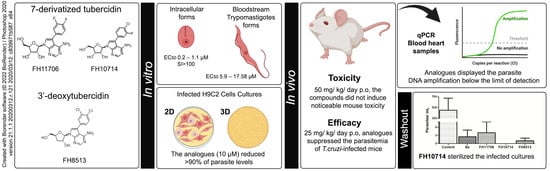
Phenotypic Evaluation of Nucleoside Analogues against Trypanosoma cruzi Infection: In Vitro and In Vivo Approaches
Abstract
1. Introduction
2. Results
2.1. In Vitro Activity and Selectivity against Intracellular Forms of T.cruzi (Tulahuen Strain)
2.2. In Vitro Activity against Bloodstream Forms of T.cruzi (Y strain)
2.3. In Vitro Cardiotoxicity
2.4. In Vitro Activity against Intracellular Forms T. cruzi (Y strain) in 2D and 3D H9C2 Cultures
2.5. In Vitro Analysis of Derivatives Uptake by TcrNT2 Thymidine Transporter
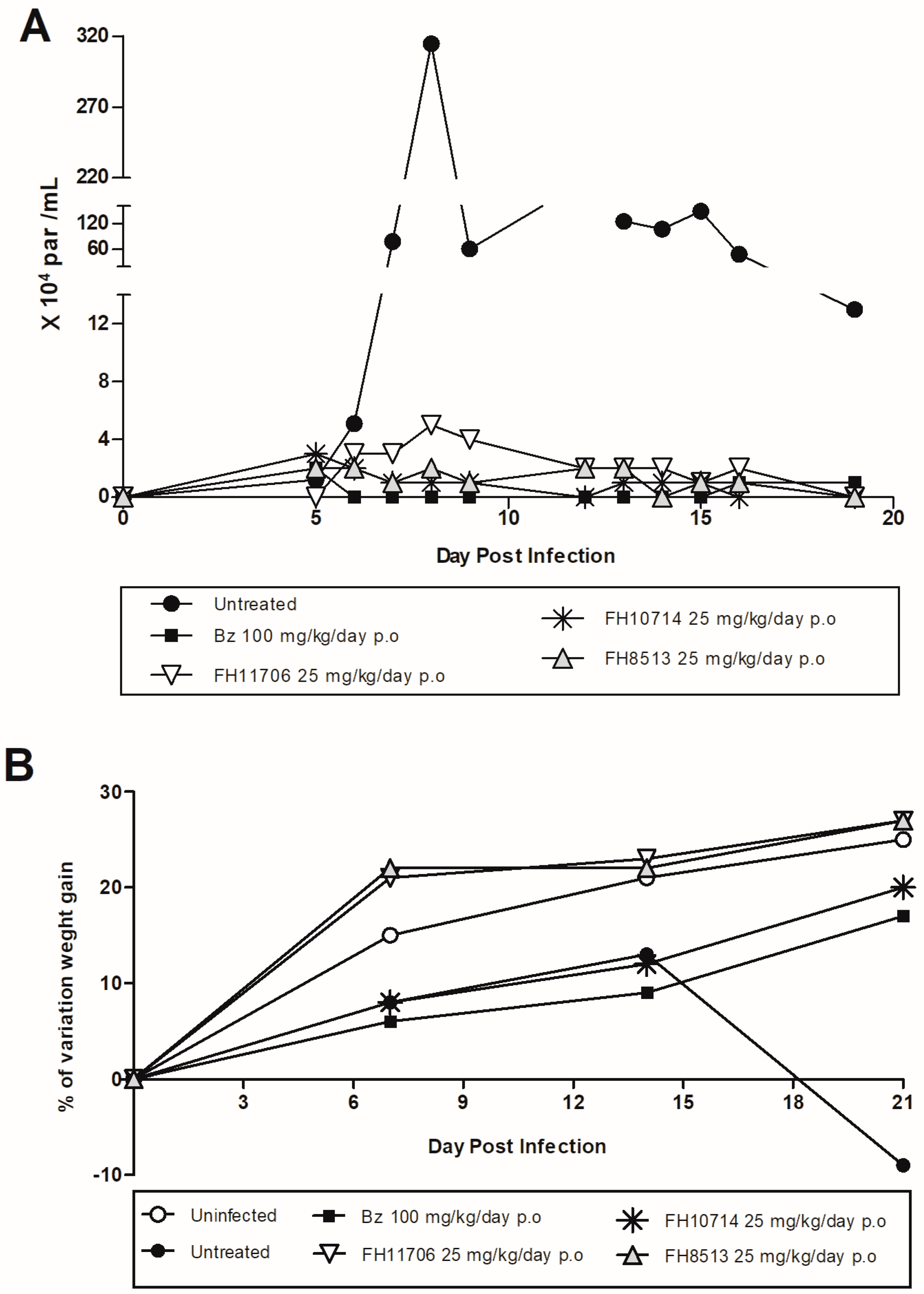
2.6. In Vivo Analysis of the Derivatives in a Mouse Model of Acute Toxicity and T. cruzi Infection
2.7. In Vitro Washout Assays in T. cruzi-Infected L929 Cultures (Tulahuen Strain)
3. Discussion
4. Conclusions
5. Material and Methods
5.1. The Studied Compounds
5.2. In Vitro Cultures of Mammalian Cells
5.3. The Obtention and Maintenance of the Parasites
5.4. The In Vitro Toxicity on Mammalian Cell Cultures
5.5. The In Vitro Activity of the Compounds against Intracellular Forms of T. cruzi (Tulahuen and Y strains) in L929 and H9C2 Monolayers, Respectively
5.6. The In Vitro Activity of the Compounds against Intracellular Forms of T. cruzi (Y strain) in H9C2 Organoids
5.7. The In Vitro Activity of the Compounds against Bloodstream Forms of T. cruzi (Y strain)
5.8. The In Vitro Activity of the Compounds against Leishmania mexicana Promastigotes
5.9. In Vitro Analysis of Derivatives Uptake by TcrNT2 Thymidine Transporter
5.10. In Vitro Washout Assays in T. cruzi-Infected L929 Cultures (Tulahuen Strain)
5.11. In Vivo Analysis of the Derivatives in a Mouse Model of Acute Toxicity and T. cruzi Infection
5.12. Statistical Analysis
5.13. Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- WHO Neglected Tropical Diseases—GLOBAL. Available online: https://www.who.int/health-topics/neglected-tropical-diseases (accessed on 9 October 2022).
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- DNDi Chagas Disease|DNDi. Available online: https://dndi.org/diseases/chagas/ (accessed on 9 October 2022).
- Hotez, P.J.; Booker, C. STOP: Study, Treat, Observe, and Prevent Neglected Diseases of Poverty Act. PLoS Negl. Trop. Dis. 2020, 14, e0008064. [Google Scholar] [CrossRef] [PubMed]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Restrepo Zambrano, M.; Rouset, F.; Carrasco, O.F.; Echeverría Murillo, D.; Costales, J.A.; Brenière, S.F. Congenital Chagas Disease in the Ecuadorian Amazon: Maternal Screening at Delivery and Evaluation of Risk Factors Associated with Vector Exposure. Am. J. Trop. Med. Hyg. 2019, 101, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J. Chagas Disease as Example of a Reemerging Parasite. Semin. Diagn. Pathol. 2019, 36, 164–169. [Google Scholar] [CrossRef]
