Mass Spectrometry-Based Metabolomic and Lipidomic Analysis of the Effect of High Fat/High Sugar Diet and GreenshellTM Mussel Feeding on Plasma of Ovariectomized Rats
Abstract
:1. Introduction
2. Results
Metabolomic Profile Change in Rats Fed with HFHS Diet and GSM
3. Discussion
4. Methods
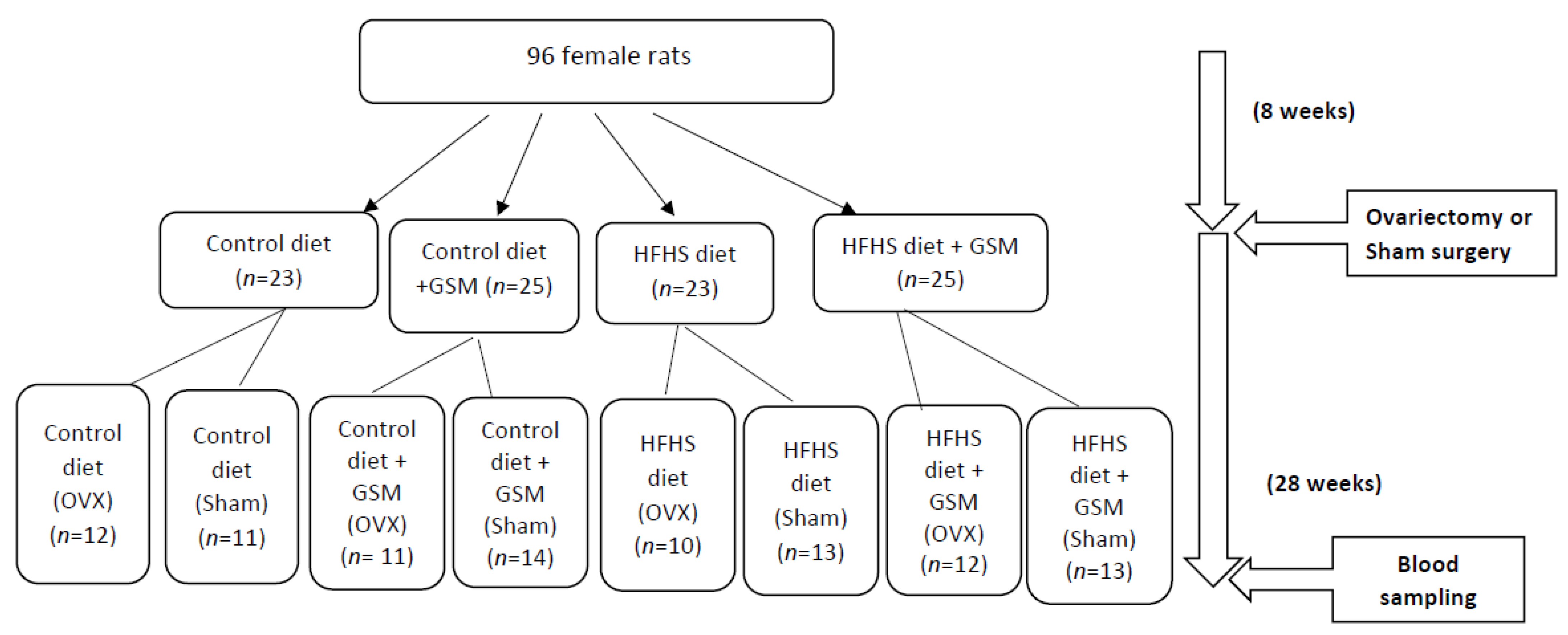
4.1. Experimental Methods
4.2. Metabolomic Analysis
4.2.1. Chemicals and Reagents
4.2.2. Sample Preparation
4.2.3. Instruments and Conditions
4.2.4. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Berenbaum, F.; Sellam, J. The phenotypic approach to osteoarthritis: A look at metabolic syndrome-associated osteoarthritis. Joint. Bone Spine 2019, 86, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Zhang, Y.; Parousis, A.; Sharma, A.; Rossomacha, E.; Endisha, H.; Wu, B.; Kacprzak, I.; Mahomed, N.N.; Gandhi, R. High-fat diet-induced acceleration of osteoarthritis is associated with a distinct and sustained plasma metabolite signature. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Herzog, W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J. Orthop. Res. 2016, 34, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.-L.; Blanchet, T.; Peluso, D.; Hopkins, B.; Morris, E.; Glasson, S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr. Cartil 2007, 15, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.S.; Wolber, F.M. Effects of Greenshell Mussel (Perna canaliculus) Intake on Pathological Markers of Multiple Phenotypes of Osteoarthritis in Rats. Appl. Sci. 2020, 10, 6131. [Google Scholar] [CrossRef]
- Gertsman, I.; Barshop, B.A. Promises and pitfalls of untargeted metabolomics. J. Inherit. Metab. Dis. 2018, 41, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Kimmerling, K.A.; Little, D.; Guilak, F. Serum and synovial fluid lipidomic profiles predict obesity-associated osteoarthritis, synovitis, and wound repair. Sci. Rep. 2017, 7, 44315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treschow, A.; Hodges, L.; Wright, P.; Wynne, P.; Kalafatis, N.; Macrides, T. Novel anti-inflammatory ω-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. CBPB 2007, 147, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Coulson, S.