Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement
Abstract
:1. Introduction and Aims
2. Histidine, Chemical, and Biological Properties
2.1. HIS as a pH Buffer
2.2. HIS and Metal Ion Chelation
2.3. HIS as an Antioxidant
3. HIS Requirements and Sources
3.1. Effects of a HIS-Deficient Diet
3.2. Requirements and Sources of Dietary HIS
4. HIS Metabolism
4.1. Catabolism of HIS
4.1.1. HIS Catabolism in the Skin
4.1.2. HIS Catabolism in the Liver
4.1.3. Role of HIS Aminotransferase
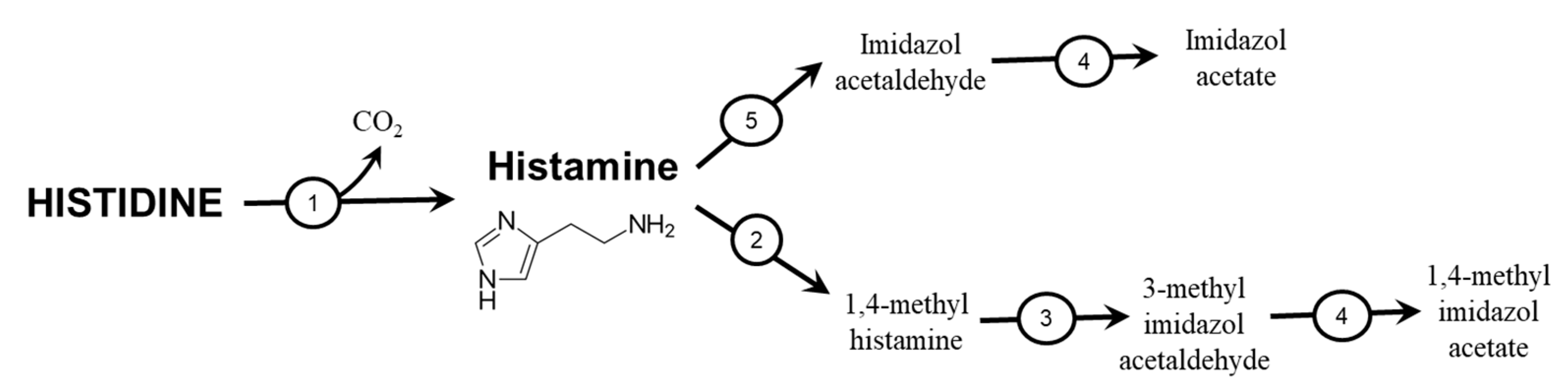
4.2. Histamine
Effects of Dietary HIS on Histamine Levels
4.3. Methyl- and Sulphur-Containing Derivatives of HIS
4.3.1. 3-Methylhistidine (3-MH)
4.3.2. 1-Methylhistidine (1-MH)
4.3.3. Ergothioneine
4.4. HIS-Rich Proteins and Peptides
4.5. HIS-Containing Dipeptides (HIS-CD)
4.5.1. L-Carnosine (CAR)
4.5.2. Homocarnosine
4.5.3. Other HIS-CD
5. HIS and HIS-Containing Substances as Nutritional Supplements
5.1. Effects on Muscle Performance and Fatigue
5.2. Effects on Neurodegenerative and Age-Related Disorders
5.3. Metabolic Syndrome
5.4. Rheumatoid Arthritis
5.5. Inflammatory Bowel Disease
5.6. Organ Preservation for Transplantation and Myocardial Protection in Cardiac Surgery
5.7. Modulation of the Sensitivity of Cancer Cells to Methotrexate
5.8. Atopic Dermatitis
5.9. Anaemia of Patients with Uraemia
6. Side Effects of Increased HIS Intake
7. Summary and Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Pinals, R.S.; Harris, E.D.; Burnett, J.B.; Gerber, D.A. Treatment of rheumatoid arthritis with L-histidine: A randomized, placebo-controlled, double-blind trial. J. Rheumatol. 1977, 4, 414–419. [Google Scholar] [PubMed]
- Giordano, C.; De Santo, N.G.; Rinaldi, S.; Acone, D.; Esposito, R.; Gallo, B. Histidine for treatment of uraemic anaemia. Br. Med. J. 1973, 4, 714–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.P.; Brown, S.B.; Griffiths, C.E.; Weller, R.B.; Gibbs, N.K. Feeding filaggrin: Effects of l-histidine supplementation in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNicolantonio, J.J.; McCarty, M.F.; OKeefe, J.H. Role of dietary histidine in the prevention of obesity and metabolic syndrome. Open Heart 2018, 5, e000676. [Google Scholar] [CrossRef] [Green Version]
- Matsukura, T.; Tanaka, H. Applicability of zinc complex of L-carnosine for medical use. Biochemistry (Mosc.) 2000, 65, 817–823. [Google Scholar]
- Babizhayev, M.A.; Guiotto, A.; Kasus-Jacobi, A. N-Acetylcarnosine and histidyl-hydrazide are potent agents for multitargeted ophthalmic therapy of senile cataracts and diabetic ocular complications. J. Drug. Target 2009, 17, 36–63. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of anserine/carnosine supplementation on verbal episodic memory in elderly people. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.; Fedorova, T.; Stepanova, M.; Dobrotvorskaya, I.; Kozlova, E.; Boldanova, N.; Bagyeva, G.; Ivanova-Smolenskaya, I.; Illarioshkin, S. Carnosine [corrected] increases efficiency of DOPA therapy of Parkinson’s disease: A pilot study. Rejuvenation Res. 2008, 11, 821–827. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakao, T.; Maemura, H.; Sato, M.; Kamahara, K.; Morimatsu, F.; Takamatsu, K. Carnosine and anserine ingestion enhances contribution of nonbicarbonate buffering. Med. Sci. Sports Exerc. 2006, 38, 334–338. [Google Scholar]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef] [Green Version]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc.) 2000, 65, 757–765. [Google Scholar] [PubMed]
- Edelman, J.J.; Seco, M.; Dunne, B.; Matzelle, S.J.; Murphy, M.; Joshi, P.; Yan, T.D.; Wilson, M.K.; Bannon, P.G.; Vallely, M.P.; et al. Custodiol for myocardial protection and preservation: A systematic review. Ann. Cardiothorac. Surg. 2013, 2, 717–728. [Google Scholar] [PubMed]
- Kahn, J.; Schemmer, P. Comprehensive review on Custodiol-N (HTK-N) and its molecular side of action for organ preservation. Curr. Pharm. Biotechnol. 2017, 18, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.; Yang, J.; Gong, C.; Huang, R.; Wang, Q.; Shen, J. Systematic review of preservation solutions for allografts for liver transplantation based on a network meta-analysis. Int. J. Surg. 2018, 54 Pt A, 1–6. [Google Scholar] [CrossRef]
- Poon, I.K.; Patel, K.K.; Davis, D.S.; Parish, C.R.; Hulett, M.D. Histidine-rich glycoprotein: The Swiss Army knife of mammalian plasma. Blood 2011, 117, 2093–2101. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.A.; Aldini, G.; Wim, D. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Smolik, S.; Nogaj, P.; Szpakowska, A.; Lodowska, J.; Weglarz, L. The role of amino acids supplementation of protective Na2H2EDTA containing ointments in chelation of allergenic metal ions. Acta Pol. Pharm. 2010, 67, 737–740. [Google Scholar]
