The Effects of Rhizosphere Inoculation with Pseudomonas mandelii on Formation of Apoplast Barriers, HvPIP2 Aquaporins and Hydraulic Conductance of Barley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Bacterial Strain and Cultural Media
2.2. Determining the Phosphate-Mobilizing Activity, Antagonistic Activity of the Strain to Microscopic Fungi, Chitinase and Cellulase Activity, Halophilicity of Bacteria
2.3. ABA and IAA Assay in Cultural Media
2.4. Plant–Water Relations: Transpiration Rate, Water Potential, Hydraulic Conductivity
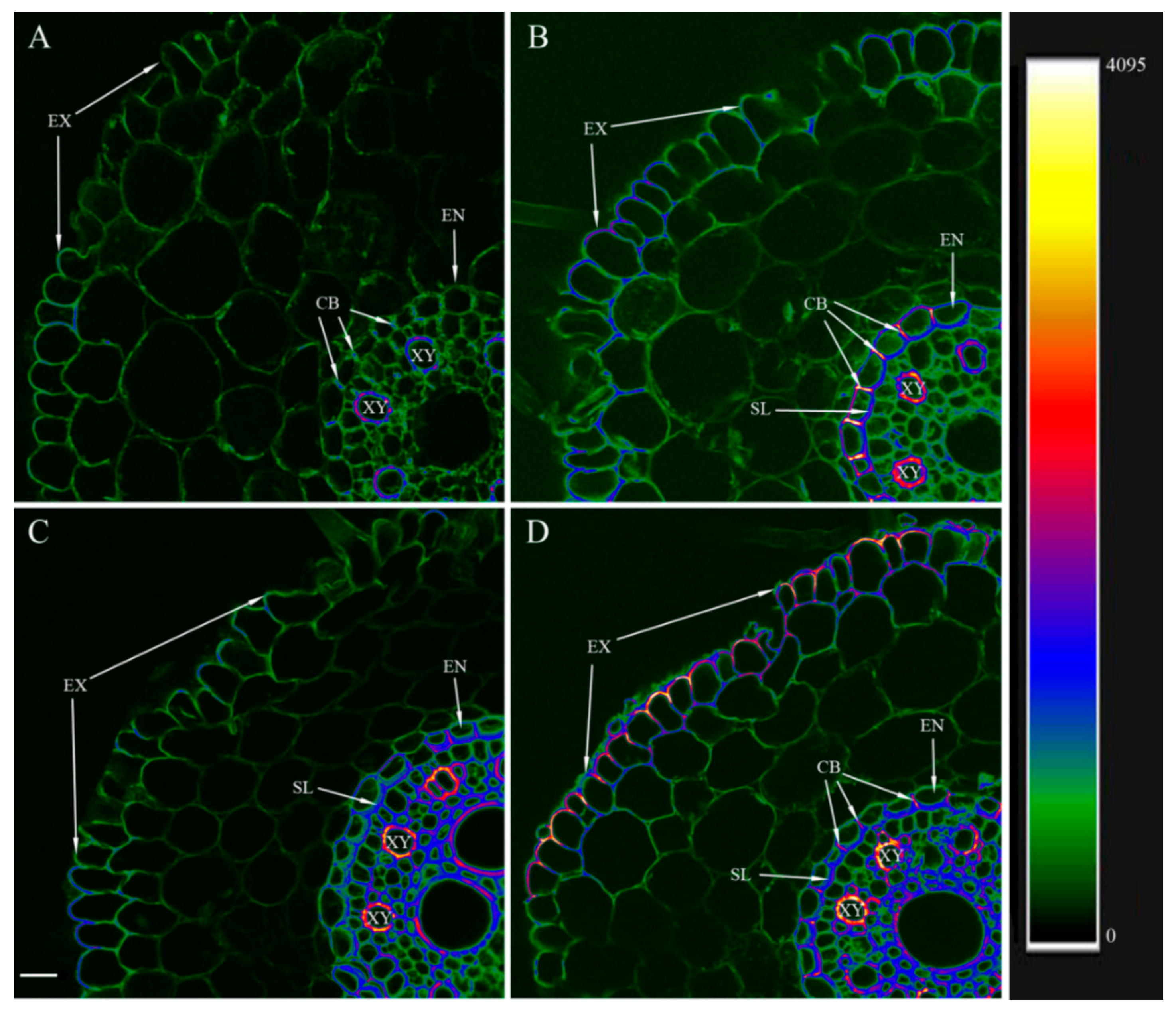
2.5. Visualization of Lignin and Suberin
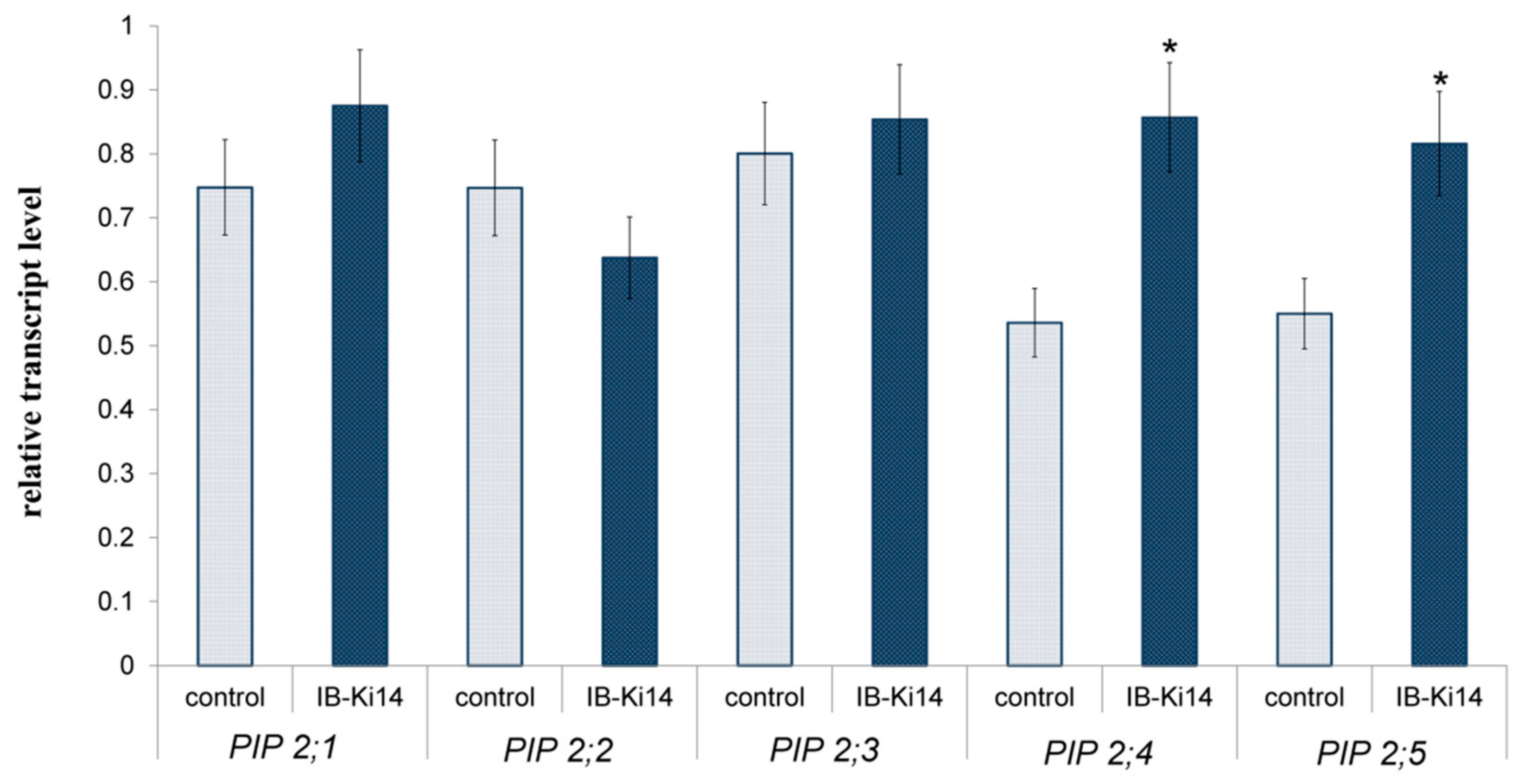
2.6. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.7. Generation of Antibodies against AQPs
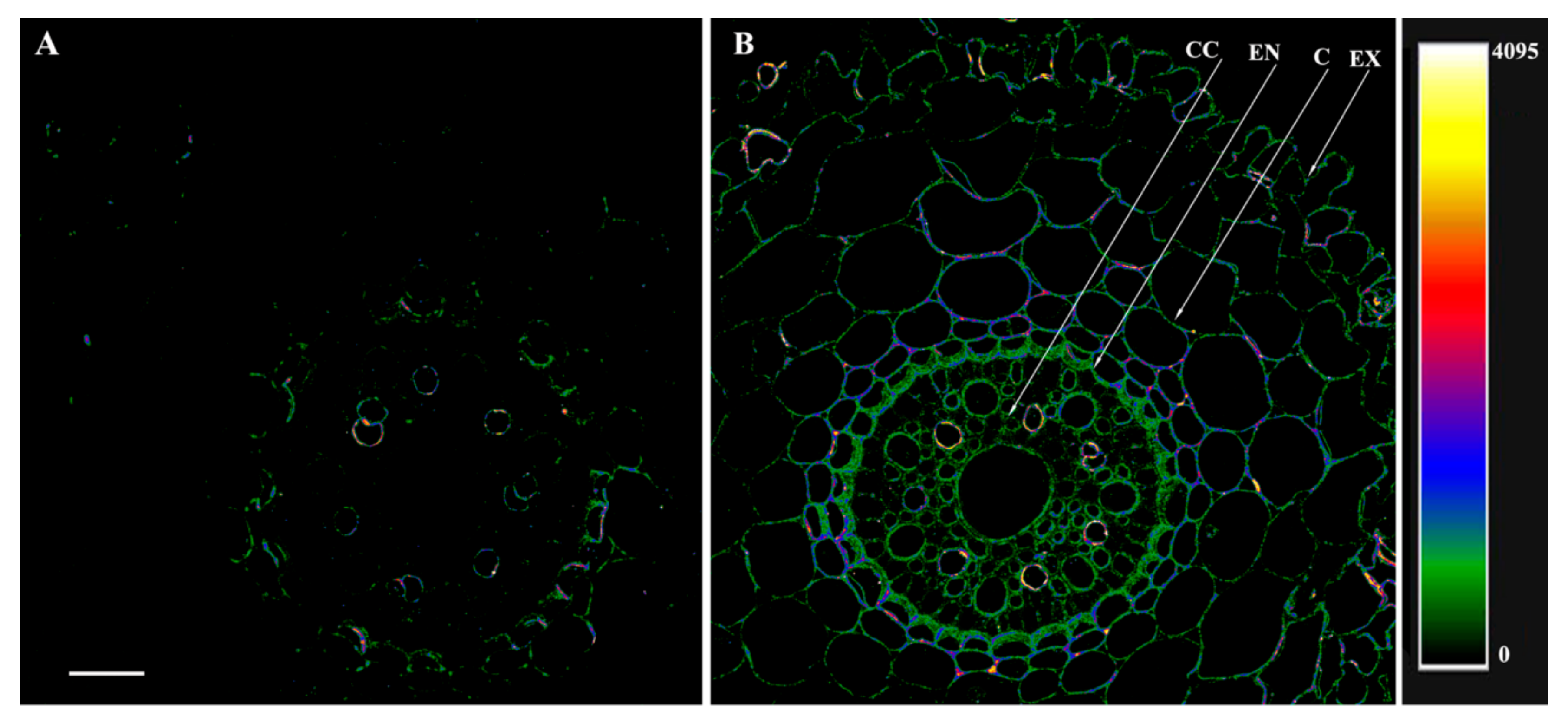
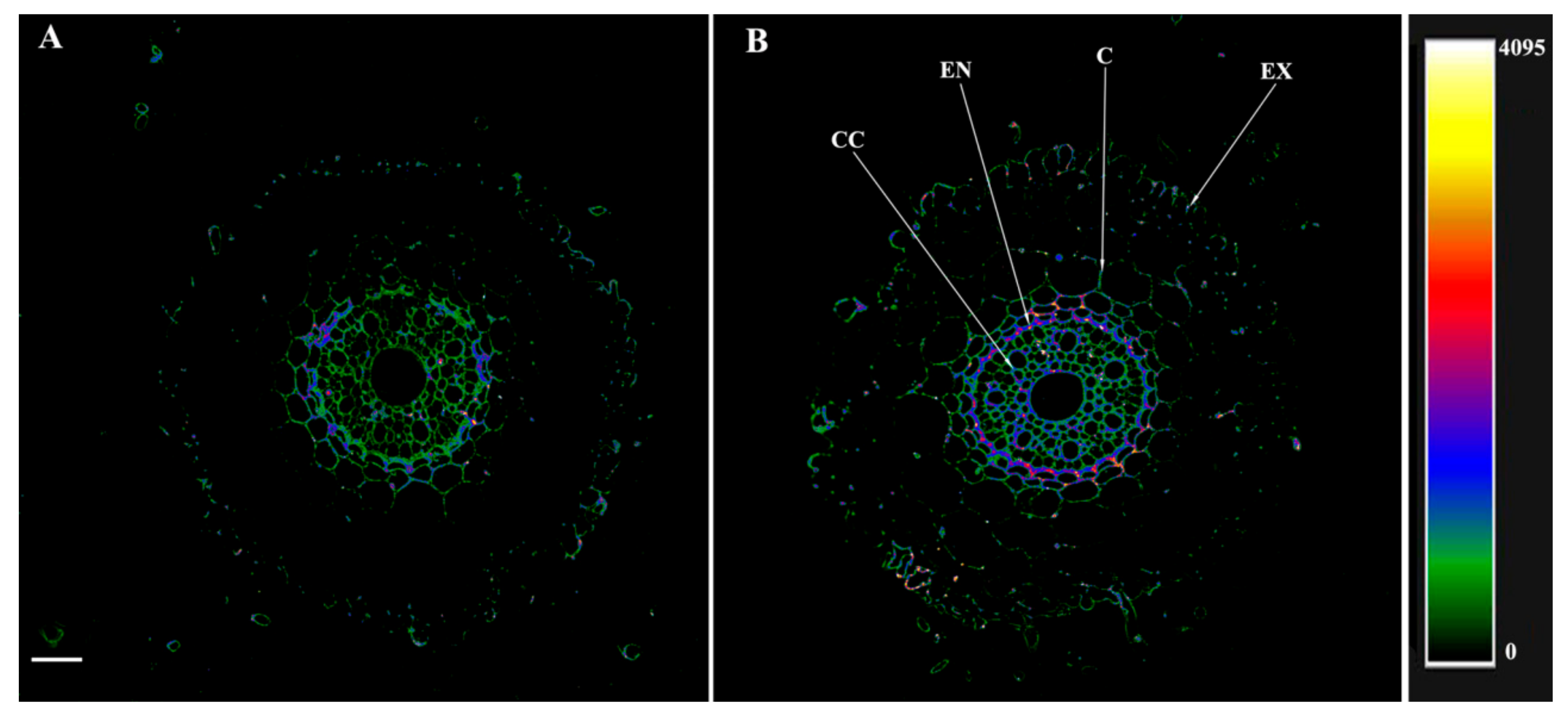
2.8. Immunolocalization of Aquaporins, IAA and ABA
2.9. Membrane Lipid Peroxidation
2.10. Statistics
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 2019, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarebanadkouki, M.; Trtik, P.; Hayat, F.; Carminati, A.; Kaestner, A. Root water uptake and its pathways across the root: Quantification at the cellular scale. Sci. Rep. 2019, 9, 12979. [Google Scholar] [CrossRef]
- Martynenko, E.; Arkhipova, T.; Safronova, V.; Seldimirova, O.; Galin, I.; Akhtyamova, Z.; Veselov, D.; Ivanov, R.; Kudoyarova, G. Effects of Phytohormone-Producing Rhizobacteria on Casparian Band Formation, Ion Homeostasis and Salt Tolerance of Durum Wheat. Biomolecules 2022, 12, 230. [Google Scholar] [CrossRef]
