Bacteriome Diversity of Blackflies’ Gut and Association with Onchocerca volvulus, the Causative Agent of Onchocerciasis in Mbam Valley (Center Region, Cameroon)
Abstract
:1. Introduction
2. Results
2.1. Blackflies Infection by Onchocerca volvulus
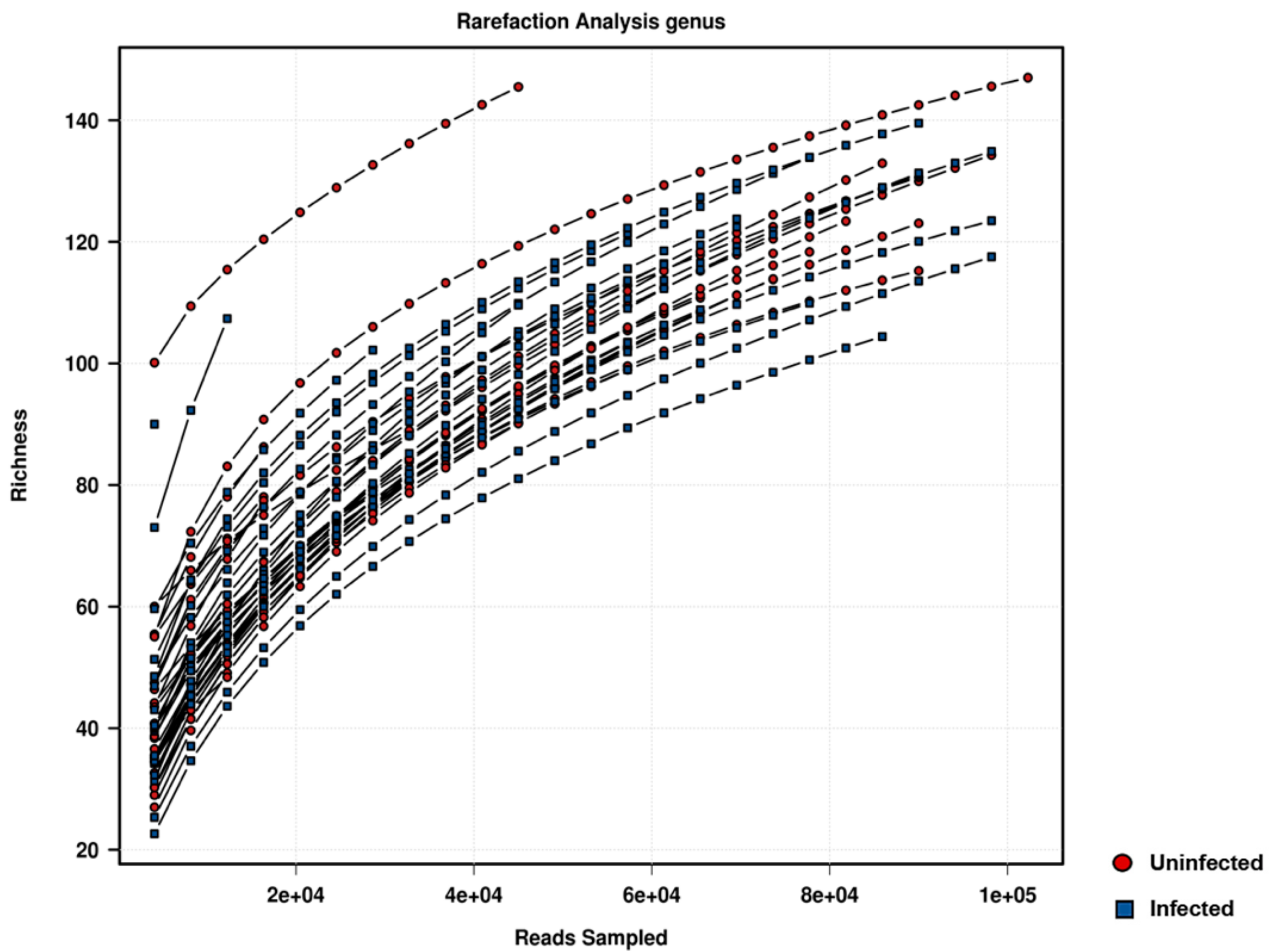
2.2. Sequencing Data Analysis
2.3. Taxonomic Assignment
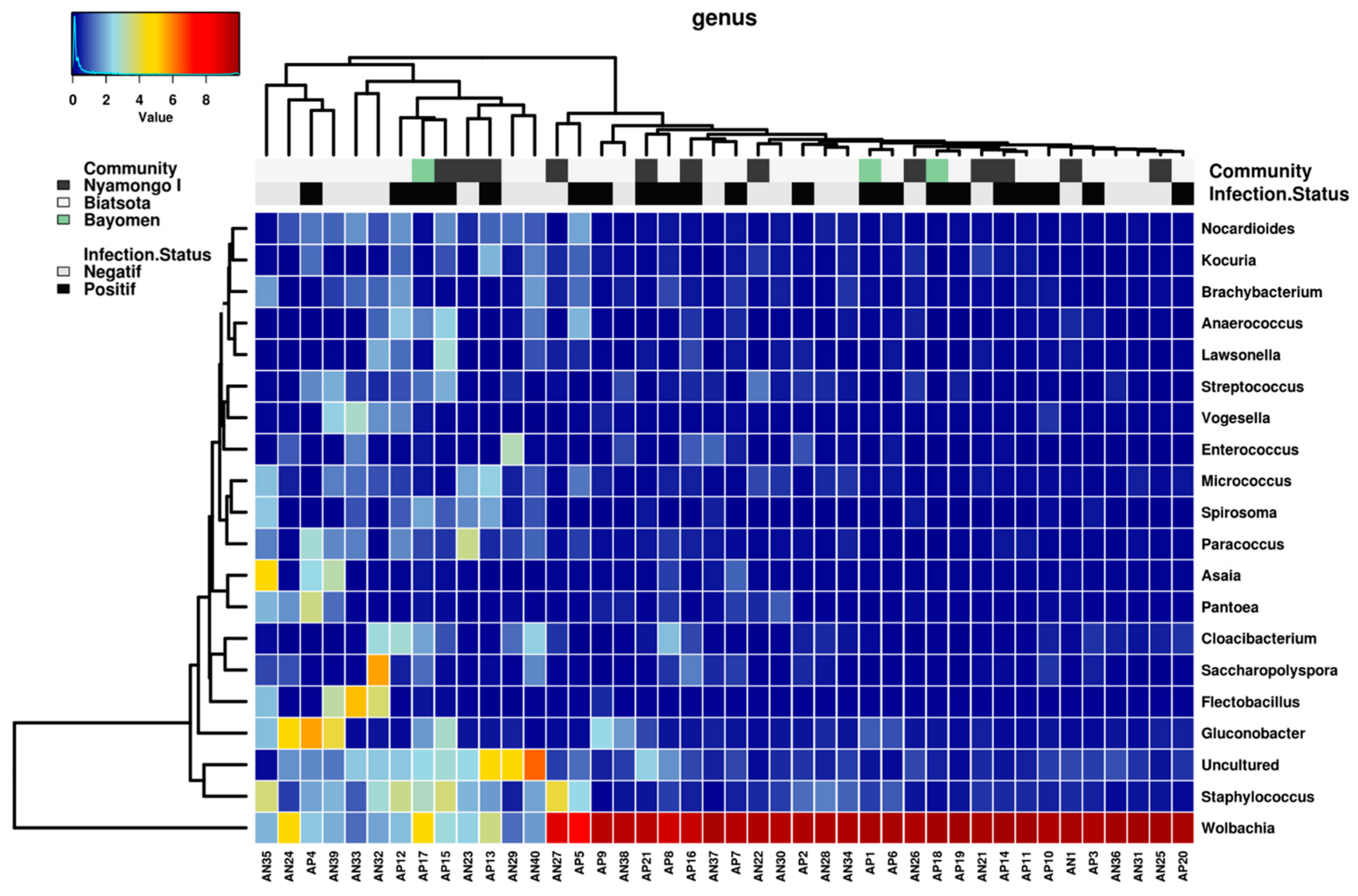
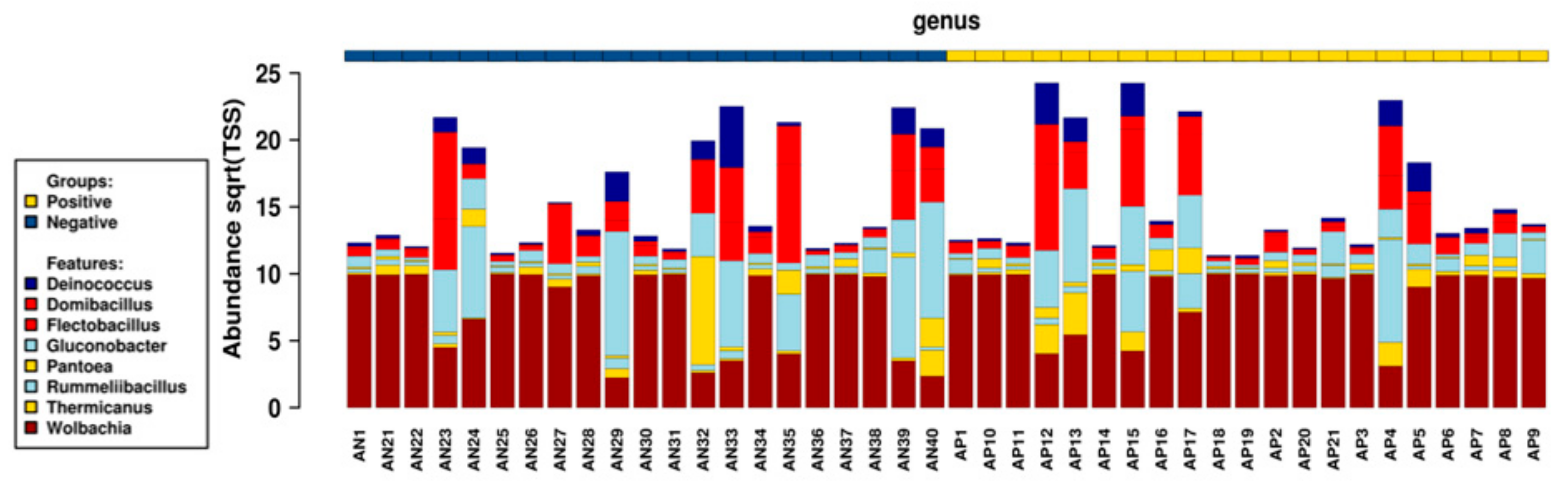
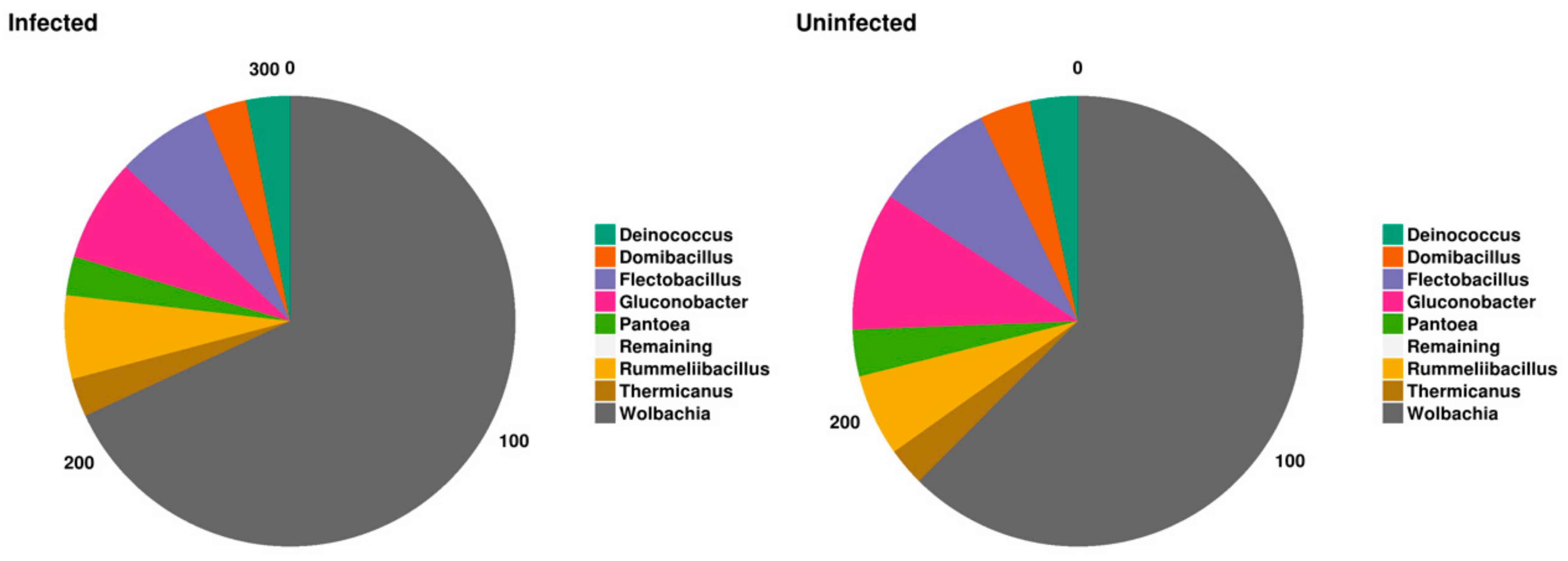
2.4. Bacterial Genera
2.4.1. Global Distribution
2.4.2. Blackfly Gut Core Bacteriome
