2.1. Synthesis, Conformation and Configuration of Diastereomeric Trans- and cis-2-Halogeno-4-methyl-1,3,2-dioxaphosphorinan-2-thiones 6
Our initial investigations were focused on the efficient preparation and assignment of stereochemistry (ring conformation, configuration of the C4 and phosphorus stereocentres) to 2-chloro-, 2-bromo- and 2-fluoro-4-methyl-1,3,2-dioxaphosphorinan-2-thiones 6, which were intended to use as model compounds in subsequent studies of the stereochemistry of nucleophilic substitution at phosphorus.
At first,
trans-2-chloro-4-methyl-1,3,2-dioxaphosphorinan-2-thione
6(
Cl) was prepared by treatment of
trans-2-chloro-4-methyl-1,3,2-dioxaphosphorinan
7 [
16,
17,
18] with acetylsulfenyl chloride. The reaction was carried out in ether at 0 °C and afforded
trans-
6(
Cl) as a pure diastereomer (
31P-NMR assay) in 91% yield (
Scheme 3).
This reaction, as other reactions of P
III-compounds with sulfenyl chlorides [
19], gave the desired product
6(
Cl) in a stereospecific way with retention of configuration at phosphorus via the intermediate quasi-phosphonium chloride (
A). Hence, the isomer of
6(
Cl) obtained has the same
trans-relation between the C
4-methyl group and the chlorine atom as in the starting phosphorochloridite (
7).
To gain more detailed insight into the ring stereochemistry and conformation the 270 MHz
1H-NMR spectrum of
trans-
6(
Cl) was recorded. It showed that all the resonance signals of the ring protons were well separated from each other, thus allowing the first order analysis of the spectrum without use of spin decoupling. In
Table 1, all of the chemical shifts and coupling constants, derived from the spectrum, are summarized.
These values and particularly very distinct differences between the long-range
31P-
1H couplings observed for axial and equatorial protons indicated that the dioxaphosphorinan ring in
trans-
6(
Cl) adopts the chair conformation with the equatorial C
4-methyl group and thiophosphoryl sulfur. Thus, the methyl protons appeared in the spectrum as a double doublet with coupling constants with the axial methine proton,
3JCH3-CH = 6.2 Hz, and with phosphorus,
4JCH3-P = 2.6 Hz. These values are diagnostic for equatorial position of the methyl group in the dioxaphosphorinan ring. The
31P-NMR spectrum of
trans-
6(
Cl) showed the resonance signal shaped like a double multiplet at δ
P = 59.0 ppm (see
Figure 4, which is characteristic and typical for the 1,3,2-dioxaphosphorinan ring with the methyl group and thiophosphoryl sulfur in equatorial positions.) Interestingly, almost the same shape of the
31P-spectrum was observed for the phosphoryl analogues of our compounds [
12,
20].
The second, convenient approach to the same isomer
trans-
6(
Cl) consists in chlorination of an equimolar mixture of
cis- and
trans-2-hydrogen-4-methyl-1,3,2-dioxaphosphorinan-2-thiones
8 [21] by means of sulfuryl chloride. The chlorination reaction was performed in benzene at 0 °C and afforded, rather unexpectedly, a diastereomerically pure
trans-
6(
Cl) in 80% yield. However, when the reaction was carried out at −40 °C, a mixture of
trans-
6(
Cl) (85%) and
cis-
6(
Cl) (15%) was formed as revealed by
31P-NMR spectra.
The results of the chlorination reaction of diastereomeric thiophosphites
8 summarized in
Scheme 4 indicated that the diastereomer
trans-
6(
Cl) is thermodynamically more stable than
cis-
6(
Cl) and that epimerization at phosphorus was occurring in the reaction course. A possibility of epimerization of the starting thiophosphites
8 (
trans-
8→
cis-
8) was excluded since treatment of a 1:1 mixture of isomeric
8 with gaseous hydrogen chloride under the reaction conditions comparable to the chlorination reaction did not affect the isomeric ratio of
8. Most probably,
cis-
6(
Cl), when formed from
trans-
8, undergoes conversion to the more stable
trans-
6(
Cl) via the chloride–chloride exchange reaction at phosphorus as shown below (
Scheme 5).
The observation of a fully stereospecific formation of
trans-
6(
Cl) upon chlorination of a mixture of isomeric thiophosphites
8 prompted us to investigate their bromination (
Scheme 6). It was found that the reaction of thiophosphite
8 consisting of 73%
trans-
8 and 27%
cis-
8 with elemental bromine carried out at 30 °C in benzene gave only one crystalline isomer of phosphorobromidate trans-
6(
Br) in 85% yield. As in the case of
trans-
6(
Cl), the C
4-methyl protons of the crystalline isomer appeared in
1H-NMR spectrum as a double doublet with characteristic coupling constants,
3JCH3-H = 6.9 Hz and
4JCH3-P = 2.9 Hz, indicating that it exists in a chair conformation with both the C
4-methyl and P=S groups in equatorial position. Moreover,
Figure 4 shows that the shape of the resonance signal in the
31P-NMR spectrum of the more stable crystalline thiophosphoryl bromide
trans-
6(
Br) is almost the same as that of the corresponding chloride
trans-
6(
Cl). When the same mixture of isomeric thiophosphites
8 was reacted with bromine at −15°C, a mixture of both isomeric phosphorobromidates was formed, i.e.,
trans-
6(
Br) (64%) and
cis-
6(
Br) (36%).