- Cui, B.; Liu, R.; Flowers, T.J.; Song, J. Casparian bands and suberin lamellae: Key targets for breeding salt tolerant crops? Environ. Exp. Bot. 2021, 191, 104600. [Google Scholar] [CrossRef]
- Li, L.; Pan, S.; Melzer, R.; Fricke, W. Apoplastic barriers, aquaporin gene expression and root and cell hydraulic conductivity in phosphate-limited sheepgrass plants. Physiol. Plant. 2020, 168, 118–132. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Revx. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Chen, K.; Wang, S.; Mur, L.A.J.; Guo, S. Exploring the Roles of Aquaporins in Plant-Microbe Interactions. Cells 2018, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Zawoznik, M.S.; Ameneiros, M.; Benavides, M.P.; Vázquez, S.; Groppa, M.D. Response to saline stress and aquaporin expression in Azospirillum-inoculated barley seedlings. Appl. Microbiol. Biot. 2011, 90, 1389–1397. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef]
- Josef, K.; Joséantonio, H.; Fuensanta, C.; Antonio, R. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct. Plant Biol. 2008, 35, 141–151. [Google Scholar] [CrossRef]
- Verhille, S.; Baida, N.; Dabboussi, F.; Izard, D.; Leclerc, H. Taxonomic study of bacteria isolated from natural mineral waters: Proposal of Pseudomonas jessenii sp. nov. and Pseudomonas mandelii sp. nov. Syst. Appl. Microbiol. 1999, 22, 45–58. [Google Scholar] [CrossRef]