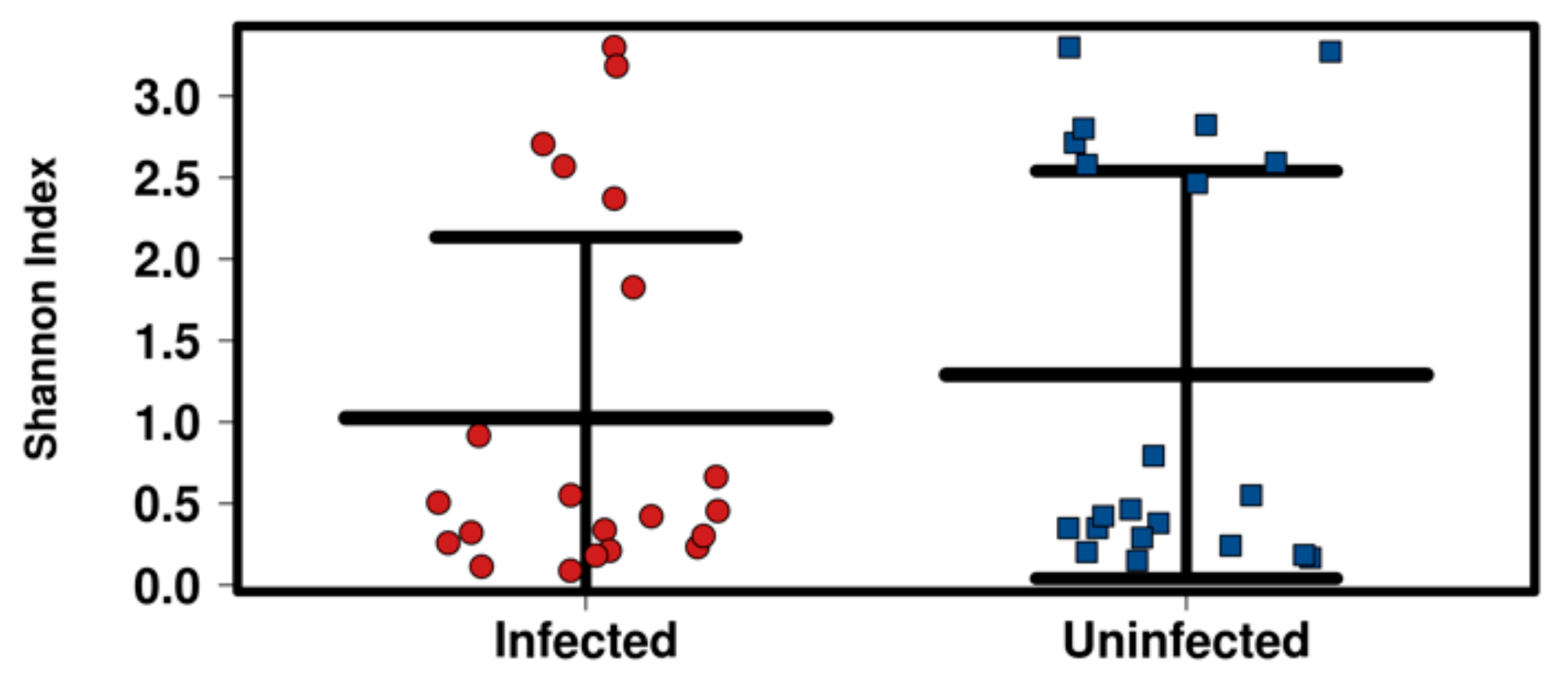
2.5. Association between Blackflies’ Bacterial Diversity with Infection Status
2.6. Multivariate Association between Bacterial Diversity in Blackflies and O. volvulus Infection Status
2.7. Potential Biomarker According to Blackfly Infection Status
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Ethics Approval and Consent to Participate
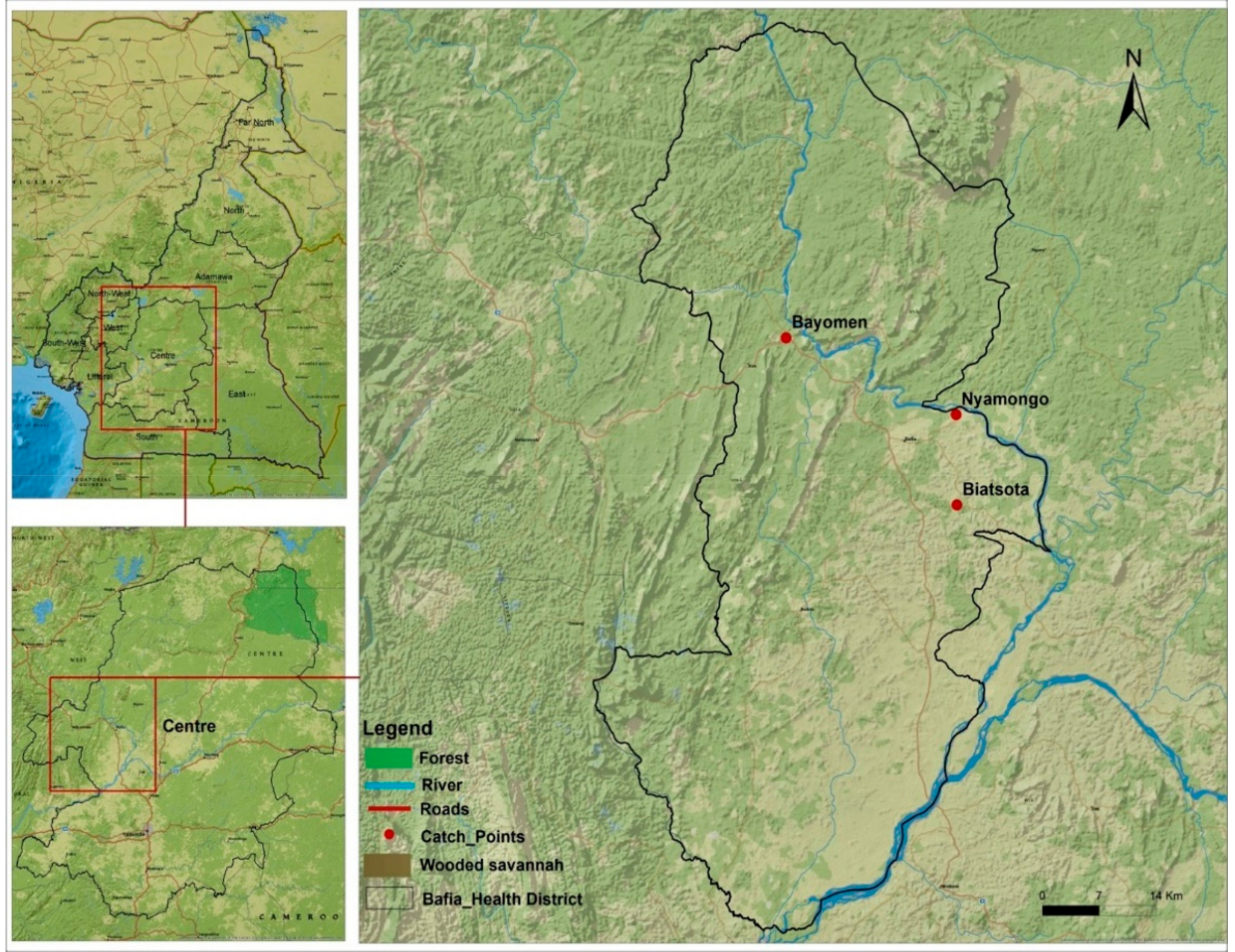
5.2. Study Area
5.3. Capture, Dissection and Preservation of Blackflies
5.4. DNA Extraction and O. volvulus PCR Amplification
5.5. High Throughput Sequencing and Meta-Barcoding Analysis
5.6. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takaoka, H. Review of the Biology and Ecology of Adult Blackflies in Relation to the Transmission of Onchocerciasis in Guatemala. Trop. Med. Health 2015, 43, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Plaisier, A.P.; van Oortmarssen, G.J.; Remme, J.; Habbema, J.D.F. The Reproductive Lifespan of Onchocerca Volvulus in West African Savanna. Acta Trop. 1991, 48, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Basáñez, M.-G.; Boussinesq, M. Population Biology of Human Onchocerciasis. Phil. Trans. R. Soc. Lond. B 1999, 354, 809–826. [Google Scholar] [CrossRef] [Green Version]
- Udall, D.N. Recent Updates on Onchocerciasis: Diagnosis and Treatment. Clin. Infect. Dis. 2007, 44, 53–60. [Google Scholar] [CrossRef]
- Resnikoff, S.; Keys, T. Future Trends in Global Blindness. Indian J. Ophthalmol. 2012, 60, 387. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation Onchocerciasis. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis (accessed on 21 December 2021).