- Coura, J.R.; Viñas, P.A.; Junqueira, A.C. Ecoepidemiology, Short History and Control of Chagas Disease in the Endemic Countries and the New Challenge for Non-Endemic Countries. Mem. Inst. Oswaldo Cruz 2014, 109, 856–862. [Google Scholar] [CrossRef]
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Marcondes de Rezende, J. American Trypanosomiasis (Chagas Disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas Disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on Experimental Treatment Strategies Against Trypanosoma cruzi. JEP J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef]
- Bahia, M.T.; Diniz, L.d.F.; Mosqueira, V.C.F. Therapeutical Approaches under Investigation for Treatment of Chagas Disease. Expert Opin. Investig. Drugs 2014, 23, 1225–1237. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Moraes, C.B.; Luquetti, A.; Guhl, F.; Schijman, A.G.; Ribeiro, I. Drug Discovery for Chagas Disease Should Consider Trypanosoma cruzi Strain Diversity. Mem. Inst. Oswaldo Cruz 2014, 109, 828–833. [Google Scholar] [CrossRef]
- Campagnaro, G.D.; De Koning, H.P. Purine and Pyrimidine Transporters of Pathogenic Protozoa—Conduits for Therapeutic Agents. Med. Res. Rev. 2020, 40, 1679–1714. [Google Scholar] [CrossRef] [PubMed]
- Campagnaro, G.D.; de Freitas Nascimento, J.; Girard, R.B.M.; Silber, A.M.; De Koning, H.P. Cloning and Characterisation of the Equilibrative Nucleoside Transporter Family of Trypanosoma cruzi: Ultra-High Affinity and Selectivity to Survive in the Intracellular Niche. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2750–2763. [Google Scholar] [CrossRef] [PubMed]
- Aldfer, M.M.; AlSiari, T.A.; Elati, H.A.A.; Natto, M.J.; Alfayez, I.A.; Campagnaro, G.D.; Sani, B.; Burchmore, R.J.S.; Diallinas, G.; De Koning, H.P. Nucleoside Transport and Nucleobase Uptake Null Mutants in Leishmania mexicana for the Routine Expression and Characterization of Purine and Pyrimidine Transporters. Int. J. Mol. Sci. 2022, 23, 8139. [Google Scholar] [CrossRef] [PubMed]
- Finley, R.W.; Cooney, D.A.; Dvorak, J.A. Nucleoside Uptake in Trypanosoma cruzi: Analysis of a Mutant Resistant to Tubercidin. Mol. Biochem. Parasitol. 1988, 31, 133–140. [Google Scholar] [CrossRef]