In search of a stereospecific synthesis of the less stable cyclic thiophosphoryl chloride
cis-
6(
Cl), we turned our attention on the reaction of monothiophosphoric acids with phosphorus pentachloride. This reaction with the enantiomers of thiophosphonic acid
1 afforded exclusively enantiomerically pure thiophosphonyl chlorides
2 with inversion of configuration [
2,
4,
22]. In the hope that cyclic monothiophosphoric acids will react with phosphorus pentachloride in a similar way, the reaction of easily available
cis- and
trans-2-hydroxy-4-methyl-1,3,2-dioxaphosphorinan-2-thiones
9 [
23] withthis reagent was investigated. However, we were surprised to find that in addition to the expected isomeric chlorides
6(
Cl), two other products were formed. Their structures were dependent on the configuration at phosphorus in starting cyclic thioacids
9. Thus, treatment of
trans-
9 with PCl
5 suspended in benzene at 0–5 °C resulted in formation of three products as revealed by
31P-NMR spectra: thiophosphoryl chloride (
trans-
6(
Cl), phosphoryl chloride
trans-
5(
Cl) and unsymmetrical
trans-
cis-dithiopyrophosphate
10 (see
Scheme 7).
The formation of thiophosphoryl chloride
trans-
6(
Cl) in this reaction was expected as well as the fact that it was formed from thioacid
trans-
9 with inversion of configuration at phosphorus. However, the appearance of phosphorochloridate
trans-
5(
Cl) among the reaction products was astonishing. Moreover, the replacement of sulfur in
trans-
9 by chlorine leading to
trans-
5(
Cl) was occurring with retention at phosphorus. The third product was identified as one of the three isomeric bis-(4-methyl-1,3,2-dioxaphosphorinan-2-thionyl)oxides
10. Their synthesis, configuration and conformation were reported in a separate paper [
24].
A somewhat different outcome of the reaction of thioacid
cis-
9 with PCl
5 was observed. To our satisfaction the major product in this case was the desired thermodynamically less stable thiophosphoryl chloride
cis-
6(
Cl) formed in 60% yield. It was accompanied by the more stable isomer
trans-
6(
Cl) (21%). The latter was undoubtedly the product of epimerization at phosphorus in
cis-
6(
Cl) taking place in the presence of chloride anions in a reaction mixture. Interestingly, the same dithiopyrophosphate
trans-
cis-
10 was also formed in this reaction in comparable amounts (19%) (
Scheme 8). Distillation of a mixture of these three reaction products allowed us to isolate thiophosphoryl chloride
cis-
6(
Cl) containing 5%
trans-isomer as the lowest boiling fraction. This almost diastereomerically pure
cis-chloride
6 was used in our further experiments.
Although elucidation of all mechanistic details of the reaction under discussion was beyond the scope of the present work, we wish to rationalize herein its multidirectional course and stereochemistry of the products formation (
Scheme 9). Taking into account the well-known ambident reactivity of monothiophosphoric acids and their anions, it is reasonable to assume that cyclic thioacids, like acyclic ones, react with phosphorus pentachloride at the oxygen atom. This initial reaction, for example with
trans-
9, results in formation of
O-trichlorophosphonium chloride (
A) as the first reaction intermediate. This, in turn, upon a nucleophilic attack of the chloride anion at the phosphorinanyl phosphorus and the concomitant four-membered ring closure between sulfur and the trichlorophosphonium phosphorus atom, is converted into a second intermediate (
B). Its formation and relative stability are due to the presence of two six- and four-membered rings, which are known to stabilize hypervalent phosphorus compounds. Interestingly, almost the same type of structure was postulated for the intermediate found in the reaction between oxophosphoranesulfenyl chlorides and phosphorus trichloride [
25].
This trigonal bipyramidal intermediate (
B) may undergo decomposition in two ways. The first accompanied by elimination of phosphoryl trichloride affords the expected thiophosphoryl chloride
trans-
6(
Cl) with the experimentally confirmed inversion of configuration. On the other hand, simultaneous elimination of thiophosphoryl trichloride from (
B) results in formation of the unexpected phosphoryl chloride
trans-
5(
Cl) with retention of configuration at phosphorus. This stereochemistry of the conversion
trans-
9→trans-
5(
Cl) is also in a full agreement with that experimentally found (see
Scheme 9). Finally, the formation of dithiopyrophosphate
10 as a third reaction product is worthy of notice. It is undoubtedly formed in the reaction between starting thioacid
trans-
9 and the first reaction intermediate (
A). The latter, having a good leaving group, undergoes nucleophilic substitution by thioacid
trans-
9 with inversion of configuration at phosphorus, which affords unsymmetrical
trans-
cis-diastereomer of dithiopyrophosphate
10.