- Siewe Fodjo, J.N.; Mandro, M.; Mukendi, D.; Tepage, F.; Menon, S.; Nakato, S.; Nyisi, F.; Abhafule, G.; Wonya’rossi, D.; Anyolito, A.; et al. Onchocerciasis-Associated Epilepsy in the Democratic Republic of Congo: Clinical Description and Relationship with Microfilarial Density. PLoS Negl. Trop. Dis. 2019, 13, e0007300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colebunders, R.; Njamnshi, A.K.; van Oijen, M.; Mukendi, D.; Kashama, J.M.; Mandro, M.; Gumisiriza, N.; Preux, P.-M.; Suykerbuyk, P.; Idro, R. Onchocerciasis-Associated Epilepsy: From Recent Epidemiological and Clinical Findings to Policy Implications. Epilepsia Open 2017, 2, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Little, M.P.; Wagner, K.S.; Soumbey-Alley, E.W.; Boatin, B.A.; Basáñez, M.-G. Density-Dependent Mortality of the Human Host in Onchocerciasis: Relationships between Microfilarial Load and Excess Mortality. PLoS Negl. Trop. Dis. 2012, 6, e1578. [Google Scholar] [CrossRef] [Green Version]
- Little, M.; Breitling, L.; Basáñez, M.-G.; Alley, E.; Boatin, B. Association between Microfilarial Load and Excess Mortality in Onchocerciasis: An Epidemiological Study. Lancet 2004, 363, 1514–1521. [Google Scholar] [CrossRef]
- Boussinesq, M. Ivermectine. J. Trop. Med. 2005, 65, 69–79. [Google Scholar]
- Colebunders, R.; Basáñez, M.-G.; Siling, K.; Post, R.J.; Rotsaert, A.; Mmbando, B.; Suykerbuyk, P.; Hopkins, A. From River Blindness Control to Elimination: Bridge over Troubled Water. Infect. Dis. Poverty 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, M.A.; Fernández-Santos, N.A.; Orozco-Algarra, M.E.; Rodríguez-Atanacio, J.A.; Domínguez-Vázquez, A.; Rodríguez-Morales, K.B.; Real-Najarro, O.; Prado-Velasco, F.G.; Cupp, E.W.; Richards, F.O.; et al. Elimination of Onchocerciasis from Mexico. PLoS Negl. Trop. Dis. 2015, 9, e0003922. [Google Scholar] [CrossRef] [Green Version]
- Gustavsen, K.; Hopkins, A.; Sauerbrey, M. Onchocerciasis in the Americas: From Arrival to (near) Elimination. Parasites Vectors 2011, 4, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouré, H.G.; Noma, M.; Tekle, A.H.; Amazigo, U.V.; Diggle, P.J.; Giorgi, E.; Remme, J.H. The Geographic Distribution of Onchocerciasis in the 20 Participating Countries of the African Programme for Onchocerciasis Control: (2) Pre-Control Endemicity Levels and Estimated Number Infected. Parasites Vectors 2014, 7, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekle, A.H.; Zouré, H.G.M.; Noma, M.; Boussinesq, M.; Coffeng, L.E.; Stolk, W.A.; Remme, J.H.F. Progress towards Onchocerciasis Elimination in the Participating Countries of the African Programme for Onchocerciasis Control: Epidemiological Evaluation Results. Infect. Dis. Poverty 2016, 5, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebrezgabiher, G.; Mekonnen, Z.; Yewhalaw, D.; Hailu, A. Reaching the Last Mile: Main Challenges Relating to and Recommendations to Accelerate Onchocerciasis Elimination in Africa. Infect. Dis. Poverty 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senyonjo, L.; Oye, J.; Bakajika, D.; Biholong, B.; Tekle, A.; Boakye, D.; Schmidt, E.; Elhassan, E. Factors Associated with Ivermectin Non-Compliance and Its Potential Role in Sustaining Onchocerca Volvulus Transmission in the West Region of Cameroon. PLoS Negl. Trop. Dis. 2016, 10, e0004905. [Google Scholar] [CrossRef]
- Frempong, K.K.; Walker, M.; Cheke, R.A.; Tetevi, E.J.; Gyan, E.T.; Owusu, E.O.; Wilson, M.D.; Boakye, D.A.; Taylor, M.J.; Biritwum, N.-K.; et al. Does Increasing Treatment Frequency Address Suboptimal Responses to Ivermectin for the Control and Elimination of River Blindness? Clin. Infect. Dis. 2016, 62, 1338–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awadzi, K.; Boakye, D.A.; Edwards, G.; Opoku, N.O.; Attah, S.K.; Osei-Atweneboana, M.Y.; Lazdins-Helds, J.K.; Ardrey, A.E.; Addy, E.T.; Quartey, B.T.; et al. An Investigation of Persistent Microfilaridermias despite Multiple Treatments with Ivermectin, in Two Onchocerciasis-Endemic Foci in Ghana. Ann. Trop. Med. Parasitol. 2004, 98, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Bakajika, D.; Senyonjo, L.; Enyong, P.; Oye, J.; Biholong, B.; Elhassan, E.; Boakye, D.; Dixon, R.; Schmidt, E. On-Going Transmission of Human Onchocerciasis in the Massangam Health District in the West Region of Cameroon: Better Understanding Transmission Dynamics to Inform Changes in Programmatic Interventions. PLoS Negl. Trop. Dis. 2018, 12, e0006904. [Google Scholar] [CrossRef]