- Hulpia, F.; Van Hecke, K.; França da Silva, C.; da Gama Jaen Batista, D.; Maes, L.; Caljon, G.; de Nazaré, C.; Soeiro, M.; Van Calenbergh, S. Discovery of Novel 7-Aryl 7-Deazapurine 3′-Deoxy-Ribofuranosyl Nucleosides with Potent Activity against Trypanosoma cruzi. J. Med. Chem. 2018, 61, 9287–9300. [Google Scholar] [CrossRef] [PubMed]
- Bouton, J.; Ferreira de Almeida Fiuza, L.; Cardoso Santos, C.; Mazzarella, M.A.; Soeiro, M.d.N.C.; Maes, L.; Karalic, I.; Caljon, G.; Van Calenbergh, S. Revisiting Pyrazolo[3,4-d]Pyrimidine Nucleosides as Anti-Trypanosoma cruzi and Antileishmanial Agents. J. Med. Chem. 2021, 64, 4206–4238. [Google Scholar] [CrossRef]
- Lin, C.; Ferreira de Almeida Fiuza, L.; Cardoso Santos, C.; Ferreira Nunes, D.; Cruz Moreira, O.; Bouton, J.; Karalic, I.; Maes, L.; Caljon, G.; Hulpia, F.; et al. 6-Methyl-7-Aryl-7-Deazapurine Nucleosides as Anti-Trypanosoma cruzi Agents: Structure-Activity Relationship and in Vivo Efficacy. ChemMedChem 2021, 16, 2231–2253. [Google Scholar] [CrossRef]
- Cardoso-Santos, C.; Ferreira de Almeida Fiuza, L.; França da Silva, C.; Mazzeti, A.L.; Donola Girão, R.; Melo de Oliveira, G.; da Gama Jaen Batista, D.; Cruz Moreira, O.; Lins da Silva Gomes, N.; Maes, L.; et al. 7-Aryl-7-Deazapurine 3′-Deoxyribonucleoside Derivative as a Novel Lead for Chagas’ Disease Therapy: In Vitro and in Vivo Pharmacology. JAC-Antimicrob. Resist. 2021, 3, dlab168. [Google Scholar] [CrossRef]
- Hulpia, F.; Campagnaro, G.D.; Scortichini, M.; Van Hecke, K.; Maes, L.; De Koning, H.P.; Caljon, G.; Van Calenbergh, S. Revisiting Tubercidin against Kinetoplastid Parasites: Aromatic Substitutions at Position 7 Improve Activity and Reduce Toxicity. Eur. J. Med. Chem. 2019, 164, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Hulpia, F.; Mabille, D.; Campagnaro, G.D.; Schumann, G.; Maes, L.; Roditi, I.; Hofer, A.; De Koning, H.P.; Caljon, G.; Van Calenbergh, S. Combining Tubercidin and Cordycepin Scaffolds Results in Highly Active Candidates to Treat Late-Stage Sleeping Sickness. Nat. Commun. 2019, 10, 5564. [Google Scholar] [CrossRef] [PubMed]
- Vodnala, S.K.; Lundbäck, T.; Yeheskieli, E.; Sjöberg, B.; Gustavsson, A.-L.; Svensson, R.; Olivera, G.C.; Eze, A.A.; De Koning, H.P.; Hammarström, L.G.J.; et al. Structure–Activity Relationships of Synthetic Cordycepin Analogues as Experimental Therapeutics for African Trypanosomiasis. J. Med. Chem. 2013, 56, 9861–9873. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.J.M.; Candlish, D.; De Koning, H.P. Different Substrate Recognition Motifs of Human and Trypanosome Nucleobase Transporters. J. Biol. Chem. 2002, 277, 26149–26156. [Google Scholar] [CrossRef]