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel (Perna canaliculus) extract efficacy in knee osteoarthritis and improvement in gastrointestinal dysfunction: A pilot study. Inflammopharmacology 2012, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.S.; Wolber, F.M. The Preventive Effects of Greenshell Mussel (Perna canaliculus) on Early-Stage Metabolic Osteoarthritis in Rats with Diet-Induced Obesity. Nutrients 2019, 11, 1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, H.B.; Gangadaran, S.; Cheema, S.K. A high fat-high sucrose diet enriched in blue mussels protects against systemic inflammation, metabolic dysregulation and weight gain in C57BL/6 mice. Food Res. Int. 2017, 100, 78–85. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Gao, Z.-J.; Chen, Z.; Abe, T.; Hori, S.; Fukiya, S.; Ishizuka, S.; Yokota, A.; Chiba, H.; Hui, S.-P. Untargeted lipidomic analysis of plasma from high fat diet-induced obese rats using UHPLC-linear trap quadrupole-Orbitrap MS. Anal. Sci. 2020, 19P442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Pearce, L.; Bettjeman, B.I. Detailed distribution of lipids in Greenshell™ Mussel (Perna canaliculus). Nutrients 2014, 6, 1454–1474. [Google Scholar] [CrossRef] [Green Version]
- Pousinis, P.; Gowler, P.R.; Burston, J.J.; Ortori, C.A.; Chapman, V.; Barrett, D.A. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.W.; Heden, T.D.; Morris, E.M.; Fritsche, K.L.; Vieira-Potter, V.J.; Thyfault, J.P. High-fat diet alters serum fatty acid profiles in obesity prone rats: Implications for invitro studies. Lipids 2015, 50, 997–1008. [Google Scholar] [CrossRef] [Green Version]
- Zhukova, N.V.; Novgorodtseva, T.P.; Denisenko, Y.K. Effect of the prolonged high-fat diet on the fatty acid metabolism in rat blood and liver. Lipids Health Dis. 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pati, S.; Krishna, S.; Lee, J.H.; Ross, M.K.; Claire, B.; Ham, A.H., Jr.; Wagner, J.J.; Filipov, N.M.; Cummings, B.S. Effects of high-fat diet and age on the blood lipidome and circulating endocannabinoids of female C57BL/6 mice. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 26–39. [Google Scholar] [CrossRef]