- Pion, S.D.S.; Nana-Djeunga, H.C.; Kamgno, J.; Tendongfor, N.; Wanji, S.; Njiokou, F.; Prichard, R.K.; Boussinesq, M. Dynamics of Onchocerca Volvulus Microfilarial Densities after Ivermectin Treatment in an Ivermectin-Naïve and a Multiply Treated Population from Cameroon. PLoS Negl. Trop. Dis. 2013, 7, e2084. [Google Scholar] [CrossRef] [Green Version]
- Nana-Djeunga, H.C.; Bourguinat, C.; Pion, S.D.; Bopda, J.; Kengne-Ouafo, J.A.; Njiokou, F.; Prichard, R.K.; Wanji, S.; Kamgno, J.; Boussinesq, M. Reproductive Status of Onchocerca Volvulus after Ivermectin Treatment in an Ivermectin-Naïve and a Frequently Treated Population from Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e2824. [Google Scholar] [CrossRef]
- Boussinesq, M.; Fobi, G.; Kuesel, A.C. Alternative Treatment Strategies to Accelerate the Elimination of Onchocerciasis. Int. Health 2018, 10, i40–i48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, B.G.; Loum, D.; Lakwo, T.L.; Katholi, C.R.; Habomugisha, P.; Byamukama, E.; Tukahebwa, E.; Cupp, E.W.; Unnasch, T.R. Community-Directed Vector Control to Supplement Mass Drug Distribution for Onchocerciasis Elimination in the Madi Mid-North Focus of Northern Uganda. PLoS Negl. Trop. Dis. 2018, 12, e0006702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Programme for the Elimination of Neglected Diseases in Africa (PENDA). 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/275500/JAF19.8-eng.pdf (accessed on 21 December 2021).
- Wilke, A.B.B.; Marrelli, M.T. Paratransgenesis: A Promising New Strategy for Mosquito Vector Control. Parasites Vectors 2015, 8, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, J.; Shyma, K.; Ranjan, S.; Gaur, G.; Bhushan, B. Genetic Manipulation of Endosymbionts to Control Vector and Vector Borne Diseases. Vet. World 2012, 5, 571. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Makepeace, B.L.; Benjamin, L.; Baylis, M.; Solomon, T. The Potential Role of Wolbachia in Controlling the Transmission of Emerging Human Arboviral Infections. Curr. Opin. Infect. Dis. 2017, 30, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, F.; Melachio, T.T.; Njitchouang, G.R.; Gimonneau, G.; Njiokou, F.; Abate, L.; Christen, R.; Reveillaud, J.; Geiger, A. Intestinal Bacterial Communities of Trypanosome-Infected and Uninfected Glossina Palpalis Palpalis from Three Human African Trypanomiasis Foci in Cameroon. Front. Microbiol. 2017, 8, 1464. [Google Scholar] [CrossRef]
- Tsagmo Ngoune, J.M.; Reveillaud, J.; Sempere, G.; Njiokou, F.; Melachio, T.T.; Abate, L.; Tchioffo, M.T.; Geiger, A. The Composition and Abundance of Bacterial Communities Residing in the Gut of Glossina Palpalis Palpalis Captured in Two Sites of Southern Cameroon. Parasites Vectors 2019, 12, 151. [Google Scholar] [CrossRef]
- Azambuja, P.; Feder, D.; Garcia, E.S. Isolation of Serratia Marcescens in the Midgut of Rhodnius Prolixus: Impact on the Establishment of the Parasite Trypanosoma Cruzi in the Vector. Exp. Parasitol. 2004, 107, 89–96. [Google Scholar] [CrossRef]
- Bando, H.; Okado, K.; Guelbeogo, W.M.; Badolo, A.; Aonuma, H.; Nelson, B.; Fukumoto, S.; Xuan, X.; Sagnon, N.; Kanuka, H. Intra-Specific Diversity of Serratia Marcescens in Anopheles Mosquito Midgut Defines Plasmodium Transmission Capacity. Sci. Rep. 2013, 3, 1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caragata, E.P.; Moreira, L.A. Using an Endosymbiont to Control Mosquito-Transmitted Disease. In Arthropod Vector: Controller of Disease Transmission, Volume 1; Elsevier: Amsterdam, The Netherlands, 2017; pp. 123–142. ISBN 978-0-12-805350-8. [Google Scholar]