- Romanha, A.J.; de Castro, S.L.; Soeiro, M.d.N.C.; Lannes-Vieira, J.; Ribeiro, I.; Talvani, A.; Bourdin, B.; Blum, B.; Olivieri, B.; Zani, C.; et al. In Vitro and in Vivo Experimental Models for Drug Screening and Development for Chagas Disease. Mem. Inst. Oswaldo Cruz 2010, 105, 233–238. [Google Scholar] [CrossRef]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and Lead Criteria in Drug Discovery for Infectious Diseases of the Developing World. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef]
- Vasudevan, G.; Carter, N.S.; Drew, M.E.; Beverley, S.M.; Sanchez, M.A.; Seyfang, A.; Ullman, B.; Landfear, S.M. Cloning of Leishmania Nucleoside Transporter Genes by Rescue of a Transport-Deficient Mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 9873–9878. [Google Scholar] [CrossRef]
- Alzahrani, K.J.H.; Ali, J.A.M.; Eze, A.A.; Looi, W.L.; Tagoe, D.N.A.; Creek, D.J.; Barrett, M.P.; De Koning, H.P. Functional and Genetic Evidence That Nucleoside Transport Is Highly Conserved in Leishmania Species: Implications for Pyrimidine-Based Chemotherapy. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 206–226. [Google Scholar] [CrossRef]
- Kratz, J.M.; Gonçalves, K.R.; Romera, L.M.; Moraes, C.B.; Bittencourt-Cunha, P.; Schenkman, S.; Chatelain, E.; Sosa-Estani, S. The Translational Challenge in Chagas Disease Drug Development. Mem. Inst. Oswaldo Cruz 2022, 117, e200501. [Google Scholar] [CrossRef]
- Soeiro, M.d.N.C. Perspectives for a new drug candidate for Chagas disease therapy. Mem Inst Oswaldo Cruz. 2022, 117, e220004. [Google Scholar] [CrossRef]
- Soeiro, M.d.N.C.; de Castro, S.L. Screening of Potential Anti-Trypanosoma cruzi Candidates: In Vitro and In Vivo Studies. Open Med. Chem. J. 2011, 5, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D Platform to Explore Cell–ECM Interactions and Drug Resistance of Epithelial Ovarian Cancer Cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. JNCI J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; Bridges, D.J.; Burchmore, R.J.S. Purine and Pyrimidine Transport in Pathogenic Protozoa: From Biology to Therapy. FEMS Microbiol. Rev. 2005, 29, 987–1020. [Google Scholar] [CrossRef]
- De Koning, H.P. Pyrimidine Transporters of Trypanosomes—A Class Apart? Trends Parasitol. 2007, 23, 190. [Google Scholar] [CrossRef]
- Campagnaro, G.D.; Alzahrani, K.J.; Munday, J.C.; De Koning, H.P. Trypanosoma brucei Bloodstream Forms Express Highly Specific and Separate Transporters for Adenine and Hypoxanthine; Evidence for a New Protozoan Purine Transporter Family? Mol. Biochem. Parasitol. 2018, 220, 46–56. [Google Scholar] [CrossRef]