- Choi, S.; Snider, A.J. Sphingolipids in high fat diet and obesity-related diseases. Mediat. Inflamm. 2015, 2015, 520618. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Kowalski, G.; Leslie, S.J.; Risis, S.; Yang, C.; Lee-Young, R.S.; Babb, J.R.; Meikle, P.J.; Lancaster, G.I.; Henstridge, D.C. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013, 56, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Li, Z.; Hailemariam, T.K.; Chakraborty, M.; Jiang, K.; Qiu, D.; Bui, H.H.; Peake, D.A.; Kuo, M.-S.; et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Sokolowska, E.; Blachnio-Zabielska, A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatini, M.; Rolland, G.; Léonce, S.; Thomas, M.; Lesur, C.; Pérez, V.; de Nanteuil, G.; Bonnet, J. Effects of ceramide on apoptosis, proteoglycan degradation, and matrix metalloproteinase expression in rabbit articular cartilage. Biochem. Biophys. Res. Commun. 2000, 267, 438–444. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. Sphingolipids in human synovial fluid-a lipidomic study. PLoS ONE 2014, 9, e91769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.-S.; Chen, W.-C.; Kuo, T.-C.; Ho, C.-T.; Kuo, C.-H.; Tseng, Y.J.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Sheen, L.-Y. Mass-spectrometry-based serum metabolomics of a C57BL/6J mouse model of high-fat-diet-induced non-alcoholic fatty liver disease development. J. Agric. Food Chem. J Agr. Food Chem. 2015, 63, 7873–7884. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, B.; Qu, H.; Zhang, L.; Lu, Y.; Xu, Y.; Lyu, Z.; Zheng, H. A High Level of Circulating Valine Is a Biomarker for Type 2 Diabetes and Associated with the Hypoglycemic Effect of Sitagliptin. Mediat. Inflamm. 2019, 2019, 8247019. [Google Scholar] [CrossRef]
- Saha, A.K.; Xu, X.J.; Lawson, E.; Deoliveira, R.; Brandon, A.E.; Kraegen, E.W.; Ruderman, N.B. Downregulation of AMPK accompanies leucine-and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 2010, 59, 2426–2434. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.-T.; Park, J.H.; Yang, H.J.; Kim, M.-S.; Kwon, D.Y. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2011, 10, 722–731. [Google Scholar] [CrossRef]