- Kamga, G.-R.; Dissak-Delon, F.N.; Nana-Djeunga, H.C.; Biholong, B.D.; Mbigha-Ghogomu, S.; Souopgui, J.; Zoure, H.G.M.; Boussinesq, M.; Kamgno, J.; Robert, A. Still Mesoendemic Onchocerciasis in Two Cameroonian Community-Directed Treatment with Ivermectin Projects despite More than 15 Years of Mass Treatment. Parasites Vectors 2016, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A. Blackfly Ecology and Onchocerca Volvulus Transmission in Three Formerly Hyperendemic Foci in Uganda, Tanzania and Cameroon; University of Antwerp, Faculty of Pharmaceutical, Biomedical, and Veterinary Sciences, Department of Biomedical Sciences: Antwerp, Belgium, 2018. [Google Scholar]
- Cheke, R.A. Factors Affecting Onchocerciasis Transmission: Lessons for Infection Control. Expert Rev. Anti-Infect. Ther. 2017, 15, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito Vector-associated Microbiota: Metabarcoding Bacteria and Eukaryotic Symbionts across Habitat Types in Thailand Endemic for Dengue and Other Arthropod-borne Diseases. Ecol. Evol. 2018, 8, 1352–1368. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.C.; Ballard, J.W.O. Wolbachia Associations with Insects: Winning or Losing Against a Master Manipulator. Front. Ecol. Evol. 2016, 3, 153. [Google Scholar] [CrossRef] [Green Version]
- Emerson, D.; Rentz, J.A.; Lilburn, T.G.; Davis, R.E.; Aldrich, H.; Chan, C.; Moyer, C.L. A Novel Lineage of Proteobacteria Involved in Formation of Marine Fe-Oxidizing Microbial Mat Communities. PLoS ONE 2007, 2, e667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Tyagi, I.; Tyagi, K.; Chandra, K. Diversity and Structure of Bacterial Communities in the Gut of Spider: Thomisidae and Oxyopidae. Front. Ecol. Evol. 2020, 8, 588102. [Google Scholar] [CrossRef]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How Many Species Are Infected with Wolbachia?—A Statistical Analysis of Current Data: Wolbachia Infection Rates. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahantarig, A.; Kittayapong, P. Endosymbiotic Wolbachia Bacteria as Biological Control Tools of Disease Vectors and Pests: Endosymbiotic Wolbachia Bacteria as Biological Control. J. Appl. Entomol. 2011, 135, 479–486. [Google Scholar] [CrossRef]
- Werren, J.H.; Zhang, W.; Li Rong, G. Evolution and Phylogeny of Wolbachia: Reproductive Parasites of Arthropods. Proc. R. Soc. Lond. B 1995, 261, 55–63. [Google Scholar] [CrossRef]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia Genome of Brugia Malayi: Endosymbiont Evolution within a Human Pathogenic Nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, Y.I.; Dembele, B.; Diallo, A.A.; Lipner, E.M.; Doumbia, S.S.; Coulibaly, S.Y.; Konate, S.; Diallo, D.A.; Yalcouye, D.; Kubofcik, J.; et al. A Randomized Trial of Doxycycline for Mansonella Perstans Infection. N. Engl. J. Med. 2009, 361, 1448–1458. [Google Scholar] [CrossRef] [Green Version]
- McGarry, H.F.; Pfarr, K.; Egerton, G.; Hoerauf, A.; Akue, J.-P.; Enyong, P.; Wanji, S.; Kläger, S.L.; Bianco, A.E.; Beeching, N.J.; et al. Evidence against Wolbachia Symbiosis in Loa Loa. Filar J. 2003, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, C.A.; Cerqueira, G.C.; Goldberg, J.M.; Hotopp, J.C.D.; Haas, B.J.; Zucker, J.; Ribeiro, J.M.C.; Saif, S.; Levin, J.Z.; Fan, L.; et al. Genomics of Loa Loa, a Wolbachia-Free Filarial Parasite of Humans. Nat. Genet. 2013, 45, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Tchioffo, M.T.; Boissière, A.; Abate, L.; Nsango, S.E.; Bayibéki, A.N.; Awono-Ambéné, P.H.; Christen, R.; Gimonneau, G.; Morlais, I. Dynamics of Bacterial Community Composition in the Malaria Mosquito’s Epithelia. Front. Microbiol. 2016, 6, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksoy, E.; Telleria, E.L.; Echodu, R.; Wu, Y.; Okedi, L.M.; Weiss, B.L.; Aksoy, S.; Caccone, A. Analysis of Multiple Tsetse Fly Populations in Uganda Reveals Limited Diversity and Species-Specific Gut Microbiota. Appl. Environ. Microbiol. 2014, 80, 4301–4312. [Google Scholar] [CrossRef] [Green Version]
- Traoré-Lamizana, M.; Somiari, H.B.M.; Vagime, C.G.; Post, R.G. Sex Chromosomes Variation and Cytotaxonomy of Onchocerciasis Vector Simulium Squamosum in Cameroon and Nigeria. Med. Vet. Entomol. 2001, 15, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.H.; Crosskey, R.W. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory. 2021. Available online: https://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf (accessed on 22 December 2021).