- Alfayez, I.A. An Investigation of 5-Fluorouracil Resistance in Leishmania and Trypanosoma Species. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2021. [Google Scholar]
- Lin, C.; Hulpia, F.; da Silva, C.F.; Batista, D.d.G.J.; Van Hecke, K.; Maes, L.; Caljon, G.; Soeiro, M.d.N.C.; Van Calenbergh, S. Discovery of Pyrrolo[2,3-b]Pyridine (1,7-Dideazapurine) Nucleoside Analogues as Anti-Trypanosoma cruzi Agents. J. Med. Chem. 2019, 62, 8847–8865. [Google Scholar] [CrossRef]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan Persister-like Cells and Drug Treatment Failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef]
- Sánchez-Valdéz, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous Dormancy Protects Trypanosoma cruzi during Extended Drug Exposure. eLife 2018, 7, e34039. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Rizzi, M.; Caeiro, L.D.; Masip, Y.E.; Perrone, A.; Sánchez, D.O.; Búa, J.; Tekiel, V. Transmigration of Trypanosoma cruzi Trypomastigotes through 3D Cultures Resembling a Physiological Environment. Cell. Microbiol. 2020, 22, e13207. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.M.; Thomas, J.; Lewis, M.D.; Cotillo, I.; Gray, D.W.; De Rycker, M. Development of Trypanosoma cruzi in Vitro Assays to Identify Compounds Suitable for Progression in Chagas’ Disease Drug Discovery. PLoS Negl. Trop. Dis. 2018, 12, e0006612. [Google Scholar] [CrossRef]
- Natto, M.J.; Hulpia, F.; Kalkman, E.R.; Baillie, S.; Alhejeli, A.; Miyamoto, Y.; Eckmann, L.; Van Calenbergh, S.; De Koning, H.P. Deazapurine Nucleoside Analogues for the Treatment of Trichomonas Vaginalis. ACS Infect. Dis. 2021, 7, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Simões-Silva, M.R.; Nefertiti, A.S.G.; De Araújo, J.S.; Batista, M.M.; Da Silva, P.B.; Bahia, M.T.; Menna-Barreto, R.S.; Pavão, B.P.; Green, J.; Farahat, A.A.; et al. Phenotypic Screening In Vitro of Novel Aromatic Amidines against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2016, 60, 4701–4707. [Google Scholar] [CrossRef] [PubMed]
- Farani, P.S.G.; Ferreira, B.I.S.; Gibaldi, D.; Lannes-Vieira, J.; Moreira, O.C. Modulation of MiR-145-5p and MiR-146b-5p Levels Is Linked to Reduced Parasite Load in H9C2 Trypanosoma cruzi Infected Cardiomyoblasts. Sci. Rep. 2022, 12, 1436. [Google Scholar] [CrossRef]
- Timm, B.L.; da Silva, P.B.; Batista, M.M.; da Silva, F.H.G.; da Silva, C.F.; Tidwell, R.R.; Patrick, D.A.; Jones, S.K.; Bakunov, S.A.; Bakunova, S.M.; et al. In Vitro and In Vivo Biological Effects of Novel Arylimidamide Derivatives against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2014, 58, 3720–3726. [Google Scholar] [CrossRef]