- Jang, S.-H.; Kim, J.; Kim, J.; Hong, S.; Lee, C.W. Genome sequence of cold-adapted Pseudomonas mandelii strain JR-1. J. Bacteriol. 2012, 194, 3263. [Google Scholar] [CrossRef] [Green Version]
- Weon, H.; Dungan, R.; Kwon, S.; Kim, J. The phylogeny of fluorescent pseudomonads in an unflooded rice paddy soil. Ann. Microbiol. 2007, 57, 299–306. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Prongjunthuek, K.; Mortuza, M.F.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyoma, T. Physiological and genetic characterization of rice nitrogen fixer PGPR isolated from rhizosphere soils of different crops. Plant Soil 2014, 379, 51–66. [Google Scholar] [CrossRef]
- Katsuhara, M.; Akiyama, Y.; Koshio, K.; Shibasaka, M.; Kasamo, K. Functional analysis of water channels in barley roots. Plant Cell Physiol. 2002, 43, 885–893. [Google Scholar] [CrossRef]
- Horie, T.; Kaneko, T.; Sugimoto, G.; Sasano, S.; Panda, S.K.; Katsuhara, M. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiol. 2011, 52, 663–675. [Google Scholar] [CrossRef]
- Seldimirova, O.A.; Kudoyarova, G.R.; Katsuhara, M.; Galin, I.R.; Zaitsev, D.Y.; Kruglova, N.N.; Veselov, D.S.; Veselov, S.Y. Dynamics of the contents and distribution of ABA, auxins and aquaporins in developing caryopses of an ABA-deficient barley mutant and its parental cultivar. Seed Sci. Res. 2019, 29, 261–269. [Google Scholar] [CrossRef]
- Sharipova, G.; Veselov, D.; Fricke, W.; Dodd, I.; Katsuhara, M.; Furuichi, T.; Ivanov, I.; Veselov, S. Exogenous application of abscisic acid (ABA) increases root and cell hydraulic conductivity and abundance of some aquaporin isoforms in the ABA deficient barley mutant Az34. Ann. Bot. 2016, 118, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Chaumont, F.; Barrieu, F.; Jung, R.; Chrispeels, M.J. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000, 122, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Kuzmina, L.; Gilvanova, E.; Galimzyanova, N.; Arkhipova, T.; Ryabova, A.; Aktuganov, G.; Sidorova, L.; Kudoyarova, G.; Melent’ev, A. Characterization of the Novel Plant Growth-Stimulating Strain Advenella kashmirensis IB-K1 and Evaluation of Its Efficiency in Saline Soil. Microbiology 2022, 91, 204–216. [Google Scholar] [CrossRef]
- Moawad, M.E.P.; Vleck, P.L.G. Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13–23. [Google Scholar] [CrossRef]
- Veselov, D.S.; Sharipova, G.V.; Veselov, S.U.; Kudoyarova, G.R. The effects of NaCl treatment on water relations, growth and ABA content in barley cultivars di_ering in drought tolerance. J. Plant Growth Regul. 2008, 27, 380–386. [Google Scholar] [CrossRef]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Musielak, T.J.; Schenkel, L.; Kolb, M.; Henschen, A.; Bayer, M. A simple and versatile cell wall staining protocol to study plant reproduction. Plant Reprod. 2015, 28, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezrukova, M.; Kildibekova, A.; Shakirova, F. WGA reduces the level of oxidative stress in wheat seedlings under salinity. Plant Growth Regul. 2008, 54, 195–201. [Google Scholar] [CrossRef]