- Siriarchavatana, P. The Preventive Effect of Greenshell Mussel Meat Against Osteoarthritis In Vivo. Doctoral Thesis, Massey University, Palmerston North, New Zealand, 2019; p. 145. [Google Scholar]
- Lai, Q.; Yuan, G.; Wang, H.; Liu, Z.; Kou, J.; Yu, B.; Li, F. Metabolomic profiling of metoprolol-induced cardioprotection in a murine model of acute myocardial ischemia. Biomed. Pharm. 2020, 124, 109820. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Naito, H.; Yetti, H.; Tamada, H.; Kitamori, K.; Hayashi, Y.; Wang, D.; Yanagiba, Y.; Wang, J.; Ikeda, K. Dysregulated bile acid synthesis, metabolism and excretion in a high fat-cholesterol diet-induced fibrotic steatohepatitis in rats. Dig. Dis. Sci. 2013, 58, 2212–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Zhang, H.; Dong, X.; Chen, X.-F.; Zhu, Z.-Y.; Hong, Z.-Y.; Chai, Y.-F. UPLC-Q-TOF/MS based metabolomic profiling of serum and urine of hyperlipidemic rats induced by high fat diet. J. Pharm. Anal. 2014, 4, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.C.; Kim, W.C.; Ryu, R.; Wu, Z.; Seo, J.S.; Choi, M.S.; Liu, K.H. Plasma lipidomics reveals insights into anti-obesity effect of Chrysanthemum morifolium Ramat leaves and its constituent luteolin in high-fat diet-induced dyslipidemic mice. Nutrients 2020, 12, 2973. [Google Scholar] [CrossRef]
- Fraser, K.; Roy, N.C.; Goumidi, L.; Verdu, A.; Suchon, P.; Leal-Valentim, F.; Trégouët, D.-A.; Morange, P.-E.; Martin, J.-C. Plasma Biomarkers and Identification of Resilient Metabolic Disruptions in Patients with Venous Thromboembolism Using a Metabolic Systems Approach. ATVB 2020, 40, 2527–2538. [Google Scholar] [CrossRef]
- Huynh, K.; Barlow, C.K.; Jayawardana, K.S.; Weir, J.M.; Mellett, N.A.; Cinel, M.; Magliano, D.J.; Shaw, J.E.; Drew, B.G.; Meikle, P.J. High-throughput plasma lipidomics: Detailed mapping of the associations with cardiometabolic risk factors. Cell Chem. Biol. 2019, 26, 71–84. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Jing, L.; Guigonis, J.M.; Borchiellini, D.; Durand, M.; Pourcher, T.; Ambrosetti, D. LC-MS based metabolomic profiling for renal cell carcinoma histologic subtypes. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. A J. Chemom. Soc. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.; Rudge, S.A.; Zhang, Q.; Wakelam, M.J. Using lipidomics analysis to determine signalling and metabolic changes in cells. Curr. Opin. Biotechnol. 2017, 43, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Gaud, C.; Sousa, B.C.; Nguyen, A.; Fedorova, M.; Ni, Z.; O’Donnell, V.B.; Wakelam, M.J.; Andrews, S.; Lopez-Clavijo, A.F. BioPAN: A web-based tool to explore mammalian lipidome metabolic pathways on LIPID MAPS. F1000Research 2021, 10. [Google Scholar] [CrossRef]
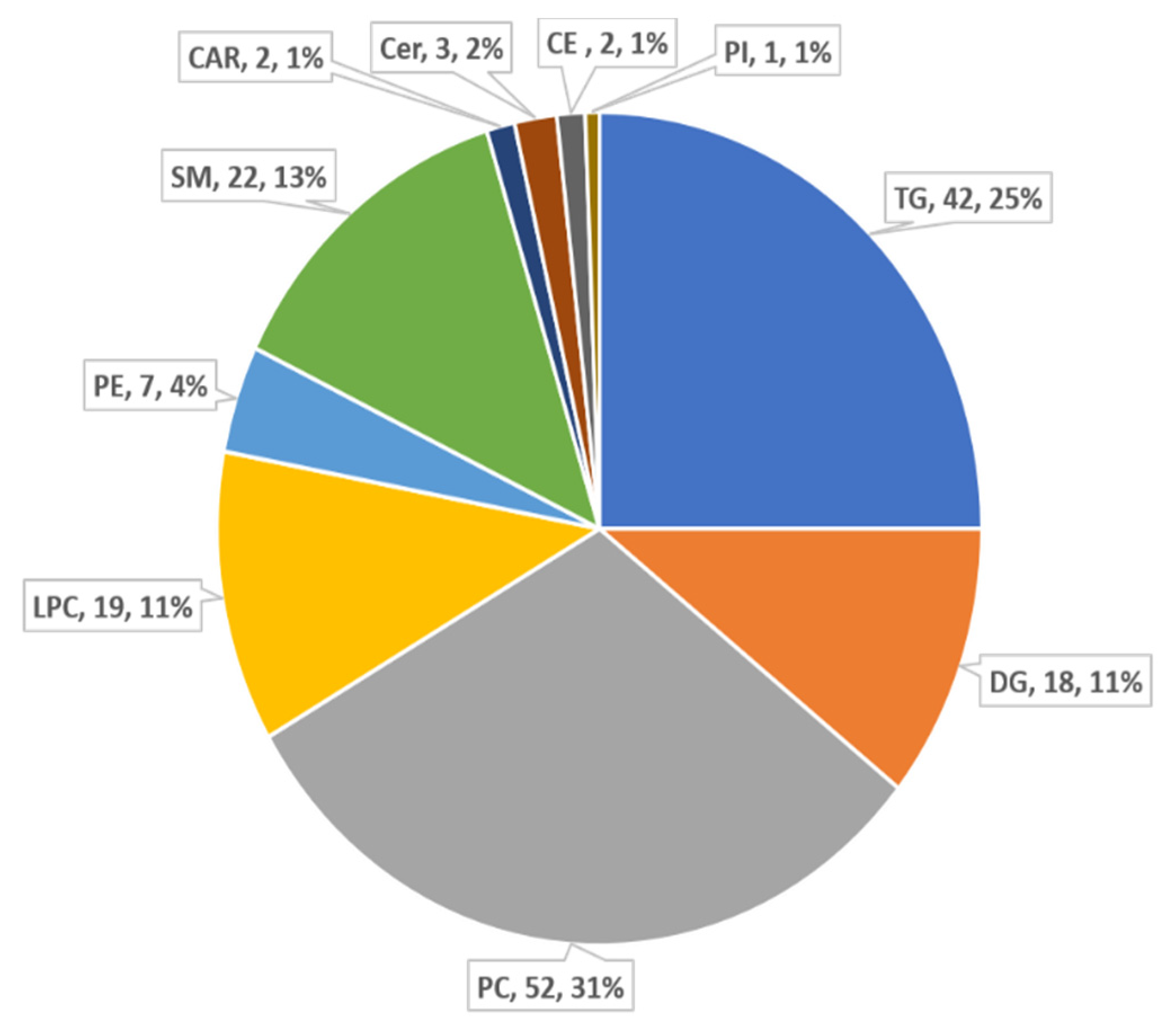




| Lipids | Average Rt (Min) | Adduct Type | Fold Change | ||
|---|---|---|---|---|---|
| HFHS/ Control | HFHS + GSM/HFHS | Control + GSM/ Control | |||
| DG 38:5 | 8.227 | [M+Na]+ | 0.54 * | 0.76 | 0.49 * |
| DG 38:6 | 7.444 | [M+Na]+ | 0.76 | 0.83 | 0.61 * |
| DG 47:10 | 6.276 | [M+Na]+ | 0.74 * | 1.09 | 1.2 * |
| DG 47:9 | 6.188 | [M+Na]+ | 0.56 * | 0.78 | 0.66 * |
| DG 49:10 | 7.184 | [M+Na]+ | 0.89 | 1.12 | 1.34 * |
| DG 49:12 | 6.177 | [M+Na]+ | 0.69 * | 0.88 | 0.76 * |
| LPC 16:1 | 2.228 | [M+H]+ | 0.33 * | 0.96 | 1.19 * |
| LPC 20:5 | 1.994 | [M+H]+ | 0.28 * | 2.6 * | 4.38 * |
| PC 32:1 | 6.065 | [M+H]+ | 0.19 * | 0.79 | 0.87 |
| PC 32:2 | 5.506 | [M+H]+ | 0.46 * | 1.00 | 1.14 |
| PC 34:3 | 5.647 | [M+H]+ | 0.42 * | 1.01 | 1.34 * |
| PC 34:4 | 5.442 | [M+H]+ | 0.37 * | 0.79 | 0.69 * |
| PC 36:5 | 6.268 | [M+H]+ | 0.73 * | 1.1 | 1.19 |
| PC 36:6 | 5.639 | [M+H]+ | 0.31 * | 1.24 | 1.32 * |
| PC 37:4 | 6.613 | [M+H]+ | 0.65 | 0.74 | 0.6 * |
| PC 38:5 | 6.264 | [M+H]+ | 0.41 * | 0.81 | 0.69 |
| PC 38:7 | 6.181 | [M+H]+ | 0.7 * | 0.87 | 0.76 * |
| PC 38:8 | 5.685 | [M+H]+ | 0.24 * | 1.29 | 2.26 * |
| PC 40:4 | 7.786 | [M+H]+ | 0.92 | 0.54 * | 0.32 * |
| PC O-36:4 | 6.702 | [M+H]+ | 0.66 * | 0.8 | 0.59 * |
| PC O-36:5 | 6.567 | [M+H]+ | 0.69 | 0.83 | 0.59 * |
| PC O-39:10 | 6.912 | [M+H]+ | 0.24 * | 0.76 | 0.87 |
| PC O-39:4 | 7.076 | [M+H]+ | 0.97 | 0.81 | 0.62 * |
| TG 48:1 | 10.944 | [M+NH4]+ | 0.2 * | 0.97 | 0.84 |
| TG 50:3 | 10.844 | [M+NH4]+ | 0.25 * | 1.08 | 1.03 |
| TG 54:7 | 10.732 | [M+NH4]+ | 0.62 * | 1.4 * | 1.88 * |