To complete the synthesis of cyclic thiophosphoryl halogenides
6(
Cl,
Br,
F) and having some experience with the preparation of chlorides
6(
Cl) and bromides
6(
Br), we turned to the synthesis of diastereomeric fluorides
6(
F). In general, the synthetic approaches to acyclic and cyclic thiophosphoryl fluorides are sparse and of limited applicability. The most practical method of their synthesis involves the halogen exchange reaction at phosphorus using inorganic fluorides [
3,
26]. In our first attempt to obtain
6(
F), a very simple reaction was tested, namely, the addition of elemental sulfur to cyclic fluorophosphite
11. The latter was prepared according to the modified procedure described by Schmutzler [
27] from
trans-chlorophosphite
7 and antimony trifluoride (
Scheme 10).
Trans-fluorophosphite
11 as a major and thermodynamically more stable product obtained in this reaction was contaminated with 5%
cis-isomer. To our surprise, it was found that the addition of elemental sulfur to
trans-
11 does not occur even in boiling toluene. When, however, acetylsulfenyl chloride was used instead of elemental sulfur, the conversion of
trans-
11 to
cis-
6(
F) was achieved efficiently under mild reaction conditions (
Scheme 11).
As expected, the sulfurization of
trans-fluorophosphite
11 occurred with a full retention of configuration at phosphorus and afforded the crude thiophosphoryl fluoride
cis-
6(
F) containing 5% of
trans-
6(
F). The diastereomerically pure solid
cis-isomer (m.p. 33–34 °C) was isolated from this mixture by distillation and crystallization. The NMR (
1H,
31P) data of
cis-fluoride
6(
F) were consistent with a rigid chair conformation in which the thiophosphoryl sulfur and the methyl group on C
4 are in equatorial positions on the 1,3,2-dioxaphosphorinan ring. For instance, the resonance signal (double doublet) of the methyl protons with characteristic coupling constants,
3JCH3-H = 6.2 Hz and
4JCH3-P = 2.6 Hz, shows that the methyl group is equatorial. The
31P-NMR spectrum of
cis-
6(
F) shows a typical doublet with the coupling constant
1JP-F= 1086 Hz due to the presence of fluorine directly bonded to phosphorus. The shape of each arm of this doublet is almost identical with the
31P-NMR resonance signals of
trans-
6(
Cl) and
trans-
6(
Br) (see
Figure 1). This observation may be taken as additional evidence that all the thiophosphoryl halogenides mentioned above exist in the same chair conformation with the equatorial C
4-methyl and thiophosphoryl group. Finally, a single-crystal X-ray analysis of
cis-
6(
F) revealed [
28] that the 1,3,2-dioxaphosphorinan ring also adopts a chair conformation in the solid state and confirmed our configurational assignments based on NMR measurements (see
Figure 2 and
Figure 5).
The
trans-isomer of
6(
F) was obtained from
trans-bromide
6(
Br) and ammonium fluoride. The bromide → fluoride exchange reaction carried out in acetonitrile solution at 40 °C afforded after 8 h a mixture of
trans- and
cis-fluorides at a ratio of 84:16. When the reaction was conducted for 24 h at the same temperature, the desired
trans-fluoride
6(
F) was formed with inversion of configuration and with 94% diastereomeric purity. The diastereomerically pure
trans-
6(
F) was obtained by preparative gas chromatography. However, prolongation of the reaction time to 50 h resulted in the isomerization of
trans-
6(
F) (inversion of configuration at P) to the thermodynamically more stable
cis-
6(
F) and the latter was formed with 83% diastereomeric purity (
Scheme 12).
Most probably, the above fluoride–fluoride exchange reaction should lead to the diastereomerically pure
cis-
6(
F) after longer heating. Moreover, the fact that a trace of the less stable
trans-
6(
F) was not observed when the pure fluoride
cis-
6(
F) was heated with NH
4F (60 h, 40 °C) indicates that this identity reaction reflects a strong preference of fluorine for an axial position versus a P=S bond in the 1,3,2-dioxaphosphorinan ring. DFT calculations show that the
trans-
6(
F) isomer has a free energy of ΔG = 2.5 kcal/mol higher than
cis-
6(
F) isomer, which corresponds roughly to an equilibrium concentration ratio [
trans-
6(
F)]
eq/[
cis-
6(
F)]
eq of ca. 1.5 × 10
−2. Taking into account
1H- and
31P-NMR data, as well as DFT calculations (see
Supplementary Materials), it is reasonable to assume that the
trans-
6(
F) isomer exists in two chair conformations (
A and
B) and one boat conformation(
C) that are in a fast equilibrium as shown below (
Scheme 13).