- Trombley, P.Q.; Horning, M.S.; Blakemore, L.J. Interactions between carnosine and zinc and copper: Implications for neuromodulation and neuroprotection. Biochemistry (Mosc.) 2000, 65, 807–816. [Google Scholar]
- Dahl, T.A.; Midden, W.R.; Hartman, P.E. Some prevalent biomolecules as defenses against singlet oxygen damage. Photochem. Photobiol. 1988, 47, 357–362. [Google Scholar] [CrossRef]
- Hartman, P.E.; Hartman, Z.; Ault, K.T. Scavenging of singlet molecular oxygen by imidazole compounds: High and sustained activities of carboxy terminal histidine dipeptides and exceptional activity of imidazole-4-acetic acid. Photochem. Photobiol. 1990, 51, 59–66. [Google Scholar] [CrossRef]
- Chan, W.K.; Decker, E.A.; Chow, C.K.; Boissonneault, G.A. Effect of dietary carnosine on plasma and tissue antioxidant concentrations and on lipid oxidation in rat skeletal muscle. Lipids 1994, 29, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. On the enigma of carnosine’s anti-ageing actions. Exp. Gerontol. 2009, 44, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-carnosine and its derivatives as new therapeutic agents for the prevention and treatment of vascular complications of diabetes. Curr. Med. Chem 2019. [Google Scholar] [CrossRef] [PubMed]
- Song, B.C.; Joo, N.-S.; Aldini, G.; Yeum, K.-J. Biological functions of histidine-dipeptides and metabolic syndrome. Nutr. Res. Pract. 2014, 8, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Ihara, H.; Kakihana, Y.; Yamakage, A.; Kai, K.; Shibata, T.; Nishida, M.; Yamda, K.-I.; Uchida, K. 2-Oxo-histidine-containing dipeptides are functional oxidation products. J. Biol. Chem. 2019, 294, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Rose, W.C.; Haines, W.J.; Warner, D.T.; Johnson, J.E. The amino acid requirements of man. II. The role of threonine and histidine. J. Biol. Chem. 1951, 188, 49–58. [Google Scholar]
- Kopple, J.D.; Swendseid, M.E. Evidence that histidine is an essential amino acid in normal and chronically uremic man. J. Clin. Investig. 1975, 55, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Kopple, J.D.; Swendseid, M.E. Effect of histidine intake of plasma and urine histidine levels, nitrogen balance and N tau-methylhistidine excretion in normal and chronically uremic men. J. Nutr. 1981, 111, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Clemens, R.A.; Kopple, J.D.; Swendseid, M.E. Metabolic effects of histidine-deficient diets fed to growing rats by gastric tube. J. Nutr. 1984, 114, 2138–2146. [Google Scholar] [CrossRef]
- Anderson, H.L.; Soon Cho, E.; Krause, P.A.; Hanson, K.C.; Krause, G.F.; Wixom, R.L. Effects of dietary histidine and arginine on nitrogen retention of men. J. Nutr. 1977, 107, 2067–2077. [Google Scholar] [CrossRef]
- Cho, E.S.; Anderson, H.L.; Wixom, R.L.; Hanson, K.C.; Krause, G.F. Long-term effects of low histidine intake on men. J. Nutr. 1984, 114, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Nasset, E.S.; Gatewood, V.H. Nitrogen balance and hemoglobin of adult rats fed amino acid diets low in L- and D-histidine. J. Nutr. 1954, 53, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Bendsen, N.T.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B16–B31. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, I.; Fujimura, N.; Nozawa, Y.; Furuhata, Y.; Sato, H. The effect of histidine on mental fatigue and cognitive performance in subjects with high fatigue and sleep disruption scores. Physiol. Behav. 2015, 147, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Currell, K.; Derave, W.; Everaert, I.; McNaughton, L.; Slater, G.; Burke, L.M.; Stear, S.J.; Castell, L.M. A-Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance-Part 20. Br. J. Sports Med. 2011, 45, 530–532. [Google Scholar] [CrossRef] [Green Version]
- Nozawa, Y.; Ishizaki, T.; Kuroda, M.; Noguchi, T. Effect of dried-bonito broth intake on peripheral blood flow, mood, and oxidative stress marker in humans. Physiol. Behav. 2008, 93, 267–273. [Google Scholar] [CrossRef]
- Kuroda, M.; Ishizaki, T.; Maruyama, T.; Takatsuka, Y.; Kuboki, T. Effect of dried-bonito broth on mental fatigue and mental task performance in subjects with a high fatigue score. Physiol. Behav. 2007, 92, 957–962. [Google Scholar] [CrossRef]
- Baslow, M.H.; Guilfoyle, D.N. N-acetyl-L-histidine, a prominent biomolecule in brain and eye of poikilothermic vertebrates. Biomolecules 2015, 5, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M.; Vodeničarovová, M. Effects of histidine load on ammonia, amino acid, and adenine nucleotide concentrations in rats. Amino Acids 2019, 51, 1667–1680. [Google Scholar]