| TG 54:8 | 10.638 | [M+NH4]+ | 0.66 * | 1.36 | 2.03 * |
| TG 56:10 | 10.589 | [M+NH4]+ | 1.07 | 1.36 | 2.22 * |
| TG 60:12 | 10.677 | [M+NH4]+ | 1.46 * | 1.16 | 1.94 * |
| Fold Change | |||||
|---|---|---|---|---|---|
| Lipids | Average Rt (min) | Adduct Type | HFHS/ Control | HFHS + GSM/HFHS | Control + GSM/ Control |
| Cer 41:1 | 10.315 | [M+H-H2O]+ | 0.54 * | 0.63 | 0.69 * |
| DG 38:5 | 8.227 | [M+Na]+ | 0.62 * | 0.69 * | 0.54 * |
| DG 38:6 | 7.444 | [M+Na]+ | 0.91 | 0.74 * | 0.61 * |
| DG 47:9 | 6.188 | [M+Na]+ | 0.47 * | 0.77 | 0.71 * |
| DG 49:10 | 7.184 | [M+Na]+ | 0.91 | 1.02 | 1.2 * |
| DG 49:12 | 6.177 | [M+Na]+ | 0.65 * | 0.8 | 0.78 * |
| LPC 20:5 | 1.994 | [M+H]+ | 0.41 * | 1.58 * | 3.14 * |
| PC 32:1 | 6.065 | [M+H]+ | 0.21 * | 0.97 | 0.89 |
| PC 32:2 | 5.506 | [M+H]+ | 0.4 * | 1.18 | 1.05 |
| PC 33:2 | 5.87 | [M+H]+ | 0.47 * | 0.88 | 1.27 |
| PC 34:3 | 5.647 | [M+H]+ | 0.44 * | 1.01 | 1.19 |
| PC 34:4 | 5.442 | [M+H]+ | 0.27 * | 0.74 | 0.68 * |
| PC 35:2 | 6.695 | [M+H]+ | 0.68 * | 1.03 | 1.34 * |
| PC 36:4 | 6.186 | [M+H]+ | 0.57 * | 0.79 | 0.78 * |
| PC 36:6 | 5.639 | [M+H]+ | 0.28 * | 1.06 | 1.08 |
| PC 37:4 | 6.613 | [M+H]+ | 0.49 * | 0.83 | 0.8 |
| PC 38:5 | 6.264 | [M+H]+ | 0.38 * | 0.86 | 0.77 |
| PC 38:7 | 6.181 | [M+H]+ | 0.65 * | 0.84 | 0.78 * |
| PC 38:8 | 5.685 | [M+H]+ | 0.26 * | 1.43 | 2.16 * |
| PC 40:4 | 7.786 | [M+H]+ | 0.71 | 0.63 * | 0.32 * |
| PC O-36:4 | 6.702 | [M+H]+ | 0.5 * | 0.82 | 0.62 * |
| PC O-36:5 | 6.567 | [M+H]+ | 0.49 * | 0.84 | 0.63 * |
| PC O-39:4 | 7.076 | [M+H]+ | 0.75 * | 0.82 | 0.7 * |
| PC O-40:8 | 6.79 | [M+H]+ | 0.71 * | 0.77 * | 0.63 * |
| TG 49:2 | 10.894 | [M+NH4]+ | 0.52 * | 1.4 | 1.35 * |
| TG 51:3 | 10.905 | [M+NH4]+ | 0.96 | 1.05 | 1.47 * |
| TG 54:7 | 10.732 | [M+NH4]+ | 1.1 | 1.11 | 2 * |
| TG 54:8 | 10.638 | [M+NH4]+ | 1.12 | 1.04 | 1.8 * |
| TG 56:10 | 10.589 | [M+NH4]+ | 1.09 | 1.3 | 1.87 * |
| Metabolites | Average Rt(Min) | Adduct Type | Fold Change | ||
|---|---|---|---|---|---|
| HFHS/ Control | HFHS + GSM/ HFHS | Control + GSM/ Control | |||
| Cholic acid | 2.572 | [M+H]+ | 1.42 * | 1.02 | 0.59 |
| Serine | 11.141 | [M+H]+ | 1.28 | 1.06 | 0.65 * |
| Threonine | 10.263 | [M+H]+ | 0.94 | 1.21 | 0.58 * |
