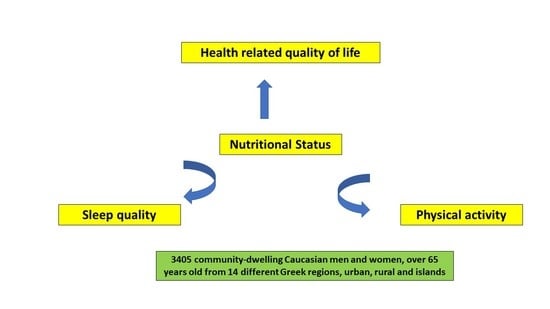
Nutritional Status Is Associated with Health-Related Quality of Life, Physical Activity, and Sleep Quality: A Cross-Sectional Study in an Elderly Greek Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic and Anthropometric Characteristics and Nutritional Status Evaluation
3.2. Health-Related Quality of Life, Physical Activity, and Sleep Quality Evaluation
3.3. Comparisons between Nutritional Status and Sociodemographic and Anthropometric Characteristics
3.4. Comparisons between Nutritional Status and Health-Related Quality of Life, Physical Activity, and Sleep Quality
3.5. Multiple Regression Analysis for Mediterranean Diet Adherence after Adjustment for Potential Confounding Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan American Health Organization. Healthy Ageing. Available online: https://www.paho.org/en/healthy-aging (accessed on 1 November 2022).
- de Morais, C.; Oliveira, B.; Afonso, C.; Lumbers, M.; Raats, M.; de Almeida, M.D.V. Nutritional risk of European elderly. Eur. J. Clin. Nutr. 2013, 67, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, L.; Walton, J.; Flynn, A. Nutritional challenges for older adults in Europe: Current status and future directions. Proc. Nutr. Soc. 2019, 78, 221–233. [Google Scholar] [CrossRef]
- Gillsjö, C.; Nyström, M.; Palmér, L.; Carlsson, G.; Dalheim-Englund, A.C.; Eriksson, I. Balance in life as a prerequisite for community-dwelling older adults’ sense of health and well-being after retirement: An interview-based study. Int. J. Qual. Stud. Health Well-Being 2021, 16, 1984376. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.J.; Claunch, K.D.; Verdeja, M.A.; Dungan, M.T.; Anderson, S.; Clayton, C.K.; Goates, M.C.; Thacker, E.L. What Does “Successful Aging” Mean to you?–Systematic Review and Cross-Cultural Comparison of Lay Perspectives of Older Adults in 13 Countries, 2010-2020. J. Cross Cult. Gerontol. 2020, 35, 455–478. [Google Scholar] [CrossRef]
- Makovski, T.T.; Schmitz, S.; Zeegers, M.P.; Stranges, S.; van den Akker, M. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res. Rev. 2019, 53, 100903. [Google Scholar] [CrossRef]
- Robinson, S.M. Improving nutrition to support healthy ageing: What are the opportunities for intervention? Proc. Nutr. Soc. 2018, 77, 257–264. [Google Scholar] [CrossRef]
- Mantzorou, M.; Vadikolias, K.; Pavlidou, E.; Serdari, A.; Vasios, G.; Tryfonos, C.; Giaginis, C. Nutritional status is associated with the degree of cognitive impairment and depressive symptoms in a Greek elderly population. Nutr. Neurosci. 2020, 23, 201–209. [Google Scholar] [CrossRef]
- Pinto de Souza Fernandes, D.; Duarte, M.S.L.; Pessoa, M.C.; Franceschini, S.D.C.; Ribeiro, A.Q. Evaluation of diet quality of the elderly and associated factors. Arch. Gerontol. Geriatr. 2017, 72, 174–180. [Google Scholar] [CrossRef]
- Naseer, M.; Forssell, H.; Fagerström, C. Malnutrition, functional ability and mortality among older people aged ≥60 years: A 7-year longitudinal study. Eur. J. Clin. Nutr. 2016, 70, 399–404. [Google Scholar] [CrossRef]
- Lesourd, B. Nutritional factors and immunological ageing. Proc. Nutr. Soc. 2006, 65, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, E.; Toniolo, L. Nutrition, Diet and Healthy Aging. Nutrients 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Margetts, B.M.; Thompson, R.L.; Elia, M.; Jackson, A.A. Prevalence of risk of undernutrition is associated with poor health status in older people in the UK. Eur. J. Clin. Nutr. 2003, 57, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Nyunt, M.S.; Gao, Q.; Wee, S.L.; Yap, K.B.; Ng, T.P. Association of Frailty and Malnutrition With Long-term Functional and Mortality Outcomes Among Community-Dwelling Older Adults: Results From the Singapore Longitudinal Aging Study 1. JAMA Netw. Open. 2018, 1, e180650. [Google Scholar] [CrossRef] [Green Version]
- Nuotio, M.; Tuominen, P.; Luukkaala, T. Association of nutritional status as measured by the Mini-Nutritional Assessment Short Form with changes in mobility, institutionalization and death after hip fracture. Eur. J. Clin. Nutr. 2016, 70, 393–398. [Google Scholar] [CrossRef]
