Yu, I.-S.; Ku, W.-C.; O’Bryan, M.K.; Lin, Y.-H. Deficiency of the Tbc1d21 gene causes male infertility with morphological abnormalities of the sperm mitochondria and flagellum in mice. PLoS Genet. 2020, 16, e1009020. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Ke, C.-C.; Wang, Y.-Y.; Chen, M.-F.; Chen, T.-M.; Ku, W.-C.; Chiang, H.-S.; Yeh, C.-H. RAB10 Interacts with the Male Germ Cell-Specific GTPase-Activating Protein during Mammalian Spermiogenesis. Int. J. Mol. Sci. 2017, 18, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef] [Green Version]
- Kwon, W.S.; Rahman, M.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Increased male fertility using fertility-related biomarkers. Sci. Rep. 2015, 5, 15654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, A.S.; Mruk, D.D. Rab8B GTPase and junction dynamics in the testis. Endocrinology 2003, 144, 1549–1563. [Google Scholar] [CrossRef]
- Mruk, D.D.; Lau, A.S. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 2009, 80, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Schluter, O.M.; Schmitz, F.; Jahn, R.; Rosenmund, C.; Sudhof, T.C. A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 2004, 24, 6629–6637. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Du, Y.-R.; Qin, W.-H.; Hu, Y.-G.; Huang, Y.-N.; Bao, L.; Han, D.; Mansouri, A.; Xu, G.-L. RIM-BP3 is a manchette-associated protein essential for spermiogenesis. Development 2009, 136, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Salas-Huetos, A.; Oud, M.S.; Aston, K.I.; Veltman, J.A. Disease gene discovery in male infertility: Past, present and future. Hum. Genet. 2021, 140, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell Mol. Life Sci. 2020, 77, 2029–2048. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Wang, Y.-Y.; Chen, Y.-L.; Chen, M.-F.; Chiang, H.-S.; Kuo, P.-L.; Lin, Y.-H. CDC42 Negatively Regulates Testis-Specific SEPT12 Polymerization. Int. J. Mol. Sci. 2018, 19, 2627. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-L.; Lai, T.-H.; Liu, H.-C.; Wang, Y.-Y.; Lin, Y.-H.; Ke, C.-C.; Chung, M.-T.; Chan, C.-C.; Lin, Y.-H. Localization Patterns of RAB3C Are Associated with Murine and Human Sperm Formation. Medicina 2022, 58, 1408. https://doi.org/10.3390/medicina58101408
Tsai Y-L, Lai T-H, Liu H-C, Wang Y-Y, Lin Y-H, Ke C-C, Chung M-T, Chan C-C, Lin Y-H. Localization Patterns of RAB3C Are Associated with Murine and Human Sperm Formation. Medicina. 2022; 58(10):1408. https://doi.org/10.3390/medicina58101408
Chicago/Turabian StyleTsai, Yieh-Loong, Tsung-Hsuan Lai, Hsuan-Che Liu, Ya-Yun Wang, Yu-Hua Lin, Chih-Chun Ke, Ming-Ting Chung, Chying-Chyuan Chan, and Ying-Hung Lin. 2022. "Localization Patterns of RAB3C Are Associated with Murine and Human Sperm Formation" Medicina 58, no. 10: 1408. https://doi.org/10.3390/medicina58101408
APA StyleTsai, Y.-L., Lai, T.-H., Liu, H.-C., Wang, Y.-Y., Lin, Y.-H., Ke, C.-C., Chung, M.-T., Chan, C.-C., & Lin, Y.-H. (2022). Localization Patterns of RAB3C Are Associated with Murine and Human Sperm Formation. Medicina, 58(10), 1408. https://doi.org/10.3390/medicina58101408