- Steinert, P.M.; Cantieri, J.S.; Teller, D.C.; Lonsdale-Eccles, J.D.; Dale, B.A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc. Natl. Acad. Sci. USA 1981, 78, 4097–4101. [Google Scholar] [CrossRef] [Green Version]
- Hug, D.H.; Hunter, J.K.; Dunkerson, D.D. The potential role for urocanic acid and sunlight in the immune suppression associated with protein malnutrition. J. Photochem. Photobiol. B. 1998, 44, 117–123. [Google Scholar] [CrossRef]
- Kang-Lee, Y.A.; Harper, A.E. Effect of induction of histidase on histidine metabolism in vivo. J. Nutr. 1979, 109, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Martínez, L.; Alemán, G.; Bourges, H.; Tovar, A.R. Histidase expression is regulated by dietary protein at the pretranslational level in rat liver. J. Nutr. 1998, 128, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luhby, A.L.; Cooperman, J.M.; Teller, D.N. Urinary excretion of formiminoglutamic acid: Application in diagnosis of clinical folic acid deficiency. Am. J. Clin. Nutr. 1959, 7, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, M.; Gardine, R.C.; Condit, P.T. A method for the detection of N-formiminoglutamic acid in urine. J. Natl. Cancer Inst. 1958, 20, 71–77. [Google Scholar]
- Meléndez-Hevia, E.; De Paz-Lugo, P.; Cornish-Bowden, A.; Cárdenas, M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009, 34, 853–872. [Google Scholar]
- Fell, D.; Steele, R.D. Effect of methionine on in vivo histidine metabolism in rats. J. Nutr. 1983, 113, 860–866. [Google Scholar] [CrossRef]
- Billings, R.E.; Noker, P.E.; Tephly, T.R. The role of methionine in regulating folate-dependent reactions in isolated rat hepatocytes. Arch. Biochem. Biophys. 1981, 208, 108–120. [Google Scholar] [CrossRef]
- Cynober, L. Metabolism of dietary glutamate in adults. Ann. Nutr. Metab. 2018, 73, 5–14. [Google Scholar] [CrossRef]
- Noguchi, T.; Minatogawa, Y.; Okuno, E.; Kido, R. Organ distribution of rat histidine-pyruvate aminotransferase isoenzymes. Biochem. J. 1976, 157, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. (1985). 2017, 122, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Schayer, R.W. Histamine and autonomous responses of the microcirculation; relationship to glucocorticoid action. Ann. N. Y. Acad. Sci. 1964, 116, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Ayada, K.; Tsuchiya, M.; Yoneda, H.; Yamaguchi, K.; Kumamoto, H.; Sasaki, S.; Tadano, T.; Watanabe, M.; Endo, Y. Induction of the histamine-forming enzyme histidine decarboxylase in skeletal muscles by prolonged muscular work: Histological demonstration and mediation by cytokines. Biol. Pharm. Bull. 2017, 40, 1326–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savany, A.; Cronenberger, L. Properties of histidine decarboxylase from rat gastric mucosa. Eur. J. Biochem. 1982, 123, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Moon, P.D.; Kim, S.J.; Seo, J.U.; Kang, T.H.; Kim, I.J.; Kang, I.C.; Um, J.Y.; Kim, H.M.; Hong, S.H. Activation of hypoxia-inducible factor-1 regulates human histidine decarboxylase expression. Cell. Mol. Life. Sci. 2009, 66, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Maejima, D.; Nagai, T.; Meininger, C.J.; Gashev, A.A. Histamine as an endothelium-derived relaxing factor in aged mesenteric lymphatic vessels. Lymphat. Res. Biol. 2017, 15, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.S.; Fitzpatrick, D.; Meier, E.; Fisher, H. Influence of dietary histidine on tissue histamine concentration, histidine decarboxylase and histamine methyltransferase activity in the rat. Agents Actions 1981, 11, 307–311. [Google Scholar] [CrossRef]
- Martin, S.K.; Harmon, D.L.; Conway, C.E.; Vanzant, E.S.; McLeod, K.R. Influence of dietary histidine on basophil release, circulating concentration, and urinary excretion of histamine in domestic felines. J. Appl. Res. Vet. Med. 2012, 10, 289–299. [Google Scholar]
- Lozeva, V.; Tarhanen, J.; Attila, M.; Männistö, P.T.; Tuomisto, L. Brain histamine and histamine H3 receptors following repeated L-histidine administration in rats. Life Sci. 2003, 73, 1491–1503. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Nakamura, T.; Shibakusa, T.; Sugita, M.; Naganuma, F.; Iida, T.; Miura, Y.; Mohsen, A.; Harada, R.; Yanai, K. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J. Nutr. 2014, 144, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Kasaoka, S.; Tsuboyama-Kasaoka, N.; Kawahara, Y.; Inoue, S.; Tsuji, M.; Ezaki, O.; Kato, H.; Tsuchiya, T.; Okuda, H.; Nakajima, S. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition 2004, 20, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kasaoka, S.; Takizawa, M.; Ogawa, M.; Tsuchiya, T.; Nakajima, S. Bitter taste and blood glucose are not involved in the suppressive effect of dietary histidine on food intake. Neurosci. Lett. 2007, 420, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Tanaka, K.; Fujimi, T.J.; Kanzawa, N.; Nakajima, S. Proline decreases the suppressive effect of histidine on food intake and fat accumulation. J. Nutr. Sci. Vitaminol. (Tokyo) 2016, 62, 277–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okusha, Y.; Hirai, Y.; Maezawa, H.; Hisadome, K.; Inoue, N.; Yamazaki, Y.; Funahashi, M. Effects of intraperitoneally administered L-histidine on food intake, taste, and visceral sensation in rats. J. Physiol. Sci. 2017, 67, 467–474. [Google Scholar] [CrossRef]
- Sheiner, J.B.; Morris, P.; Anderson, G.H. Food intake suppression by histidine. Pharmacol. Biochem. Behav. 1985, 23, 721–726. [Google Scholar] [CrossRef]
- Vaziri, P.; Dang, K.; Anderson, G.H. Evidence for histamine involvement in the effect of histidine loads on food and water intake in rats. J. Nutr. 1997, 127, 1519–1526. [Google Scholar] [CrossRef] [Green Version]
- Yoshimatsu, H.; Chiba, S.; Tajima, D.; Akehi, Y.; Sakata, T. Histidine suppresses food intake through its conversion into neuronal histamine. Exp. Biol. Med. (Maywood) 2002, 227, 63–68. [Google Scholar] [CrossRef]
- Nagasawa, T.; Yoshizawa, F.; Nishizawa, N. Plasma N tau-methylhistidine concentration is a sensitive index of myofibrillar protein degradation during starvation in rats. Biosci. Biotechnol. Biochem. 1996, 60, 501–502. [Google Scholar] [CrossRef] [Green Version]
- Myint, T.; Fraser, G.E.; Lindsted, K.D.; Knutsen, S.F.; Hubbard, R.W.; Bennett, H.W. Urinary 1-methylhistidine is a marker of meat consumption in Black and in White California Seventh-day Adventists. Am. J. Epidemiol. 2000, 152, 752–755. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta 2012, 1822, 784–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotgia, S.; Zinellu, A.; Mangoni, A.A.; Pintus, G.; Attia, J.; Carru, C.; McEvoy, M. Clinical and biochemical correlates of serum L-ergothioneine concentrations in community-dwelling middle-aged and older adults. PLoS ONE 2014, 9, e84918. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Saiki, S.; Okuzumi, A.; Mohney, R.P.; Hattori, N. Identification of novel biomarkers for Parkinson’s disease by metabolomic technologies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.; Spézia, F.; Papineau, D.; Sabadie, C.; Erdelmeier, I.; Moutet, M.; Yadan, J.C. Reproductive safety evaluation of L-Ergothioneine. Food Chem. Toxicol. 2015, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—a diet—derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berenbrink, M. Evolution of vertebrate haemoglobins: Histidine side chains, specific buffer value and Bohr effect. Respir. Physiol. Neurobiol. 2006, 154, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Santone, C.; Di Nardo, P.; Sarkar, B. Histatins: Salivary peptides with copper(II)- and zinc(II)-binding motifs: Perspectives for biomedical applications. FEBS J. 2014, 281, 657–672. [Google Scholar] [CrossRef]
- Arvanitis, D.A.; Vafiadaki, E.; Johnson, D.M.; Kranias, E.G.; Sanoudou, D. The histidine-rich calcium binding protein in regulation of cardiac rhythmicity. Front. Physiol. 2018, 9, 1379. [Google Scholar] [CrossRef]
- González-Estrada, M.T.; Freeman, W.J. Effects of carnosine on olfactory bulb EEG, evoked potentials and DC potentials. Brain Res. 1980, 202, 373–386. [Google Scholar]
- Hill, C.A.; Harris, R.C.; Kim, H.J.; Harris, B.D.; Sale, C.; Boobis, L.H.; Kim, C.K.; Wise, J.A. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 2007, 32, 225–233. [Google Scholar] [CrossRef]
- Harris, R.C.; Wise, J.A.; Price, K.A.; Kim, H.J.; Kim, C.K.; Sale, C. Determinants of muscle carnosine content. Amino Acids 2012, 43, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teufel, M.; Saudek, V.; Ledig, J.-P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, K. Carnosine and homocarnosine, the forgotten, enigmatic peptides of the brain. Neurochem. Res. 2005, 30, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Volpe, S.L.; Decker, E.A. Quantitation of carnosine in humans plasma after dietary consumption of beef. J. Agric. Food. Chem. 2005, 53, 4736–4739. [Google Scholar] [CrossRef]
- Jackson, M.C.; Scollard, D.M.; Mack, R.J.; Lenney, J.F. Localization of a novel pathway for the liberation of GABA in the human CNS. Brain Res. Bull. 1994, 33, 379–385. [Google Scholar] [CrossRef]