- Valentini, A.; Federici, M.; Cianfaran, I.M.A.; Tarantino, U.; Bertoli, A. Frailty and nutritional status in older people: The Mini Nutritional Assessment as a screening tool for the identification of frail subjects. Clin. Interv. Aging. 2018, 13, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.H.; Chou, M.Y.; Chu, C.S.; Liao, M.C.; Wang, Y.C.; Lin, Y.T.; Chen, L.K.; Liang, C.K. Predictive Effect of Malnutrition on Long-Term Clinical Outcomes among Older Men: A Prospectively Observational Cohort Study. J. Nutr. Health Aging 2019, 23, 876–882. [Google Scholar] [CrossRef]
- Eckstrom, E.; Neukam, S.; Kalin, L.; Wright, J. Physical Activity and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 671–683. [Google Scholar] [CrossRef]
- Daskalopoulou, C.; Stubbs, B.; Kralj, C.; Koukounari, A.; Prince, M.; Prina, A.M. Physical activity and healthy ageing: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2017, 38, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Public Health England. Guidance Physical activity: Applying All Our Health. Available online: https://www.gov.uk/government/publications/physical-activity-applying-all-our-health/physical-activity-applying-all-our-health (accessed on 1 November 2022).
- Paluska, S.A.; Schwenk, T.L. Physical activity and mental health: Current concepts. Sports Med. 2000, 29, 167–180. [Google Scholar] [CrossRef]
- Vagetti, G.C.; Barbosa Filho, V.C.; Moreira, N.B.; Oliveira, V.D.; Mazzardo, O.; Campos, W.D. Association between physical activity and quality of life in the elderly: A systematic review, 2000–2012. Braz. J. Psychiatry 2014, 36, 76–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mok, A.; Khaw, K.T.; Luben, R.; Wareham, N.; Brage, S. Physical activity trajectories and mortality: Population based cohort study. BMJ 2019, 365, l2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology. 2016, 17, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekelund, U.; Brown, W.J.; Steene-Johannessen, J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.E.; Lee, I.M. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br. J. Sports Med. 2019, 53, 886–894. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.M.; Lancet Physical Activity Series 2 Executive Committe; Lancet Sedentary Behaviour Working Group. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yan, W.; Wang, T.; Zhu, L.J.; Xu, Y.; Zhao, J.; Yu, L.L.; Bao, H.H.; Cheng, X.S. Independent and joint association of physical activity and sedentary behavior on all-cause mortality. Chin. Med. J. 2021, 134, 2857–2864. [Google Scholar] [CrossRef]
- Lok, N.; Lok, S.; Canbaz, M. The effect of physical activity on depressive symptoms and quality of life among elderly nursing home residents: Randomized controlled trial. Arch. Gerontol. Geriatr. 2017, 70, 92–98. [Google Scholar] [CrossRef]
- Ebeling, P.R.; Cicuttini, F.; Scott, D.; Jones, G. Promoting mobility and healthy aging in men: A narrative review. Osteoporos Int. 2019, 30, 1911–1922. [Google Scholar] [CrossRef]
- Hosker, D.K.; Elkins, R.M.; Potter, M.P. Promoting Mental Health and Wellness in Youth Through Physical Activity, Nutrition, and Sleep. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 171–193. [Google Scholar] [CrossRef]
- Wolkove, N.; Elkholy, O.; Baltzan, M.; Palayew, M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ 2007, 176, 1299–1304. [Google Scholar] [CrossRef]
- Arora, T.; Choudhury, S.; Taheri, M.S. The Relationships Among Sleep, Nutrition, and Obesity. Curr. Sleep Med. Rep. 2015, 1, 218–225. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, S.; Gonzalez, K.; Lee-Ang, L.; Young, M.C.; Tamez, M.; Mattei, J. Diet and Sleep Physiology: Public Health and Clinical Implications. Front. Neurol. 2017, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485. [Google Scholar]
- Mantzorou, M.; Vadikolias, K.; Pavlidou, E.; Tryfonos, C.; Vasios, G.; Serdari, A.; Giaginis, C. Mediterranean diet adherence is associated with better cognitive status and less depressive symptoms in a Greek elderly population. Aging Clin. Exp. Res. 2021, 33, 1033–1040. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization. 2006. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 1 November 2022).