The conformer (
B) with the axial methyl group at C
4 should be present in equilibrium in substantial proportions, which is reflected in the different shape of the diagnostic
1H-NMR resonance signal of the C
4-Me from that of the
cis-isomer
6(
F). Thus, the methyl protons of
trans-
6(
F) appeared in the spectrum as a doublet of triplets due to characteristic splitting by the C
4-proton (
3JCH3-H = 6.5 Hz), then by phosphorus (
4JCH3-P = 0.9 Hz) and additionally by the vicinal axial proton at C
5 with the same coupling constant value (0.9 Hz) as with phosphorus. The observation of the last coupling and a small value of the coupling constant with phosphorus is indicative of the presence of the conformer (
B) in equilibrium. On the other hand, the
31P-NMR spectrum of
trans-
6(
F) shows a typical doublet due to splitting by fluorine with the coupling constant
1JP-F = 1094 Hz. Both arms of this doublet are dense multiplets, which are characteristic of the dioxaphosphorinan rings, having methyl and thiophosphoryl groups
trans situated. The
31P-NMR spectra of a mixture of
trans-
6(
F) (84%) and
cis-
6(
F) (16%) (shown in
Figure 5) in conjunction with
Figure 4 best illustrate the relationship between the shape of the
31P-NMR resonance signals and configuration at phosphorus and C
4-carbon in the 1,3,2-dioxaphosphorinan systems.
These observations are supported by our DFT calculations. We performed full conformational analysis of one pair of diastereomers, namely (
RP,SC4) and (
SP,SC4), as the other pair, (
SP,RC4) and (
RP,RC4), respectively, are their equivalent enantiomeric forms. For both diastereomers, two stable low energy chair conformations and one boat conformation were identified. The structures and relative free energies of the
cis- and
trans-conformers of
6(
F) in aqueous solution (in kcal/mol) are given in
Supplementary Materials, Figure S2 and Table S2. The calculated geometry of
cis-2-fluoro-4-methyl-1,3,2-dioxaphosphorinan-2-thione in the aqueous solution agrees very well with its crystal structure reported [
24], which proves the reliability of the theoretical method used (
Supplementary Materials, Table S4).
The most stable conformer of the two diastereomers (
Scheme 11) is the conformer
cis-
6(
F) in which fluorine occupies an axial position and the methyl group on the C
4 carbon occupies an equatorial position.
2.2. Stereochemistry of Nucleophilic Displacement Reactions at Phosphorus in 2-Halogeno-4-methyl-1,3,2-dioxaphosphorinan-2-thiones 6
Having in hand the diastereomeric pairs of thiophosphoryl chloride
6(
Cl) and fluoride
6(
F), we could start with investigation of the stereochemical course of nucleophilic displacement processes occurring at phosphorus. In the present work, three basic substitution reactions were examined, namely alkaline hydrolysis, methanolysis and aminolysis. The choice of the first two reactions was due to the fact that they produce diastereomeric thioacids
9 [23] and thionophosphates
12 [19], respectively, which were obtained by us in an independent way and the configuration at phosphorus in both diastereomers of
9 and
12 was firmly established. Moreover, the diastereomeric hydrolysis products
9 as well as thiophosphoryl amides
13 formed in the third reaction do not undergo epimerization at phosphorus under the reaction conditions.
At first, the stereochemical course of displacement reactions at phosphorus in the
trans-isomer of thiophosphoryl chloride
6(
Cl) was investigated. The results obtained are summarized in
Scheme 14.
Alkaline hydrolysis of the diastereomerically pure
trans-
6(
Cl) as well as other halogenides
6 was carried out in a water-dioxane solution at ca. 0 °C. The crude sodium salt of thioacid
9 isolated from the reaction mixture showed two resonance signals at δ
P = 50.5 and 54.0 ppm in the
31P-NMR spectrum, corresponding to the sodium salts of thioacid
trans-
9 and
cis-
9, respectively. They were formed in a 92:8 ratio. For further identification and characterization, this mixture of both sodium salts was converted into the corresponding dicyclohexyl ammonium salts of
trans-
9 (92%) and
cis-
9 (8%) displaying chemical shifts at δ
P = 50.5 and 53.5 ppm. The fact that thioacid
trans-
9 was formed as the major hydrolysis product indicates that the reaction under discussion occurred with inversion of configuration at phosphorus. A very small amount (8%) of thioacid
cis-
9 formed in this reaction may be explained by a concomitant formation of
cis-
6(
Cl) (via the chloride–chloride exchange reaction in the starting
trans-
6(
Cl)) and its subsequent hydrolysis to
cis-
9. Alkaline hydrolysis of the thermodynamically less stable thiophosphoryl chloride
cis-
6(
Cl) with 95% diastereomeric purity afforded a mixture of two sodium salts of thioacid
cis-
9 and
trans-
9 in a 77:23 ratio as revealed by
31P-NMR spectrum. Formation of the sodium salt of
cis-
9 as the major product also demonstrated the prevailing stereoinvertive course of hydrolysis of this stereomer (
Scheme 15).
The methanolysis reaction of trans-6(Cl) was carried out in a mixture of benzene and sodium methoxide in methanol at 0 °C. It afforded the cyclic thionophosphate trans-12 with 93% diastereomeric purity (31P-NMR assay). As in the case of alkaline hydrolysis of trans-6(Cl), the stereochemical outcome of the methanolysis was also inversion of configuration at phosphorus. However, in this case a small decrease of diastereoselectivity in this reaction may be due to the methoxy–methoxy exchange reaction at phosphorus in trans-12, resulting in formation of cis-12, the more thermodynamically stable isomer.