- Drapała, A.; Bielińska, K.; Konopelski, P.; Pączek, L.; Ufnal, M. His-Leu, an angiotensin I-derived peptide, does not affect haemodynamics in rats. J. Renin Angiotensin Aldosterone Syst. 2018, 19, 1470320318808879. [Google Scholar]
- Feng, R.N.; Niu, Y.C.; Sun, X.W.; Li, Q.; Zhao, C.; Wang, C.; Guo, F.C.; Sun, C.H.; Li, Y. Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: A randomised controlled trial. Diabetologia 2013, 56, 985–994. [Google Scholar] [CrossRef]
- del Favero, S.; Roschel, H.; Solis, M.Y.; Hayashi, A.P.; Artioli, G.G.; Otaduy, M.C.; Benatti, F.B.; Harris, R.C.; Wise, J.A.; Leite, C.C.; et al. Beta-alanine (Carnosyn™) supplementation in elderly subjects (60–80 years): Effects on muscle carnosine content and physical capacity. Amino Acids 2012, 43, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Blancquaert, L.; Everaert, I.; Missinne, M.; Baguet, A.; Stegen, S.; Volkaert, A.; Petrovic, M.; Vervaet, C.; Achten, E.; DE Maeyer, M.; et al. Effects of histidine and β-alanine supplementation on human muscle carnosine storage. Med. Sci. Sports Exerc. 2017, 49, 602–609. [Google Scholar] [CrossRef]
- Varanoske, A.N.; Hoffman, J.R.; Church, D.D.; Coker, N.A.; Baker, K.M.; Dodd, S.J.; Oliveira, L.P.; Dawson, V.L.; Wang, R.; Fukuda, D.H.; et al. β-Alanine supplementation elevates intramuscular carnosine content and attenuates fatigue in men and women similarly but does not change muscle l-histidine content. Nutr. Res. 2017, 48, 16–25. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; LaMacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports. Nutr. 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.L.; Barnett, C.T.; Davidson, J.; Maritza, B.; Fraser, W.D.; Harris, R.; Sale, C. β-alanine supplementation improves in-vivo fresh and fatigued skeletal muscle relaxation speed. Eur. J. Appl. Physiol. 2017, 117, 867–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Andrade Kratz, C.; de Salles Painelli, V.; de Andrade Nemezio, K.M.; da Silva, R.P.; Franchini, E.; Zagatto, A.M.; Gualano, B.; Artioli, G.G. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J. Sci. Med. Sport 2017, 20, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Berti Zanella, P.; Donner Alves, F.; Guerini de Souza, C. Effects of beta-alanine supplementation on performance and muscle fatigue in athletes and non-athletes of different sports: A systematic review. J. Sports Med. Phys. Fitness 2017, 57, 1132–1141. [Google Scholar]
- Lim, J.K.; Narang, P.K.; Overman, D.O.; Jacknowitz, A.I. Beneficial effects of methionine and histidine in aspirin solutions on gastric mucosal damage in rats. J. Pharm. Sci. 1979, 68, 295–298. [Google Scholar] [CrossRef]
- Adachi, N.; Liu, K.; Arai, T. Prevention of brain infarction by postischemic administration of histidine in rats. Brain Res. 2005, 1039, 220–223. [Google Scholar] [CrossRef]
- Moradi-Arzeloo, M.; Farshid, A.A.; Tamaddonfard, E.; Asri-Rezaei, S. Effects of histidine and vitamin C on isoproterenol-induced acute myocardial infarction in rats. Vet. Res. Forum 2016, 7, 47–54. [Google Scholar]
- Farshid, A.A.; Tamaddonfard, E.; Simaee, N.; Mansouri, S.; Najafi, S.; Asri-Rezaee, S.; Alavi, H. Effects of histidine and N-acetylcysteine on doxorubicin-induced cardiomyopathy in rats. Cardiovasc. Toxicol. 2014, 14, 153–161. [Google Scholar] [CrossRef]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. β-Alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybakova, Y.S.; Boldyrev, A.A. Effect of carnosine and related compounds on proliferation of cultured rat pheochromocytoma PC-12 cells. Bull. Exp. Biol. Med. 2012, 154, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, Y.S.; Kalen, A.L.; Eckers, J.C.; Fedorova, T.N.; Goswami, P.C.; Sarsour, E.H. Increased manganese superoxide dismutase and cyclin B1 expression in carnosine-induced inhibition of glioblastoma cell proliferation. Biomed. Khim. 2015, 61, 510–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikuła-Pietrasik, J.; Książek, K. L-Carnosine prevents the pro-cancerogenic activity of senescent peritoneal mesothelium towards ovarian cancer cells. Anticancer Res. 2016, 36, 665–671. [Google Scholar]
- Iovine, B.; Oliviero, G.; Garofalo, M.; Orefice, M.; Nocella, F.; Borbone, N.; Piccialli, V.; Centore, R.; Mazzone, M.; Piccialli, G.; et al. The anti-proliferative effect of L-carnosine correlates with a decreased expression of hypoxia inducible factor 1 alpha in human colon cancer cells. PLoS ONE 2014, 9, e96755. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Guo, Y.; Zhang, J.; Ding, Z.; Ha, W.; Harding, J.J. Effect of carnosine, aminoguanidine, and aspirin drops on the prevention of cataracts in diabetic rats. Mol. Vis. 2008, 14, 2282–2291. [Google Scholar]