- Mori, I.; Rhee, J.; Shibasaka, M.; Sasano, S.; Kaneko, T.; Horie, T.; Katsuhara, M. CO2 transport by PIP2 aquaporins of barley. Plant Cell Physiol. 2014, 55, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ranathunge, K.; Nayak, S.; Schreiber, L.; Mathew, M.K. Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 4215–4228. [Google Scholar] [CrossRef]
- Lee, M.-H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.-J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef]
- Akhiyarova, G.R.; Ivanov, R.S.; Ivanov, I.I.; Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Nuzhnaya, T.; Ovchinnikova, T.V.; Veselov, D.S.; Kudoyarova, G.R. Effects of Salinity and Abscisic Acid on Lipid Transfer Protein Accumulation, Suberin Deposition and Hydraulic Conductance in Pea Roots. Membranes 2021, 11, 762. [Google Scholar] [CrossRef]
- Aroca, R.; Ferrante, A.; Vernieri, P.; Chrispeels, M.J. Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann. Bot. 2006, 98, 1301–1310. [Google Scholar] [CrossRef]
- Park, Y.-G.; Mun, B.-G.; Kang, S.-M.; Hussain, A.; Shahzad, R.; Seo, C.-W.; Kim, A.-Y.; Lee, S.-U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [Green Version]
- Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-Promoting Effect of Rhizobacterium (Bacillus subtilis IB22) in Salt-Stressed Barley Depends on Abscisic Acid Accumulation in the Roots. Int. J. Mol. Sci. 2021, 22, 10680. [Google Scholar] [CrossRef]
- Kraft, M.; Kuglitsch, R.; Kwiatkowski, J.; Frank, M.; Grossmann, K. Indole-3-acetic acid and auxin herbicides up-regulate 9-cis-epoxycarotenoid dioxygenase gene expression and abscisic acid accumulation in cleavers (Galium aparine): Interaction with ethylene. J. Exp. Bot. 2007, 58, 1497–1503. [Google Scholar] [CrossRef] [Green Version]