- Guedes-da-Silva, F.H.; Batista, D.G.J.; Meuser, M.B.; Demarque, K.C.; Fulco, T.O.; Araújo, J.S.; Da Silva, P.B.; Da Silva, C.F.; Patrick, D.A.; Bakunova, S.M.; et al. In Vitro and In Vivo Trypanosomicidal Action of Novel Arylimidamides against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2016, 60, 2425–2434. [Google Scholar] [CrossRef]
- Beneke, T.; Madden, R.; Makin, L.; Valli, J.; Sunter, J.; Gluenz, E. A CRISPR Cas9 High-Throughput Genome Editing Toolkit for Kinetoplastids. R. Soc. Open Sci. 2017, 4, 170095. [Google Scholar] [CrossRef]
- Al-Salabi, M.I.; Wallace, L.J.M.; De Koning, H.P. A Leishmania major Nucleobase Transporter Responsible for Allopurinol Uptake Is a Functional Homolog of the Trypanosoma brucei H2 Transporter. Mol. Pharm. 2003, 63, 814–820. [Google Scholar] [CrossRef]
- Tetaud, E.; Lecuix, I.; Sheldrake, T.; Baltz, T.; Fairlamb, A.H. A New Expression Vector for Crithidia fasciculata and Leishmania. Mol. Biochem. Parasitol. 2002, 120, 195–204. [Google Scholar] [CrossRef]
- de Almeida Fiuza, L.F.; Batista, D.d.G.J.; Nunes, D.F.; Moreira, O.C.; Cascabulho, C.; Soeiro, M.d.N.C. Benznidazole Modulates Release of Inflammatory Mediators by Cardiac Spheroids Infected with Trypanosoma cruzi. Exp. Parasitol. 2021, 221, 108061. [Google Scholar] [CrossRef] [PubMed]
- Nefertiti, A.S.G.; Batista, M.M.; Da Silva, P.B.; Batista, D.G.J.; Da Silva, C.F.; Peres, R.B.; Torres-Santos, E.C.; Cunha-Junior, E.F.; Holt, E.; Boykin, D.W.; et al. In Vitro and In Vivo Studies of the Trypanocidal Effect of Novel Quinolines. Antimicrob. Agents Chemother. 2018, 62, e01936-17. [Google Scholar] [CrossRef]
- Santos, C.C.; Zhang, H.; Batista, M.M.; de Oliveira, G.M.; Demarque, K.C.; da Silva, N.L.; Moreira, O.C.; Ogungbe, I.V.; Soeiro, M. de N.C. Phenotypic Investigation of 4-Nitrophenylacetyl- and 4-Nitro-1 H -Imidazoyl-Based Compounds as Antileishmanial Agents. Parasitology 2022, 149, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Bombaça, A.C.S.; Dias, F.d.A.; Ennes-Vidal, V.; Garcia-Gomes, A.d.S.; Sorgine, M.H.F.; d’Avila-Levy, C.M.; Menna-Barreto, R.F.S. Hydrogen Peroxide Resistance in Strigomonas Culicis: Effects on Mitochondrial Functionality and Aedes Aegypti Interaction. Free Radic. Biol. Med. 2017, 113, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Almeida Fiuza, L.; Peres, R.B.; Simões-Silva, M.R.; da Silva, P.B.; Batista, D.d.G.J.; da Silva, C.F.; Nefertiti Silva da Gama, A.; Krishna Reddy, T.R.; Soeiro, M.d.N.C. Identification of Pyrazolo[3,4-e][1,4]Thiazepin Based CYP51 Inhibitors as Potential Chagas Disease Therapeutic Alternative: In Vitro and in Vivo Evaluation, Binding Mode Prediction and SAR Exploration. Eur. J. Med. Chem. 2018, 149, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Rodenko, B.; Wanner, M.J.; Alkhaldi, A.A.M.; Ebiloma, G.U.; Barnes, R.L.; Kaiser, M.; Brun, R.; McCulloch, R.; Koomen, G.