In contrast to alkaline hydrolysis and methanolysis, the reaction of
trans-
6(
Cl) with dimethylamine in ether at 0 °C gave the corresponding thiophosphoryl amide
trans-
13 with complete inversion of configuration at phosphorus. As the aminolysis product (
trans-
13) was not reported in the literature, it was prepared by us in an independent way starting from
cis-2-dimethylamino-4-methyl-1,3,2-dioxaphosphorinan
14 (80% dp) [
29], which upon treatment with acetylsulfenyl chloride gave thiophosphoryl amide
trans-
13 with 94% diastereomeric purity. Addition of elemental sulfur to amidophosphite
14 also occurred with retention of configuration at phosphorus and afforded
trans-
13 with 82% diastereomeric purity (
Scheme 16).
Similar results were obtained with the thiophosphoryl bromide
trans-
6(
Br), which was used as a substrate in the three substitution reactions investigated herein (see
Scheme 14). Thus, alkaline hydrolysis and methanolysis of this bromide were found to occur with a predominant inversion of configuration at phosphorus while aminolysis, as in the case of the
trans-chloride
6(
Cl), was fully diastereoselective and gave the pure
trans-
13 with inversion of configuration. In this connection, it is interesting to mention that the condensation reactions of isomeric
cis- and
trans-chlorides and bromides,
6(
Cl) and
6(
Br) with the diastereomeric thioacid
9 anions occurred almost exclusively by inversion of P-configuration [
24].
Before discussing our present results on displacement reactions in the diastereomeric thiophosphoryl fluorides
6(
F), it is necessary to note that Inch and coworkers [
30] reported very early that the displacement of fluorine by nucleophilic reagents in the 1,3,2-dioxaphosphorinan-2-ones derived from carbohydrates occurs with preponderant retention at phosphorus. However, the stereochemical course of these reactions was examined with only one, more stable diastereomer with the axial fluorine. Therefore, the discussion on the stereochemistry-mechanism relationship was rather limited and not conclusive. As we have been successful in preparing and purifying both
cis- and
trans-fluorides
6(
F), we decided to concentrate our attention on their alkaline hydrolysis. In the first place, this reaction was investigated with the thermodynamically more stable thiophosphoryl fluoride
cis-
6(
F), and also with the axial fluorine, to compare our results with those reported by Inch. Thus, hydrolysis was carried out according to a general procedure also applied for other thiophosphoryl halogenides
6 (NaOH, H
2O-dioxane, 0 °C) and resulted in a mixture of the two sodium salts of the thioacid
cis-
9 and
trans-
9 in a ratio of 66:34 as estimated by
31P-NMR spectra [
27] (see
Scheme 17).
This result clearly showed that the fluoride
cis-
6(
F) underwent hydrolytic conversion to
cis-
9(Na) as the major product with retention of configuration at phosphorus. In this way, the results of Inch were to some extent corroborated. However, the most intriguing was a high content of the sodium salt of
trans-
9, which was simultaneously formed in this reaction with inversion of configuration, as epimerization of the more stable
cis-
6(
F) to its less stable
trans-isomer does not occur in the presence of fluoride anion. Such a stereochemical outcome of alkaline hydrolysis should be due to a different mechanism of hydrolysis of 2-fluorophosphorinanes
6(
F) as compared with their chloro- and bromo-analogues. We have also noted a negligible effect of the reaction medium and added inorganic salts on the retention-inversion ratio (see
Table 2).
The results of alkaline hydrolysis of the less stable fluoride
trans-
6(
F) (equatorial fluorine) were even more interesting. In this case, two sodium salts of the thioacid
9 were obtained in a 73:27 ratio; however, the major product,
cis-
9(
Na), was formed with inversion of configuration (see
Scheme 18).
In additional experiments, it was found that diastereomeric purity of the starting thiophosphoryl fluoride
trans-
6(
F) has practically no effect on the product ratio, which is in this case equivalent to the inversion-retention ratio (see
Table 3).
To estimate the effect of epimerization of the starting
trans-fluoride
6(
F) to
cis-isomer by fluoride ion, alkaline hydrolysis was performed with an insufficient molar amount of sodium hydroxide. The results obtained are shown in
Scheme 19. It turned out that the unreacted fluoride
trans-
6(
F) was only slightly epimerized and recovered with 84% diastereomeric purity. Therefore, this side process considered here cannot essentially change the main stereochemical outcome of hydrolysis.
Finally, for comparison purposes, the reaction of the more stable thiophosphoryl fluoride
cis-
6(
F) with sodium methoxide was briefly investigated. In accord with the results of alkaline hydrolysis of this thiofluoridate, its methanolysis afforded both isomeric thionophosphates
cis-
12 and
trans-
12, the former being the major product was formed with retention of configuration as shown in
Scheme 20.
It was also found that the product isomer ratios are practically not influenced by solvents, both protic and aprotic, indicating that they reflect the mode of substitution (
Table 4).