- Cornelli, U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener. Dis. 2010, 7, 193–202. [Google Scholar] [CrossRef]
- Baraniuk, J.N.; El-Amin, S.; Corey, R.; Rayhan, R.; Timbol, C. Carnosine treatment for gulf war illness: A randomized controlled trial. Glob. J. Health Sci. 2013, 5, 69–81. [Google Scholar] [CrossRef]
- Chengappa, K.N.; Turkin, S.R.; DeSanti, S.; Bowie, C.R.; Brar, J.S.; Schlicht, P.J.; Murphy, S.L.; Hetrick, M.L.; Bilder, R.; Fleet, D. A preliminary, randomized, double-blind, placebo-controlled trial of L-carnosine to improve cognition in schizophrenia. Schizophr. Res. 2012, 142, 145–152. [Google Scholar] [CrossRef]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar]
- Lombardi, C.; Carubelli, V.; Lazzarini, V.; Vizzardi, E.; Bordonali, T.; Ciccarese, C.; Castrini, A.I.; Dei Cas, A.; Nodari, S.; Metra, M. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition 2015, 31, 72–78. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Feng, R.; Li, Y.; Lin, S.; Zhang, W.; Li, Y.; Sun, C.; Li, S. Histidine supplementation alleviates inflammation in the adipose tissue of high-fat diet-induced obese rats via the NF-κB- and PPARγ-involved pathways. Br. J. Nutr. 2014, 112, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.J.; Negrisoli, G.; Carini, M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J. Cell. Mol. Med. 2011, 15, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Hsu, C.C.; Lin, M.H.; Liu, K.S.; Yin, M.C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef]
- Brown, B.E.; Kim, C.H.; Torpy, F.R.; Bursill, C.A.; McRobb, L.S.; Heather, A.K.; Davies, M.J.; van Reyk, D.M. Supplementation with carnosine decreases plasma triglycerides and modulates atherosclerotic plaque composition in diabetic apo E(-/-) mice. Atherosclerosis 2014, 232, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cotillard, A.; Vatier, C.; Bastard, J.-P.; Fellahi, S.; Stévant, M.; Allatif, O.; Langlois, C.; Bieuvelet, S.; Brochot, A.; et al. A dietary supplement containing cinnamon, chromium and carnosine decreases fasting plasma glucose and increases lean mass in overweight or obese pre-diabetic subjects: A randomized, placebo-controlled trial. PLoS ONE 2015, 10, e0138646. [Google Scholar] [CrossRef]
- Niu, Y.-C.; Feng, R.-N.; Hou, Y.; Li, K.; Kang, Z.; Wang, J.; Sun, C.-H.; Li, Y. Histidine and arginine are associated with inflammation and oxidative stress in obese women. Br. J. Nutr. 2012, 108, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Okubo, H.; Sasaki, S. Histidine intake may negatively correlate with energy intake in human: A cross-sectional study in Japanese female students aged 18 years. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 329–334. [Google Scholar] [CrossRef]
- Li, Y.-C.; Li, C.-L.; Qi, J.-Y.; Huang, L.-N.; Shi, D.; Du, S.-S.; Liu, L.-Y.; Feng, R.-N.; Sun, C.-H. Relationships of dietary histidine and obesity in northern Chinese adults, an internet-based cross-sectional study. Nutrients 2016, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Gerber, D.A. Low free serum histidine concentration in rheumatoid arthritis. A measure of disease activity. J. Clin. Investig. 1975, 55, 1164–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitton, N.G.; Dixon, J.S.; Astbury, C.; Francis, R.J.; Bird, H.A.; Wright, V. Kinetic investigations into the possible cause of low serum histidine in rheumatoid arthritis. Ann. Rheum. Dis. 1988, 47, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andou, A.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kamada, N.; Kobayashi, T.; Hashimoto, M.; Okutsu, T.; Shimbo, K.; Takeda, T.; et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology 2009, 136, 564–574.e2. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Iishi, H.; Sakai, N.; Yano, H.; Uedo, N.; Narahara, H.; Iseki, K.; Mikuni, T.; Ishiguro, S.; Tatsuta, M. Polaprezinc attenuates Helicobacter pylori-associated gastritis in Mongolian gerbils. Helicobacter 2002, 7, 384–389. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Ono, N.; Imaizumi, A.; Mori, M.; Suzuki, H.; Uo, M.; Hashimoto, M.; Naganuma, M.; Matsuoka, K.; Mizuno, S.; et al. Decreased plasma histidine level predicts risk of relapse in patients with ulcerative colitis in remission. PLoS ONE 2015, 10, e01407166. [Google Scholar] [CrossRef]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Scott, I.R.; Harding, C.R.; Barrett, J.G. Histidine-rich protein of the keratohyalin granules. Source of the free amino acids, urocanic acid and pyrrolidone carboxylic acid in the stratum corneum. Biochim. Biophys. Acta 1982, 719, 110–117. [Google Scholar] [CrossRef]