| Lysine | 9.533 | [M+H]+ | 1.37 * | 0.95 | 0.90 * |
| Valine | 6.481 | [M+H]+ | 1.19 | 0.82 * | 0.71 * |
| Pipecolic acid | 9.534 | [M+H]+ | 1.69 * | 0.91 | 0.33 * |
| Histidine | 8.167 | [M-H]− | 0.97 | 0.87 * | 0.93 |
| 3-Methylcytidine | 6.516 | [M+H]+ | 0.83 | 0.99 | 1.45 * |
| Metabolites | Average Rt (Min) | Adduct Type | Fold Change | ||
|---|---|---|---|---|---|
| HFHS/ Control | HFHS + GSM/ HFHS | Control + GSM/ Control | |||
| 3- hydroxybutyric acid | 6.863 | [M-H]− | 0.55 * | 0.98 | 1.16 |
| Valine | 6.481 | [M+H]+ | 1.03 | 0.80 | 0.63 * |
| Cholic acid | 2.572 | [M+H]+ | 1.38 * | 0.42 | 1.23 |
| Tryptophan | 7.968 | [M+H]+ | 0.80 * | 1.06 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abshirini, M.; Cabrera, D.; Fraser, K.; Siriarchavatana, P.; Wolber, F.M.; Miller, M.R.; Tian, H.S.; Kruger, M.C. Mass Spectrometry-Based Metabolomic and Lipidomic Analysis of the Effect of High Fat/High Sugar Diet and GreenshellTM Mussel Feeding on Plasma of Ovariectomized Rats. Metabolites 2021, 11, 754. https://doi.org/10.3390/metabo11110754
Abshirini M, Cabrera D, Fraser K, Siriarchavatana P, Wolber FM, Miller MR, Tian HS, Kruger MC. Mass Spectrometry-Based Metabolomic and Lipidomic Analysis of the Effect of High Fat/High Sugar Diet and GreenshellTM Mussel Feeding on Plasma of Ovariectomized Rats. Metabolites. 2021; 11(11):754. https://doi.org/10.3390/metabo11110754
Chicago/Turabian StyleAbshirini, Maryam, Diana Cabrera, Karl Fraser, Parkpoom Siriarchavatana, Frances M. Wolber, Matthew R. Miller, Hong Sabrina Tian, and Marlena C. Kruger. 2021. "Mass Spectrometry-Based Metabolomic and Lipidomic Analysis of the Effect of High Fat/High Sugar Diet and GreenshellTM Mussel Feeding on Plasma of Ovariectomized Rats" Metabolites 11, no. 11: 754. https://doi.org/10.3390/metabo11110754
APA StyleAbshirini, M., Cabrera, D., Fraser, K., Siriarchavatana, P., Wolber, F. M., Miller, M. R., Tian, H. S., & Kruger, M. C. (2021). Mass Spectrometry-Based Metabolomic and Lipidomic Analysis of the Effect of High Fat/High Sugar Diet and GreenshellTM Mussel Feeding on Plasma of Ovariectomized Rats. Metabolites, 11(11), 754. https://doi.org/10.3390/metabo11110754