Dissecting the results of our studies on the stereochemistry of displacement reactions at phosphorus in 2-halogeno-4-methyl-1,3,2-dioxaphosphorinan-2-thiones 6 presented above several important observations and interpretations can be inferred. First of all, there is a sharp difference in the stereochemical course of substitution reactions between diastereomeric thiophosphoryl chlorides and bromides 6(Cl, Br) on the one side and fluorides 6(F) on the other side. Both diastereomeric chlorides 6(Cl) and bromides 6(Br) react with nucleophilic reagents as a rule with full inversion of configuration at phosphorus. A small decrease of diastereoselectivity observed in alkaline hydrolysis and methanolysis is due to concomitant side epimerization reactions of a substrate and/or a product of the displacement reaction investigated. In the case of thiophosphoryl chlorides 6(Cl) and bromides 6(Br), the substitutions are kinetically controlled, which implies that inversion at phosphorus may be a consequence of a direct SN2-P type displacement.
In contrast to the diastereomeric chlorides 6(Cl), both diastereomeric fluorides (cis-6(F) and trans-6(F)) undergo alkaline hydrolysis, affording the same mixture of the corresponding thioacids 9 with the thermodynamically more stable thioacid cis-9 being a major product in both cases. It means that hydrolysis of cis-6(F) affords cis-9 with retention of configuration, while trans-6(F) upon hydrolysis gives the same major product with inversion of configuration. Such a stereochemical outcome of alkaline hydrolysis of both isomeric fluorides 6(F) and other observations presented above indicate that this reaction as well as methanolysis are controlled by thermodynamic factors. Taking into account well known facts that (a) fluorine forms a very strong bond with phosphorus, (b) the fluoride anion is a poor leaving group, and (c) fluorine as a substituent stabilizes pentacoordinate phosphorus and is strongly apicophilic, it is believed that nucleophilic substitution at phosphorus in thiophosphoryl fluorides 6(F) occurs according to the addition–elimination (A–E) mechanism.
2.3. DFT Studies on Mechanism-Stereochemistry Relationship inSubstitution of Cyclic 2-halogeno-4-methyl-1,3,2-dioxaphosphorinane-2-thiones 6
The conformation and structure of 1,3,2-dioxaphosphorinanes have been extensively studied by
1H-,
13C- and
31P-NMR, IR, and X-ray spectroscopies [
10,
31]. Although theoretical studies of various 1,3,2-dioxaphosphorinanes have also been published [
32,
33,
34], to the best of our knowledge, the hydrolysis reaction of 2-halogeno-4-methyl-1,3,2-dioxaphosphorinan-2-thiones
6 was not investigated by theoretical methods. Therefore, having in mind the results of our stereochemical studies and suggestions concerning the different mechanisms of the alkaline hydrolysis of both cyclic diastereomeric thiophosphoryl chlorides
6(
Cl) and fluorides
6(
F), we decided to concentrate on elucidation stereochemical and mechanistic details of this reaction.
To calculate the energy of this substitution reaction, we first performed conformational analysis of reagents (6(Cl), 6(F)) and the product, 2-hydroxy-4-methyl-1,3,2-dioxaphosphorinan-2-thione 9. In the case discussed here, the presence of the methyl group at C4 in the six-membered ring induces stereoisomerism in the molecule, which may result in a different reactivity of diastereomers towards the nucleophile. Therefore, we calculated chair and boat conformations for both cis- and trans- diastereomers of chlorides 6(Cl) and fluorides 6(F) and compared the Gibbs free energies of all stable minima.
The most stable diastereomer of
6(
Cl) was found to exist in a chair conformation with chlorine in the axial position and methyl in the equatorial position, denoted in this work as
trans-
6(
Cl). The remaining conformers are ΔG
rel = 2.5–5.6 kcal/mol higher in energy than
trans-
6(
Cl). The relative Gibbs free energies of all isomers are collected in
Table S1. These data show a strong preference for chlorine to the axial position. All isomers with chlorine occupying the equatorial position have much higher energies. Additional stabilization of
trans-
6(
Cl) is due to the methyl group in equatorial arrangement, which minimizes the steric congestion. These observations support the common knowledge about these ring systems [
10].
The stability order of conformers of
6(
F) is very similar in terms of relative stereochemistry. One should remember that due to the different priority of substituents, upon replacement of Cl for F, the most stable conformation is again that with axial fluorine and an equatorial methyl group, i.e.,
cis-
6(
F) (see
Supplementary Materials, Table S2 and Figure S2). Comparison of the calculated structure of this isomer in the aqueous solution agrees very well with the reported crystal structure [
28], which proves the reliability of the theoretical method used (
Supplementary Materials, Table S4).
The most stable conformer of
9 is that with the axial hydroxyl and equatorial C
4-methyl group, i.e.,
cis-
9, as in the case of fluorine and chlorine. However, only slightly less stable (by 0.7 kcal/mol) is the isomer
trans-
9 with both hydroxyl and methyl groups in equatorial positions (
Supplementary Materials, Table S3 and Figure S3).