- Jontofsohn, R.; Heinze, V.; Katz, N.; Stuber, U.; Wilke, H.; Kluthe, R. Histidine and iron supplementation in dialysis and pre-dialysis patients. Proc. Eur. Dial. Transplant. Assoc. 1975, 11, 391–397. [Google Scholar]
- Blumenkrantz, M.J.; Shapiro, D.J.; Swendseid, M.E.; Kopple, J.D. Histidine supplementation for treatment of anaemia of uraemia. Br. Med. J. 1975, 2, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Politis-Tsegos, C.; de Wardener, H. Letter: Histidine for treatment of uraemic anaemia. Br. Med. J. 1974, 1, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berns, J.S.; Mosenkis, A. Pharmacologic adjuvants to epoetin in the treatment of anemia in patients on hemodialysis. Hemodial. Int. 2005, 9, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Vera-Aviles, M.; Vantana, E.; Kardinasari, E.; Koh, N.L.; Latunde-Dada, G.O. Protective role of histidine supplementation against oxidative stress damage in the management of anemia of chronic kidney disease. Pharmaceuticals (Basel). 2018, 11, E111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Karasawa, N.; Shimizu, M.; Morimatsu, F.; Yamada, R. Safety evaluation of chicken breast extract containing carnosine and anserine. Food Chem. Toxicol. 2008, 46, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Geliebter, A.A.; Hashim, S.A.; Van Itallie, T.B. Oral L-histidine fails to reduce taste and smell acuity but induces anorexia and urinary zinc excretion. Am. J. Clin. Nutr. 1981, 34, 119–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henkin, R.I.; Patten, B.M.; Re, P.K.; Bronzert, D.A. A syndrome of acute zinc loss. Cerebellar dysfunction, mental changes, anorexia, and taste and smell dysfunction. Arch. Neurol. 1975, 32, 745–751. [Google Scholar] [CrossRef]
- Solomon, J.K.; Geison, R.L. Effect of excess dietary L-histidine on plasma cholesterol levels in weanling rats. J. Nutr. 1978, 108, 936–943. [Google Scholar] [CrossRef]
- Harvey, P.W.; Hunsaker, H.A.; Allen, K.G. Dietary L-histidine-induced hypercholesterolemia and hypocupremia in the rat. J. Nutr. 1981, 111, 639–647. [Google Scholar] [CrossRef]
- Hitomi-Ohmura, E.; Amano, N.; Aoyama, Y.; Yoshida, A. The effect of a histidine-excess diet on cholesterol synthesis and degradation in rats. Lipids 1992, 27, 755–760. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M. Effects of histidine supplementation on amino acid metabolism in rats. Physiol. Res. 2020, 69, 99–111. [Google Scholar]
- Tyfield, L.A.; Holton, J.B. The effect of high concentrations of histidine on the level of other amino acids in plasma and brain of the mature rat. J. Neurochem. 1976, 26, 101–105. [Google Scholar] [PubMed]
- Teloh, J.K.; Dohle, D.-S.; Petersen, M.; Verhaegh, R.; Waack, I.N.; Roehrborn, F.; Jakob, H.; de Groot, H. Histidine and other amino acids in blood and urine after administration of Bretschneider solution (HTK) for cardioplegic arrest in patients: Effects on N-metabolism. Amino Acids 2016, 48, 1423–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.; Sun, S.; Liu, L.; Zhang, Q.; Guo, F.; Li, C.; Feng, R.; Sun, C. Effects of histidine supplementation on global serum and urine 1H NMR-based metabolomics and serum amino acid profiles in obese women from a randomized controlled study. J. Proteome Res. 2017, 16, 2221–2230. [Google Scholar] [CrossRef] [PubMed]






| Receptor | Expression | Main Functions |
|---|---|---|
| H1 | Ubiquitously (brain, respiratory epithelium, endothelial and smooth muscle cells, and lymphocytes) | Causes bronchoconstriction and vasodilation (urticaria) and induces wakefulness in the brain. |
| H2 | Gastric parietal cells, smooth muscle, brain, and heart. | Stimulates parietal cells to produce hydrochloric acid and vasodilation. |
| H3 | Exclusively in neurons | Presynaptic receptor that inhibits the release of histamine from histaminergic neurons. Activation promotes sleep. |
| H4 | Immune cells, mast cells, intestinal epithelial cells, sensory neurons, and cancer cells | Induces chemotaxis and degranulation of mast cells. |
| HIS-Rich Protein Or Peptide | The Role | Reference |
|---|---|---|
| Haem-containing proteins (haemoproteins) | Structure of haemoglobin, myoglobin, cytochromes, haem peroxidases, nitric oxide synthase, catalases, etc. | [76] |
| HIS-rich glycoprotein | Plasma protein that interacts with many ligands, including zinc, phospholipids, fibrinogen, heparin, and immunoglobulins, plays roles in regulating several biological processes, such as coagulation and immunity. | [15] |
| Histatins | Salivary copper- and zinc-binding peptides with antibacterial, antifungal, and wound-healing properties. Investigated for the treatment of oral diseases. | [77] |