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [Green Version]
- Barile, J.P.; Horner-Johnson, W.; Krahn, G.; Zack, M.; Miranda, D.; DeMichele, K.; Ford, D.; Thompson, W.W. Measurement characteristics for two health-related quality of life measures in older adults: The SF-36 and the CDC Healthy Days items. Disabil. Health J. 2016, 9, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Beaudreau, S.A.; Spira, A.P.; Stewart, A.; Kezirian, E.J.; Lui, L.Y.; Ensrud, K.; Redline, S.; Ancoli-Israel, S.; Stone, K.L. Study of Osteoporotic Fractures. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012, 13, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Salahuddin, M.; Maru, T.T.; Kumalo, A.; Pandi-Perumal, S.R.; Bahammam, A.S.; Manzar, M.D. Validation of the Pittsburgh sleep quality index in community dwelling Ethiopian adults. Health Qual. Life Outcomes 2017, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Hickson, M. Malnutrition and ageing. Postgrad. Med. J. 2006, 82, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Lengelé, L.; Bruyère, O.; Beaudart, C.; Reginster, J.Y.; Locquet, M. Impact of Malnutrition Status on Muscle Parameter Changes over a 5-Year Follow-Up of Community-Dwelling Older Adults from the SarcoPhAge Cohort. Nutrients 2021, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Dasdemir Ilkhan, G.; Celikhisar, H. The effect of incontinence on sleep quality in the elderly. Int. J. Clin. Pract. 2021, 75, e13965. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Finato, V.; Del Corso, C.; Catania, B.; Caselli, G.M.; Egidi, M.F. Assessment of physical activity, capacity and nutritional status in elderly peritoneal dialysis patients. BMC Nephrol. 2017, 18, 180. [Google Scholar] [CrossRef] [Green Version]
- Abizanda, P.; López, M.D.; García, V.P.; de Dios Estrella, J.; da Silva González, Á.; Vilardell, N.B.; Torres, K.A. Effects of an Oral Nutritional Supplementation Plus Physical Exercise Intervention on the Physical Function, Nutritional Status, and Quality of Life in Frail Institutionalized Older Adults: The ACTIVNES Study. J. Am. Med. Dir. Assoc. 2015, 16, 439.e9–439.e16. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Süß, P.; Schulte, D.M.; Letsch, A.; Jensen, W. Supportive Care in Oncology-From Physical Activity to Nutrition. Nutrients 2022, 14, 1149. [Google Scholar] [CrossRef]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Lorés Casanova, S.; Rodríguez Mañas, J.; Lorente Aznar, T.; Pelegrín Valero, C.; Castillón Fantova, A.; Marques Lopes, I. La actividad física y el estado nutricional y psicosocial del anciano no dependiente [Physical activity and nutritional and psychosocial status in non-dependent elderly]. Semergen 2020, 46, 244–253. [Google Scholar] [CrossRef]
- Cereda, E.; Veronese, N.; Caccialanza, R. Role of muscle-targeted nutritional therapy: New data. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.; Luscombe-Marsh, N.; Ambrosi, C.; Lushington, K. Nutritional status and quality-of-life of older adults in aged care: A systematic review and meta-analysis. Exp. Gerontol. 2022, 162, 111764. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Woods, R.T. Malnutrition and quality of life in older people: A systematic review and meta-analysis. Ageing Res. Rev. 2013, 12, 561–566. [Google Scholar] [CrossRef]
- Khatami, F.; Shafiee, G.; Kamali, K.; Ebrahimi, M.; Azimi, M.; Ahadi, Z.; Sharifi, F.; Tanjani, P.T.; Heshmat, R. Correlation between malnutrition and health-related quality of life (HRQOL) in elderly Iranian adults. J. Int. Med. Res. 2020, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tański, W.; Wójciga, J.; Jankowska-Polańska, B. Association between Malnutrition and Quality of Life in Elderly Patients with Rheumatoid Arthritis. Nutrients 2021, 13, 1259. [Google Scholar] [CrossRef]