- Ursache, R.; De Jesus Vieira Teixeira, C.; Dénervaud Tendon, V.; Gully, K.; De Bellis, D.; Schmid-Siegert, E.; Andersen, T.G.; Shekhar, V.; Calderon, S.; Pradervand, S.; et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 2021, 7, 353–364. [Google Scholar] [CrossRef]
- Péret, B.; Li, G.; Zhao, J.; Zhao, J.; Band, L.R.; Voβ, U.; Postaire, O.; Luu, D.-T.; Daines, O.; Casimiro, I.; et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef]


| Genes | Strand | 5′ to 3′ Primer Sequences | GenBank Accession Number |
|---|---|---|---|
| HvPIP2;1 | Forward | GGGTCCTACAGGAGCAACTAA | AB009307 |
| Reverse | GGCCTTCTTCTTCGCACATATC | ||
| HvPIP2;2 | Forward | GTCAAGGGCATCATGAAGGA | AB377269 |
| Reverse | TGTAGACGAGGACGAAGGT | ||
| HvPIP2;3 | Forward | TGACCAAGTGGTCCCTGTA | AB275280 |
| Reverse | CTGGTGCTTGTACCCAATGA | ||
| HvPIP2;4 | Forward | GTCATGTACGCATCCAGGTAA | AB219525 |
| Reverse | GGACGTATGCTTGGGAGTAAG | ||
| HvPIP2;5 | Forward | CTGTGTCCATCCATCCAAAGA | AB377270 |
| Reverse | CAGCTGCCGACACATAAATAAC | ||
| Hv α-tubulin | Forward | GAGCGTCTCTCTGTTGACTATG | AK250165 |
| Reverse | TGGACAGGACACTGTTGTATG |
| Characteristic | ||
|---|---|---|
| IAA concentration, ng/mL in the culture medium | 220 | |
| ABA concentration, ng/mL in the culture medium | 5 | |
| Phosphate-solubilizing activity, index of solubilization | ||
| Ca3(PO4) | 2.7 | |
| Al3PO4·3H2O | 1 | |