| HIS-rich calcium-binding protein | 170 kDa protein primarily expressed in striated muscles and arteriolar smooth muscle cells with high capacity binding Ca++. Roles in the uptake, storage, and release of calcium ions by cardiac sarcoplasmic reticulum and regulation of cardiac rhythmicity. | [78] |
| Filaggrin (filament-aggregating protein) | Skin barrier protein that aggregates cytokeratin filaments of keratinocytes to form corneocytes. Degradation of filaggrin into amino acids, urocanic acid, and pyrrolidine carboxylic acid contributes to the formation of the “natural moisturizing factor” of the skin. | [40] |
| Study Design | Main Findings | Reference |
|---|---|---|
| Elderly volunteers (n = 39), anserine/CAR (3:1), 1 g/day, 3 months. A double-blind randomized controlled trial. | Positive effects on verbal episodic memory, decreased the secretion of proinflammatory cytokines, and improved brain perfusion. | [7] |
| Age-related cataract (n = 75), eye drops containing N-acetylcarnosine. Two drops, twice daily, for 9 months. | Rejuvenation of visual functions | [6] |
| Alzheimer’s disease, a mixture of antioxidants including CAR (100 mg/day) plus donepezil or a placebo plus donepezil for 6 months. A double-blind study. | Improvement of cognition functions. | [107] |
| Parkinson’s disease (n = 36), inclusion of CAR (1.5 g/day for 30 days) in the therapy. | Improvement in neurological symptoms and a decrease in blood plasma protein carbonyl and lipid hydroperoxide levels. | [8] |
| Gulf War illness (n = 25), CAR (500, 1000, and 1500 mg doses increasing at 4-week intervals) for 12 weeks. A double-blind randomized controlled trial. | Positive effect on cognitive functions. | [108] |
| Schizophrenia, administration of CAR as an adjunct treatment (2 g/day) for 3 months. A double-blind randomized controlled trial. | Improvement in the performance on cognitive tests. | [109] |
| Mental fatigue and sleep disruption (n = 20), HIS (1.65 g/day) for 2 weeks. A placebo controlled double-blind crossover trial. | Ameliorated feelings of fatigue and improved attentiveness and performance during working memory tasks. | [34] |
| Mental fatigue (n = 48), ingestion of dried bonito broth (2.45 g) for 4 weeks. A placebo controlled double-blind crossover trial. | Improved the mood state and increased performance on a simple calculation task. | [37] |
| Healthy females (n = 31), ingestion of dried bonito broth (4.5 g) for 2 weeks. A placebo controlled double-blind randomized crossover study. | Improved mood, increased peripheral blood flow, and decreased levels of urinary oxidative stress markers. | [36] |
| Elderly people (n = 56), anserine/CAR (2.5 g/day) for 13 weeks. Double blind study. | Decrease in the body mass index and improvement in cognitive functions and physical capacity. | [110] |
| Chronic heart failure (n = 50), CAR (500 mg/day orally) for 6 months. Prospective, randomized study. | Beneficial effects on exercise performance and quality of life. | [111] |
| Study Design | Main Findings | Reference |
|---|---|---|
| Subjects with prediabetes (n = 62) and supplement containing cinnamon, chromium, and CAR (200 mg/day), 4 months. Double-blind, placebo-controlled study. | Decrease in fasting plasma glucose levels and increase in the fat-free mass. | [117] |
| Obese women with metabolic syndrome, HIS (4 g/day), 12 weeks. Double-blind, placebo-controlled study. | Improved insulin sensitivity and decreased body mass index, waist circumference, body fat, and markers of systemic inflammation. | [87] |
| Examination of serum HIS concentrations in obese (n = 235) and non-obese (n = 217) women. | Lower HIS concentrations were observed in obese women than in nonobese; negative relationships with inflammation and oxidative stress were identified. | [118] |
| Examination of HIS and energy intake by female Japanese students (n = 1689) aged 18 years. | Daily HIS intake correlated inversely with energy intake. | [119] |
| Internet-based cross-sectional study in a Chinese population (n = 88). | Dietary HIS intake was inversely correlated with energy intake, the status of insulin resistance, inflammation, oxidative stress, and the prevalence of obesity. | [120] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. https://doi.org/10.3390/nu12030848
Holeček M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients. 2020; 12(3):848. https://doi.org/10.3390/nu12030848
Chicago/Turabian StyleHoleček, Milan. 2020. "Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement" Nutrients 12, no. 3: 848. https://doi.org/10.3390/nu12030848
APA StyleHoleček, M. (2020). Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients, 12(3), 848. https://doi.org/10.3390/nu12030848