- Rasheed, S.; Woods, R.T. An investigation into the association between nutritional status and quality of life in older people admitted to hospital. J. Hum. Nutr. Diet 2014, 27, 142–151. [Google Scholar] [CrossRef]
- Chen, C.; Liu, G.G.; Shi, Q.L.; Sun, Y.; Zhang, H.; Wang, M.J.; Jia, H.P.; Zhao, Y.L.; Yao, Y. Health-Related Quality of Life and Associated Factors among Oldest-Old in China. J. Nutr. Health Aging 2020, 24, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yang, S.; Liu, W.; Wang, S.; Tai, P.; Kou, F.; Jia, W.; Han, K.; Liu, M.; He, Y. Canonical Correlation Analysis on the Association Between Sleep Quality and Nutritional Status Among Centenarians in Hainan. Front. Public Health 2020, 8, 585207. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, Y.; Jia, S.L.; Ge, M.L.; Hou, L.S.; Xia, X.; Liu, X.L.; Yue, J.R.; Dong, B.R. The association of sleep quality and sleep duration with nutritional status in older adults: Findings from the WCHAT study. Maturitas 2021, 145, 1–5. [Google Scholar] [CrossRef]
- Jyväkorpi, S.K.; Urtamo, A.; Kivimäki, M.; Strandberg, T.E. Associations of sleep quality, quantity and nutrition in oldest-old men The Helsinki Businessmen Study (HBS). Eur. Geriatr. Med. 2021, 12, 117–122. [Google Scholar] [CrossRef]
- Şengül, Ş.; Uysal, H. The relationship between the nutritional status and sleep quality of patients with atrial fibrillation. Saudi Med. J. 2019, 40, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Ooms, A.; Tuffrey Wijne, I. The impact of nutrition on sleep in people with an intellectual disability: An integrative literature review. J. Appl. Res. Intellect. Disabil. 2021, 34, 1393–1407. [Google Scholar] [CrossRef] [PubMed]

| Characteristics, n = 3405 | Descriptive Statistics |
|---|---|
| Mean age (years ± SD) | 74.6 ± 8.3 |
| Gender (n, %) | |
| Female | 48.6% |
| Male | 51.4% |
| Mean BMI (kg/m2) | 27.3 ± 4.1 |
| BMI status | |
| Normal weight | 46.8% |
| Overweight | 39.2% |
| Obese | 14.0% |
| Mid arm circumference (n, %) | |
| <22 cm | 25.4% |
| ≥22 cm | 74.6% |
| Calf circumference (n, %) | |
| <31 cm | 34.7% |
| ≥31 cm | 65.3% |
| Educational level (years ± SD) | 7.3 ± 2.8 |
| Financial status (n, %) | |
| Low | 42.9% |
| Medium | 38.3% |
| High | 18.8% |
| Living status (n, %) | |
| Alone | 34.9% |
| With others | 65.1% |
| Smoking habits (n, %) | |
| Never smokers | 75.8% |
| Smokers | 24.2% |
| HRQOL (n, %) | |
| Low | 50.1% |
| High | 49.9% |
| IPAQ (n, %) | |
| Low | 41.0% |
| Moderate | 34.4% |
| High | 24.6% |
| PSQI (n, %) | |
| Inadequate | 43.1% |
| Adequate | 56.9% |
| Characteristics, n = 3405 | Nutritional Status | |||
|---|---|---|---|---|
| Malnourished (10.4%) | At Risk of Malnutrition (35.6%) | Well Nourished (54.0%) | p-Value | |
| Age (years ± SD) | 80.9 ± 7.7 | 74.9 ± 8.5 | 73.2 ± 7.8 | p < 0.0001 |
| Gender (n, %) | p = 0.0722 | |||
| Female | 158 (44.8) | 705 (58.1) | 791 (43.0) | |
| Male | 195 (55.2) | 509 (41.9) | 1047 (57.0) | |
| BMI status | p = 0.0001 | |||
| Normal weight | 119 (33.6) | 551 (45.5) | 923 (50.2) | |
| Overweight | 148 (41.8) | 421 (34.7) | 765 (41.6) | |
| Obese | 87 (24.6) | 240 (19.8) | 151 (8.2) | |
| Mid arm circumference (n, %) | p ˂ 0.0001 | |||
| <22 cm | 272 (77.0) | 483 (39.8) | 109 (5.9) | |
| ≥22 cm | 81 (23.0) | 731 (60.2) | 1729 (94.1) | |
| Calf circumference (n, %) | p ˂ 0.0001 | |||