| FePO4·2H2O | only under the colony | |
| phytin (C6H6Ca5MgO24P6) | 2.8 | |
| Chitin hydrolysis | weak | |
| Cellulose hydrolysis | no | |
| Antagonism against B. sorokiniana, F. culmoruim, F. oxysporm, F. solani | absence | |
| Growth in presence NaCl, 1–10% | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipova, T.; Sharipova, G.; Akhiyarova, G.; Kuzmina, L.; Galin, I.; Martynenko, E.; Seldimirova, O.; Nuzhnaya, T.; Feoktistova, A.; Timergalin, M.; et al. The Effects of Rhizosphere Inoculation with Pseudomonas mandelii on Formation of Apoplast Barriers, HvPIP2 Aquaporins and Hydraulic Conductance of Barley. Microorganisms 2022, 10, 935. https://doi.org/10.3390/microorganisms10050935
Arkhipova T, Sharipova G, Akhiyarova G, Kuzmina L, Galin I, Martynenko E, Seldimirova O, Nuzhnaya T, Feoktistova A, Timergalin M, et al. The Effects of Rhizosphere Inoculation with Pseudomonas mandelii on Formation of Apoplast Barriers, HvPIP2 Aquaporins and Hydraulic Conductance of Barley. Microorganisms. 2022; 10(5):935. https://doi.org/10.3390/microorganisms10050935
Chicago/Turabian StyleArkhipova, Tatiana, Guzel Sharipova, Guzel Akhiyarova, Ludmila Kuzmina, Ilshat Galin, Elena Martynenko, Oksana Seldimirova, Tatyana Nuzhnaya, Arina Feoktistova, Maxim Timergalin, and et al. 2022. "The Effects of Rhizosphere Inoculation with Pseudomonas mandelii on Formation of Apoplast Barriers, HvPIP2 Aquaporins and Hydraulic Conductance of Barley" Microorganisms 10, no. 5: 935. https://doi.org/10.3390/microorganisms10050935