DFT calculations of the alkaline hydrolysis of
trans-
6(
Cl) in water showed that indeed the preferred reaction route is the attack of hydroxide ion from the opposite side to chlorine and the ligand exchange occurs with inversion of configuration. It is a one-step reaction that proceeds via a single transition state as was found for acyclic alkoxyphosphoryl chlorides [
35,
36]. Both
trans and
cis isomers react according to the same S
N2-P mechanism and their free energy profiles are almost identical (
Figure 6).
The analogous hydrolysis of
6(
F) seems more complicated in view of the experimental results. To deeper understand the mechanistic and stereochemical details of this process, we performed analogous DFT calculations of the replacement of fluoride ion in
cis- and
trans-
6(
F) by hydroxyl anion in water solution. Anticipating that the reaction may occur stepwise according to the addition–elimination scheme [
35], we considered at first the reaction pathways starting from the approach of the nucleophile to each face of the tetrahedron formed by S, O(C
4), O(C
6), and F (
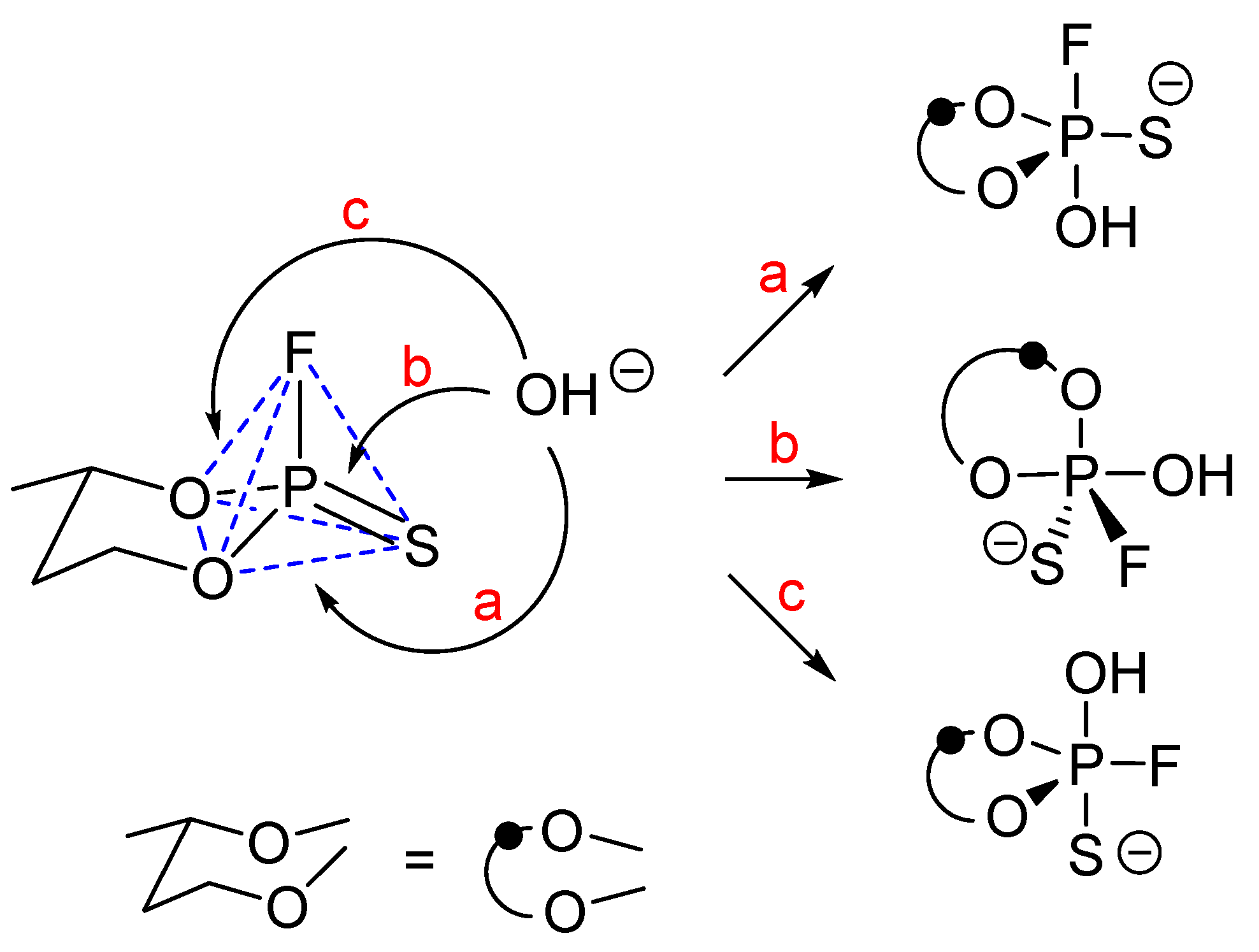
Scheme 21). The stationary points on the reaction pathway (intermediates and transition states) were identified by computing potential energy scans (PES) of the reaction. The route (
a) should lead to formation of a trigonal bipyramidal intermediate (TBPI) with OH and F in apical positions. The route (
b) consists of two directions of nucleophilic attack, from the opposite side relative to each oxygen in the ring. Energetically, both routes (
b-1 and
b-2) are almost equivalent. The route (
c) involves the attack of OH
− on phosphorus from the opposite side to sulfur (
Scheme 21).