-J.; De Koning, H.P. Targeting the Parasite’s DNA with Methyltriazenyl Purine Analogs Is a Safe, Selective, and Efficacious Antitrypanosomal Strategy. Antimicrob. Agents Chemother. 2015, 59, 6708–6716. [Google Scholar] [CrossRef]
- Duffy, T.; Cura, C.I.; Ramirez, J.C.; Abate, T.; Cayo, N.M.; Parrado, R.; Bello, Z.D.; Velazquez, E.; Muñoz-Calderon, A.; Juiz, N.A.; et al. Analytical Performance of a Multiplex Real-Time PCR Assay Using TaqMan Probes for Quantification of Trypanosoma cruzi Satellite DNA in Blood Samples. PLoS Negl. Trop. Dis. 2013, 7, e2000. [Google Scholar] [CrossRef]
- Guedes-da-Silva, F.H.; Batista, D.G.J.; Da Silva, C.F.; De Araújo, J.S.; Pavão, B.P.; Simões-Silva, M.R.; Batista, M.M.; Demarque, K.C.; Moreira, O.C.; Britto, C.; et al. Antitrypanosomal Activity of Sterol 14α-Demethylase (CYP51) Inhibitors VNI and VFV in the Swiss Mouse Models of Chagas Disease Induced by the Trypanosoma cruzi Y Strain. Antimicrob. Agents Chemother. 2017, 61, e02098-16. [Google Scholar] [CrossRef]



| Compound | Activity on Intracellular Forms | Toxicity on L929 IC50, µM | SI * | |
|---|---|---|---|---|
| (EC50, µM) | (EC90, µM) | |||
| Bz | 1.97 (1.36 to 2.86) | 5.54 (2.66 to 8.42) | >200 | >101.5 |
| FH11706 (1) | 1.11 (0.62 to 1.97) | >30 | 118.5 (75.31 to 186.4) | 106.7 |
| FH10714 (2) | 0.47 (0.24 to 0.93) | 6.19 (0.58 to 12.96) | 55.75 (48.37 to 64.25) | 118.6 |
| FH8513 (3) | 0.20 (0.10 to 0.41) | 1.61 (0.32 to 2.90) | 22.27 (13.63 to 36.38) | 111.3 |
| Compound | Activity upon Bloodstream Forms | H9C2 IC50 µM | SI * | |
|---|---|---|---|---|
| EC50, µM | EC90, µM | |||
| Bz | 6.9 (3.9 to 12.5) | 19.62 (11.5 to 33.5) | >300 | >43.48 |
| FH11706 (1) | 17.58 (12.7 to 24.4) | >30 | >300 | >17.06 |
| FH10714 (2) | 13.40 (9.1 to 19.7) | >30 | >300 | >22.39 |
| FH8513 (3) | 5.9 (3.8 to 9.3) | 16.73 (5.3 to 53) | 267.5 (235.1 to 304.4) | 45.34 |
| Compounds | IC50 (µM) | |
|---|---|---|
| 2D | 3D | |
| Bz | >200 | >200 |
| Pt | 45.98 (15.39 to 137.4) | >200 |
| FH11706 (1) | 47.71 (32.62 to 69.79) | >200 |
| FH10714 (2) | 41.82 (25.23 to 69.30) | 180.8 (153.6 to 208) |
| FH8513 (3) | 18.57 (11.36 to 30.36) | 199.8 (120.4 to 331.4) |
| Compound | % of Death Rate by Light Microscopy Quantification | % of Death Rate by qPCR 3D | |