| <31 cm | 291 (82.4) | 596 (49.1) | 293 (15.9) | |
| ≥31 cm | 62 (17.6) | 618 (50.9) | 1545 (84.1) | |
| Educational level (years ± SD) | 6.6 ± 2.0 | 7.2 ± 2.7 | 7.6 ± 3.2 | p = 0.0002 |
| Financial status (n, %) | p = 0.0001 | |||
| Low | 195 (55.2) | 589 (48.5) | 676 (36.8) | |
| Medium | 116 (32.9) | 449 (37.0) | 738 (40.1) | |
| High | 42 (11.9) | 176 (14.5) | 424 (23.1) | |
| Living status (n, %) | p = 0.0003 | |||
| Alone | 158 (44.8) | 423 (34.8) | 606 (33.0) | |
| With others | 195 (55.2) | 791 (65.2) | 1232 (67.0) | |
| Smoking habits (n, %) | p = 0.1237 | |||
| Never smokers | 286 (81.0) | 927 (76.4) | 1367 (74.4) | |
| Smokers | 67 (19.0) | 287 (23.6) | 471 (25.6) | |
| HRQOL (n, %) | p = 0.0002 | |||
| Low | 205 (58.1) | 638 (52.5) | 859 (46.7) | |
| High | 148 (41.9) | 576 (47.5) | 979 (53.3) | |
| IPAQ (n, %) | p < 0.0001 | |||
| Low | 193 (54.7) | 568 (46.8) | 636 (34.6) | |
| Moderate | 113 (32.0) | 407 (33.5) | 649 (35.3) | |
| High | 47 (13.3) | 239 (19.7) | 553 (30.1) | |
| PSQI (n, %) | p = 0.0069 | |||
| Inadequate | 157 (44.5) | 569 (46.9) | 740 (40.3) | |
| Adequate | 196 (55.5) | 645 (53.1) | 1098 (59.7) | |
| Parameters | HR (95% CI) | p-Value |
|---|---|---|
| Age (Below/Over mean value) | 0.76 (0.45–1.12) | 0.0012 |
| Gender (Male/Female) | 1.33 (0.87–1.98) | 0.0839 |
| BMI (Normal/Overweight or Obese) | 1.81 (1.53–2.18) | 0.0045 |
| Mid arm circumference (<22 cm/≥22 cm) | 0.71 (0.45–1.12) | 0.0007 |
| Calf circumference (<31 cm/≥31 cm) | 0.82 (0.54–1.11) | 0.0004 |
| Educational level (Below/Over mean value) | 1.17 (0.56–1.83) | 0.1539 |
| Financial status (Low or medium/High) | 1.12 (0.52–1.89) | 0.2270 |
| Living status (Alone/With others) | 1.58 (0.83–2.26) | 0.1808 |
| Smoking habits (No/Yes) | 0.83 (0.34–1.44) | 0.2239 |
| HRQOL (Low/High) | 2.12 (1.84–2.59) | 0.0011 |
| IPAQ (Low/Moderate or High) | 1.82 (1.49–2.28) | 0.0135 |
| PSQI (Inadequate/Adequate) | 2.11 (1.72–2.48) | 0.0202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, S.K.; Mantzorou, M.; Voulgaridou, G.; Pavlidou, E.; Vadikolias, K.; Antasouras, G.; Vorvolakos, T.; Psara, E.; Vasios, G.K.; Serdari, A.; et al. Nutritional Status Is Associated with Health-Related Quality of Life, Physical Activity, and Sleep Quality: A Cross-Sectional Study in an Elderly Greek Population. Nutrients 2023, 15, 443. https://doi.org/10.3390/nu15020443
Papadopoulou SK, Mantzorou M, Voulgaridou G, Pavlidou E, Vadikolias K, Antasouras G, Vorvolakos T, Psara E, Vasios GK, Serdari A, et al. Nutritional Status Is Associated with Health-Related Quality of Life, Physical Activity, and Sleep Quality: A Cross-Sectional Study in an Elderly Greek Population. Nutrients. 2023; 15(2):443. https://doi.org/10.3390/nu15020443
Chicago/Turabian StylePapadopoulou, Sousana K., Maria Mantzorou, Gavriela Voulgaridou, Eleni Pavlidou, Konstantinos Vadikolias, Georgios Antasouras, Theofanis Vorvolakos, Evmorfia Psara, Georgios K. Vasios, Aspasia Serdari, and et al. 2023. "Nutritional Status Is Associated with Health-Related Quality of Life, Physical Activity, and Sleep Quality: A Cross-Sectional Study in an Elderly Greek Population" Nutrients 15, no. 2: 443. https://doi.org/10.3390/nu15020443
APA StylePapadopoulou, S. K., Mantzorou, M., Voulgaridou, G., Pavlidou, E., Vadikolias, K., Antasouras, G., Vorvolakos, T., Psara, E., Vasios, G. K., Serdari, A., Poulios, E., & Giaginis, C. (2023). Nutritional Status Is Associated with Health-Related Quality of Life, Physical Activity, and Sleep Quality: A Cross-Sectional Study in an Elderly Greek Population. Nutrients, 15(2), 443. https://doi.org/10.3390/nu15020443