It should be mentioned that the frontside attack, although usually associated with a much higher energy barrier than the backside attack [
37], is in this case possible as it leads to the TBPI with electronegative oxygen in the apical position. Moreover, the ring in such TBPI occupies the apical-equatorial position, which is the preferred arrangement in terms of the ring strain. In contrast to chloride substitution, it was found that all reaction routes involving fluoride
6(
F) proceed via pentacoordinate intermediates, as suggested by stereochemical studies (taking into account the high electronegativity and apicophilicity of fluorine). Below, all P
V intermediate structures identified for reaction pathways (
a)–(
c) of
cis and
trans diastereomers of
6(F) are presented in
Figure 7 and
Figure 8).
The lowest free energy barrier in the case of
cis-
6(
F) is associated with the classical backside attack of hydroxyl relative to fluorine (path
a). The side attack (
b), which places the ring in apical-equatorial position, has the barrier by ca. 1 kcal/mol higher (
Table 5). The approach of OH
− from the back direction relative to sulfur is associated with the highest free energy barrier (by 8 kcal/mol higher than (
a)), so it is the least likely. However, calculations showed that this pathway leads to the most stable TBPI, which also loses fluoride most readily to give the thioacid
9, as can be seen in
Supplementary Materials, Figure S7. Thus, from the kinetic point of view, the formation of intermediate (
a) seems preferable. Most probably, the pentacoordinate intermediate formed as a result of backside attack (
a) can undergo a sequence of reversible pseudorotations, eventually leading to a major product with retention of configuration on P, in accord with thermodynamic equilibrium [
38]. Indeed, we have identified at least one sequence of pseudorotations linking both intermediates
6(
F)-
a and
6(
F)-
c (
Scheme 14).
The reactivity of the diastereomer
trans-
6(
F) is somewhat different. The attack of OH
− from the opposite side to fluoride (
a) has the lowest free energy barrier (by 7 kcal/mol lower than the attack (
b)). The attack from the opposite side to sulfur (
c) is again the most unfavourable. This suggests that the reaction with inversion of configuration should kinetically dominate, which is in accord with observations. The thermodynamic parameters of hydrolysis of both diastereomers are presented in
Table 5 and
Table 6 and the free energy profiles for routes (
a) and (
b) of
cis- and
trans-
6(
F) hydrolysis are compared in
Figure 9. The difference in energies of the free products comes from the fact that in route (
a) the free fluoride anion is formally produced as the byproduct while in routes (
b) and (
c) it is HF and thiophosphate anion. The reaction leading to hydrogen fluoride and thiophosphate anion is much more favourable thermodynamically but in alkaline medium; this of course is meaningless as the fast equilibrium between these species is immediately established.
Route (a): >P(S)F + OH(−) → >P(S)OH + F(−), ΔG = −18.6 (cis); −19.2 kcal/mol (trans).
Route (b) and (c): >P(S)F + OH(−) → >P(S)O(−) + HF, ΔG = −41.8 (cis); −44.4 kcal/mol (trans).
The DFT calculations presented above provided convincing evidence that alkaline hydrolysis of cyclic thiophosphoryl fluorides
6(
F) proceeds according to the addition–elimination mechanism (A–E) and allowed us to rationalize the stereochemical course and thermodynamic control of this reaction. Taking into account the Westheimer’s rules (apical attack, apical departure, pseudorotation, microscopic reversibility), we formulated the most likely mechanism of the alkaline hydrolysis of fluorophosphorinans, which is presented in
Scheme 22. Thus, the attack of a hydroxyl anion on phosphorus in
cis-
6(
F) results in formation of the first trigonal bipyramidal intermediate TBPI-A with apical fluorine and OH group and diequatorial six-membered ring. It decomposes slowly with inversion of configuration to thioacid
trans-
9, which is the minor product of this reaction. The fast Berry pseudorotation of TBPI-A leads to TBPI-B, which, in turn, undergoes permutational isomerization to TBPI-C and TBPI-D. Decomposition of the latter by departure of HF is virtually barrierless (see
Supplementary Materials, Figure S7) and leads to the thermodynamically more stable thioacid
cis-
9 with retention of configuration. This thioacid is the major and stable product. In the case of hydrolysis of
trans-
6(
F), the more stable thioacid
cis-
9 is formed from the first TBPI-A’ with inversion of configuration. The thioacid
trans-
9 as the minor product of hydrolysis is formed after three consecutive pseudorotations with retention of P-configuration. As the phosphorane intermediate TBPI-B (and TBPI-B’) with the apical S are energetically unstable and could not be localized, it is possible that the transformation of TBPI-A into TBPI-C can take place directly via the tetragonal pyramidal intermediate (which may be a 2nd order saddle point) transiently formed from TBPI-A in the process of pseudorotation.