|---|---|---|---|
| 2D | 3D | ||
| Bz | 100 | 98.4 (98.1 to 98.7) | 100 |
| FH11706 (1) | 99.25 (98.83 to 99.67) | 91.6 (86.2 to 97) | 85 (79 to 91) |
| FH10714 (2) | 100 | 93.5 (93.46 to 93.54) | 67 (38 to 96) |
| FH8513 (3) | 99.14 (97.9 to 100) | 83.8 (67.5 to 100) | 94 (90 to 98) |
| Cas9 | Cas9ΔNT1 | LmexΔNT1+TcrNT2 | ||||
|---|---|---|---|---|---|---|
| EC50 (μM) | EC50 (μM) | EC50 (μM) | Sensitization (fold) | p vs. Cas9ΔNT1 | ||
| Tubercidin | 1.02 ± 0.16 | 25.7 ± 0.94 | 0.82 ± 0.13 | 31.2 | <0.001 |  |
| 1 (FH11706) | >100 | >100 | ND |  | ||
| 2 (FH10714) | >100 | >100 | >100 |  | ||
| TH1012 | 52.5 ± 0.3 | 280 ± 18 | 96.6 ± 0.3 | 2.9 | <0.001 |  |
| FH3147 | 8.41 ± 1.61 | >100 | 13.7 ± 1.25 | 7.3 | <0.001 |  |
| FH3169 | 0.24 ± 0.06 | 2.00 ± 0.19 | 0.32 ± 0.05 | 6.3 | <0.01 |  |
| TH1004 | 37.0 ± 0.23 | 138 ± 10 | 101 ± 0.5 | 1.37 | <0.05 |  |
| 3 (FH8513) | >100 | 100.5 ± 0.9 | 31.1 ± 0.5 | 3.2 | <0.01 |  |
| Pentamidine | 0.87 ± 0.10 | 1.25 ± 0.22 | 1.12 ± 0.17 | 1.11 | >0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiuza, L.F.d.A.; Batista, D.G.J.; Girão, R.D.; Hulpia, F.; Finamore-Araújo, P.; Aldfer, M.M.; Elmahallawy, E.K.; De Koning, H.P.; Moreira, O.; Van Calenbergh, S.; et al. Phenotypic Evaluation of Nucleoside Analogues against Trypanosoma cruzi Infection: In Vitro and In Vivo Approaches. Molecules 2022, 27, 8087. https://doi.org/10.3390/molecules27228087
Fiuza LFdA, Batista DGJ, Girão RD, Hulpia F, Finamore-Araújo P, Aldfer MM, Elmahallawy EK, De Koning HP, Moreira O, Van Calenbergh S, et al. Phenotypic Evaluation of Nucleoside Analogues against Trypanosoma cruzi Infection: In Vitro and In Vivo Approaches. Molecules. 2022; 27(22):8087. https://doi.org/10.3390/molecules27228087
Chicago/Turabian StyleFiuza, Ludmila F. de A., Denise G. J. Batista, Roberson D. Girão, Fabian Hulpia, Paula Finamore-Araújo, Mustafa M. Aldfer, Ehab Kotb Elmahallawy, Harry P. De Koning, Otacílio Moreira, Serge Van Calenbergh, and et al. 2022. "Phenotypic Evaluation of Nucleoside Analogues against Trypanosoma cruzi Infection: In Vitro and In Vivo Approaches" Molecules 27, no. 22: 8087. https://doi.org/10.3390/molecules27228087
APA StyleFiuza, L. F. d. A., Batista, D. G. J., Girão, R. D., Hulpia, F., Finamore-Araújo, P., Aldfer, M. M., Elmahallawy, E. K., De Koning, H. P., Moreira, O., Van Calenbergh, S., & Soeiro, M. d. N. C. (2022). Phenotypic Evaluation of Nucleoside Analogues against Trypanosoma cruzi Infection: In Vitro and In Vivo Approaches. Molecules, 27(22), 8087. https://doi.org/10.3390/molecules27228087