- Apte-Deshpande, A.D.; Paingankar, M.S.; Gokhale, M.D.; Deobagkar, D.N. Serratia Odorifera Mediated Enhancement in Susceptibility of Aedes Aegypti for Chikungunya Virus. Indian J. Med. Res. 2014, 139, 762–768. [Google Scholar] [PubMed]
- Apte-Deshpande, A.; Paingankar, M.; Gokhale, M.D.; Deobagkar, D.N. Serratia Odorifera a Midgut Inhabitant of Aedes Aegypti Mosquito Enhances Its Susceptibility to Dengue-2 Virus. PLoS ONE 2012, 7, e40401. [Google Scholar] [CrossRef]
- Chaudhury, M.F.B. Blackflies: The Future for Biological Methods in Integrated Control. Ed. by M. Laird Xiv + 400 Pp., Academic Press, London, 1981, £28.20 (U.K.) ($58.00). Int. J. Trop. Insect Sci. 1983, 4, 299. [Google Scholar] [CrossRef]
- Yule, C.M.; Yong, H.S. Simuliidae Dipteria. 2012. Available online: https://www.researchgate.net/publication/233727136 (accessed on 22 December 2021).
- Wilson, M.D.; Post, R.J.; Gomulski, L.M. Multivariate Morphotaxonomy in the Identification of Adult Females of the Simulium Damnosum Theobald Complex (Diptera: Simuliidae) in the Onchocerciasis Control Programme Area of West Africa. Ann. Trop. Med. Parasitol. 1993, 87, 65–82. [Google Scholar] [CrossRef]

| Geographic Origin | No. Dissected Flies | No. Parous Flies (Prop; 95% CI) | No. Parous Flies with O. volvulus (Prop; 95% CI) |
|---|---|---|---|
| Bayomen | 200 | 34 (17.0%; 12.1–23.0) | 3 (8.9%; 1.9–23.7) |
| Biatsota | 558 | 100 (18.0%; 14.9–21.4) | 13 (13.0%; 7.1–21.2) |
| Nyamongo | 512 | 73 (14.3%; 11.3–17.6) | 5 (6.9%; 2.3–15.3) |
| Total | 1270 | 207 (16.3%; 14.3–18.4) | 21 (10.1%; 6.4–15.1) |
| Bacterial Genera | p | AUC (Lower–Upper) | Odds Ratio Infected/Uninfected | Mean Infected | Mean Uninfected |
|---|---|---|---|---|---|
| Fructobacillus | 0.0009 | 0.79 (0.65–0.93) | 0.45 | 0.06 | 0.18 |
| Roseomonas | 0.01 | 0.73 (0.57–0.89) | 1.41 | 0.36 | 0.17 |
| Acidomonas | 0.01 | 0.72 (0.56–0.87) | 7.12 | 0.09 | 0.07 |
| Phaseolibacter | 0.014 | 0.72 (056–0.88) | 2.97 | 0.07 | 0.09 |
| Sanguibacter | 0.016 | 0.71 (0.55–0.87) | 3.80 | 0.05 | 0.08 |
| Jatrophihabitans | 0.017 | 0.68 (0.54–0.07) | 0.07 | 0.01 | 0.07 |
| Leuconostoc | 0.019 | 0.66 (0.54–0.79) | 0.26 | 0.01 | 0.07 |
| HdN1 | 0.021 | 0.67 (0.54–0.81) | 0.26 | 0.01 | 0.06 |
| Brevibacterium | 0.028 | 0.70 0.54–0.86) | 0.27 | 0.09 | 0.35 |
| Tanticharoenia | 0.033 | 0.69 (0.53–0.85) | 2.24 | 0.08 | 0.10 |
| Cnuella | 0.04 | 0.35 (0.22–0.49) | 6.25 | 0.08 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efon Ekangouo, A.; Nana Djeunga, H.C.; Sempere, G.; Kamgno, J.; Njiokou, F.; Moundipa Fewou, P.; Geiger, A. Bacteriome Diversity of Blackflies’ Gut and Association with Onchocerca volvulus, the Causative Agent of Onchocerciasis in Mbam Valley (Center Region, Cameroon). Pathogens 2022, 11, 44. https://doi.org/10.3390/pathogens11010044
Efon Ekangouo A, Nana Djeunga HC, Sempere G, Kamgno J, Njiokou F, Moundipa Fewou P, Geiger A. Bacteriome Diversity of Blackflies’ Gut and Association with Onchocerca volvulus, the Causative Agent of Onchocerciasis in Mbam Valley (Center Region, Cameroon). Pathogens. 2022; 11(1):44. https://doi.org/10.3390/pathogens11010044
Chicago/Turabian StyleEfon Ekangouo, Arnauld, Hugues C. Nana Djeunga, Guilhem Sempere, Joseph Kamgno, Flobert Njiokou, Paul Moundipa Fewou, and Anne Geiger. 2022. "Bacteriome Diversity of Blackflies’ Gut and Association with Onchocerca volvulus, the Causative Agent of Onchocerciasis in Mbam Valley (Center Region, Cameroon)" Pathogens 11, no. 1: 44. https://doi.org/10.3390/pathogens11010044






